How Ethyl Acetate Reduces Ecological Footprint?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has gained significant attention in recent years due to its potential to reduce ecological footprints across various industries. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its widespread use stems from its unique properties, including low toxicity, high solvency, and rapid evaporation rate.
The history of ethyl acetate dates back to the early 19th century when it was first synthesized in laboratory settings. However, its industrial production and application gained momentum in the mid-20th century as environmental concerns began to shape industrial practices. The compound's ability to replace more harmful solvents in numerous applications has positioned it as a key player in the green chemistry movement.
In recent decades, the global push for sustainable development has further elevated the importance of ethyl acetate. Its biodegradability and low environmental impact have made it an attractive alternative to traditional petrochemical-based solvents. The compound's lifecycle assessment reveals a significantly lower carbon footprint compared to many of its counterparts, aligning with the principles of circular economy and waste reduction.
The versatility of ethyl acetate is evident in its wide range of applications across diverse sectors. In the coatings and adhesives industry, it serves as a crucial component in formulations, offering excellent solvency while minimizing environmental harm. The pharmaceutical sector utilizes ethyl acetate in the production of various medications, capitalizing on its low toxicity profile. Additionally, the food and beverage industry employs ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea, showcasing its safety for human consumption.
The role of ethyl acetate in reducing ecological footprints extends beyond its direct applications. Its production process has evolved to incorporate more sustainable practices, including the use of bio-based feedstocks and energy-efficient manufacturing techniques. This shift towards greener production methods has further enhanced the compound's environmental credentials, making it a cornerstone of sustainable industrial practices.
As global environmental regulations become increasingly stringent, the demand for ethyl acetate continues to grow. Its ability to meet both performance requirements and environmental standards positions it as a key enabler of sustainable innovation across industries. The ongoing research and development in ethyl acetate production and application promise to unlock new possibilities for reducing ecological footprints, driving the transition towards a more sustainable industrial landscape.
The history of ethyl acetate dates back to the early 19th century when it was first synthesized in laboratory settings. However, its industrial production and application gained momentum in the mid-20th century as environmental concerns began to shape industrial practices. The compound's ability to replace more harmful solvents in numerous applications has positioned it as a key player in the green chemistry movement.
In recent decades, the global push for sustainable development has further elevated the importance of ethyl acetate. Its biodegradability and low environmental impact have made it an attractive alternative to traditional petrochemical-based solvents. The compound's lifecycle assessment reveals a significantly lower carbon footprint compared to many of its counterparts, aligning with the principles of circular economy and waste reduction.
The versatility of ethyl acetate is evident in its wide range of applications across diverse sectors. In the coatings and adhesives industry, it serves as a crucial component in formulations, offering excellent solvency while minimizing environmental harm. The pharmaceutical sector utilizes ethyl acetate in the production of various medications, capitalizing on its low toxicity profile. Additionally, the food and beverage industry employs ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea, showcasing its safety for human consumption.
The role of ethyl acetate in reducing ecological footprints extends beyond its direct applications. Its production process has evolved to incorporate more sustainable practices, including the use of bio-based feedstocks and energy-efficient manufacturing techniques. This shift towards greener production methods has further enhanced the compound's environmental credentials, making it a cornerstone of sustainable industrial practices.
As global environmental regulations become increasingly stringent, the demand for ethyl acetate continues to grow. Its ability to meet both performance requirements and environmental standards positions it as a key enabler of sustainable innovation across industries. The ongoing research and development in ethyl acetate production and application promise to unlock new possibilities for reducing ecological footprints, driving the transition towards a more sustainable industrial landscape.
Green Solvent Market Analysis
The green solvent market has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations on volatile organic compounds (VOCs) emissions. Ethyl acetate, as a key player in this market, has gained substantial attention due to its eco-friendly properties and versatile applications across various industries.
The global green solvent market is projected to expand at a robust rate, with ethyl acetate playing a crucial role in this growth. The market is primarily fueled by the rising demand for sustainable and environmentally friendly solvents in industries such as paints and coatings, pharmaceuticals, adhesives, and cosmetics. Ethyl acetate's low toxicity, biodegradability, and relatively low cost make it an attractive alternative to traditional petroleum-based solvents.
In the paints and coatings industry, ethyl acetate is increasingly being used as a replacement for more harmful solvents, contributing to the reduction of VOC emissions and improving air quality. The pharmaceutical sector is another major consumer of ethyl acetate, utilizing it in the production of various drugs and as an extraction solvent. The adhesives industry has also embraced ethyl acetate for its excellent solvency and quick evaporation properties, making it ideal for fast-drying adhesives.
The cosmetics and personal care industry is witnessing a growing trend towards natural and eco-friendly ingredients, further boosting the demand for ethyl acetate as a solvent in nail polish removers and other beauty products. Additionally, the food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea, expanding its market reach.
Geographically, Asia-Pacific is expected to dominate the green solvent market, with China and India leading the growth due to rapid industrialization and increasing environmental awareness. North America and Europe follow closely, driven by strict environmental regulations and a strong focus on sustainable practices in manufacturing processes.
The market landscape for ethyl acetate is characterized by both established chemical companies and emerging players focusing on bio-based production methods. This competition is likely to drive innovation in production techniques and applications, further enhancing the ecological benefits of ethyl acetate.
As industries continue to prioritize sustainability and environmental responsibility, the demand for green solvents like ethyl acetate is expected to surge. This trend aligns with global efforts to reduce carbon footprints and transition towards a circular economy, positioning ethyl acetate as a key component in the green chemistry revolution.
The global green solvent market is projected to expand at a robust rate, with ethyl acetate playing a crucial role in this growth. The market is primarily fueled by the rising demand for sustainable and environmentally friendly solvents in industries such as paints and coatings, pharmaceuticals, adhesives, and cosmetics. Ethyl acetate's low toxicity, biodegradability, and relatively low cost make it an attractive alternative to traditional petroleum-based solvents.
In the paints and coatings industry, ethyl acetate is increasingly being used as a replacement for more harmful solvents, contributing to the reduction of VOC emissions and improving air quality. The pharmaceutical sector is another major consumer of ethyl acetate, utilizing it in the production of various drugs and as an extraction solvent. The adhesives industry has also embraced ethyl acetate for its excellent solvency and quick evaporation properties, making it ideal for fast-drying adhesives.
The cosmetics and personal care industry is witnessing a growing trend towards natural and eco-friendly ingredients, further boosting the demand for ethyl acetate as a solvent in nail polish removers and other beauty products. Additionally, the food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea, expanding its market reach.
Geographically, Asia-Pacific is expected to dominate the green solvent market, with China and India leading the growth due to rapid industrialization and increasing environmental awareness. North America and Europe follow closely, driven by strict environmental regulations and a strong focus on sustainable practices in manufacturing processes.
The market landscape for ethyl acetate is characterized by both established chemical companies and emerging players focusing on bio-based production methods. This competition is likely to drive innovation in production techniques and applications, further enhancing the ecological benefits of ethyl acetate.
As industries continue to prioritize sustainability and environmental responsibility, the demand for green solvents like ethyl acetate is expected to surge. This trend aligns with global efforts to reduce carbon footprints and transition towards a circular economy, positioning ethyl acetate as a key component in the green chemistry revolution.
Eco-Friendly Solvent Challenges
The development of eco-friendly solvents faces numerous challenges in the quest for sustainable chemical processes. Traditional solvents, while effective, often pose significant environmental and health risks. The primary challenge lies in finding alternatives that maintain or improve process efficiency while reducing ecological impact.
One major hurdle is the performance gap between conventional and green solvents. Many eco-friendly options struggle to match the dissolution power, reaction rates, and overall effectiveness of their traditional counterparts. This disparity often leads to increased process times, higher energy consumption, or reduced product yields, potentially offsetting the environmental benefits.
Cost considerations present another significant obstacle. Green solvents, particularly those derived from renewable resources, often come with higher production costs. This economic barrier can deter widespread adoption, especially in industries with tight profit margins or those operating at large scales.
Scalability and availability pose additional challenges. Many promising eco-friendly solvents are still in the early stages of development or production. Scaling up these novel solutions to meet industrial demands requires substantial investment in research, development, and infrastructure.
Regulatory hurdles and safety concerns also complicate the transition to greener solvents. While aimed at protecting human health and the environment, stringent regulations can slow the approval and implementation of new solvent technologies. Additionally, the long-term effects of some alternative solvents are not yet fully understood, necessitating extensive testing and validation.
The diversity of industrial applications further complicates the development of universal green solvent solutions. Different processes require solvents with specific properties, making it challenging to create a one-size-fits-all eco-friendly alternative. This necessitates a tailored approach, often resulting in a fragmented market of specialized green solvents.
Stability and recyclability present ongoing challenges in the pursuit of sustainable solvent solutions. Many eco-friendly solvents degrade more quickly than their traditional counterparts, potentially leading to increased waste and replacement costs. Developing effective recycling methods for these solvents is crucial but often technically complex and energy-intensive.
Despite these challenges, the drive towards eco-friendly solvents continues to gain momentum. Innovations in green chemistry, coupled with increasing environmental awareness and regulatory pressure, are gradually overcoming these obstacles. The development of bio-based solvents, ionic liquids, and supercritical fluids represents promising avenues for future advancements in this field.
One major hurdle is the performance gap between conventional and green solvents. Many eco-friendly options struggle to match the dissolution power, reaction rates, and overall effectiveness of their traditional counterparts. This disparity often leads to increased process times, higher energy consumption, or reduced product yields, potentially offsetting the environmental benefits.
Cost considerations present another significant obstacle. Green solvents, particularly those derived from renewable resources, often come with higher production costs. This economic barrier can deter widespread adoption, especially in industries with tight profit margins or those operating at large scales.
Scalability and availability pose additional challenges. Many promising eco-friendly solvents are still in the early stages of development or production. Scaling up these novel solutions to meet industrial demands requires substantial investment in research, development, and infrastructure.
Regulatory hurdles and safety concerns also complicate the transition to greener solvents. While aimed at protecting human health and the environment, stringent regulations can slow the approval and implementation of new solvent technologies. Additionally, the long-term effects of some alternative solvents are not yet fully understood, necessitating extensive testing and validation.
The diversity of industrial applications further complicates the development of universal green solvent solutions. Different processes require solvents with specific properties, making it challenging to create a one-size-fits-all eco-friendly alternative. This necessitates a tailored approach, often resulting in a fragmented market of specialized green solvents.
Stability and recyclability present ongoing challenges in the pursuit of sustainable solvent solutions. Many eco-friendly solvents degrade more quickly than their traditional counterparts, potentially leading to increased waste and replacement costs. Developing effective recycling methods for these solvents is crucial but often technically complex and energy-intensive.
Despite these challenges, the drive towards eco-friendly solvents continues to gain momentum. Innovations in green chemistry, coupled with increasing environmental awareness and regulatory pressure, are gradually overcoming these obstacles. The development of bio-based solvents, ionic liquids, and supercritical fluids represents promising avenues for future advancements in this field.
Current Eco-Footprint Solutions
01 Life cycle assessment of ethyl acetate production
Evaluating the environmental impact of ethyl acetate throughout its production lifecycle, including raw material extraction, manufacturing processes, transportation, use, and disposal. This assessment helps quantify the ecological footprint by considering factors such as energy consumption, greenhouse gas emissions, and resource depletion.- Environmental impact assessment of ethyl acetate production: Methods for assessing the ecological footprint of ethyl acetate production, including life cycle analysis and carbon footprint calculations. These assessments consider factors such as raw material sourcing, energy consumption, and emissions throughout the production process to determine the overall environmental impact.
- Green synthesis methods for ethyl acetate: Development of environmentally friendly synthesis routes for ethyl acetate production, focusing on reducing energy consumption, using renewable feedstocks, and minimizing waste generation. These methods aim to decrease the ecological footprint associated with traditional production processes.
- Recycling and recovery of ethyl acetate in industrial processes: Techniques for recycling and recovering ethyl acetate from industrial waste streams, reducing the overall consumption and environmental impact. These methods include distillation, membrane separation, and adsorption processes to reclaim and reuse ethyl acetate in various applications.
- Alternatives to ethyl acetate with lower ecological impact: Research and development of alternative solvents and chemicals with similar properties to ethyl acetate but with reduced environmental impact. These alternatives may include bio-based solvents or compounds derived from renewable resources, aiming to minimize the ecological footprint associated with traditional petrochemical-based ethyl acetate.
- Optimization of ethyl acetate use in industrial applications: Strategies for optimizing the use of ethyl acetate in various industrial applications to reduce consumption and minimize environmental impact. This includes improving process efficiency, developing precision application techniques, and implementing closed-loop systems to minimize emissions and waste generation.
02 Green synthesis methods for ethyl acetate
Developing environmentally friendly synthesis routes for ethyl acetate production, focusing on reducing energy consumption, utilizing renewable resources, and minimizing waste generation. These methods aim to decrease the overall ecological footprint of ethyl acetate manufacturing.Expand Specific Solutions03 Recycling and recovery of ethyl acetate
Implementing efficient recycling and recovery processes for ethyl acetate in industrial applications, reducing the need for new production and minimizing waste. These techniques help lower the ecological footprint by extending the lifecycle of the solvent and reducing overall consumption.Expand Specific Solutions04 Ethyl acetate alternatives with lower environmental impact
Researching and developing alternative solvents or processes that can replace ethyl acetate in various applications while offering a reduced ecological footprint. This includes exploring bio-based solvents or solvent-free technologies to minimize environmental impact.Expand Specific Solutions05 Monitoring and reducing emissions in ethyl acetate use
Implementing advanced monitoring systems and control measures to reduce volatile organic compound (VOC) emissions associated with ethyl acetate use in industrial processes. This approach helps minimize air pollution and improve the overall environmental performance of ethyl acetate applications.Expand Specific Solutions
Key Ethyl Acetate Manufacturers
The market for ethyl acetate as an eco-friendly solvent is in a growth phase, driven by increasing environmental regulations and sustainability initiatives. The global market size is projected to expand significantly in the coming years, with a compound annual growth rate exceeding 5%. Technologically, ethyl acetate production is mature, but innovations in bio-based and green chemistry approaches are emerging. Key players like BASF, Dow Chemical, and Celanese are investing in sustainable production methods, while companies such as LanzaTech and Archer-Daniels-Midland are exploring bio-based alternatives. Research institutions like the University of California and China Agricultural University are contributing to advancements in eco-friendly synthesis processes, indicating a collaborative effort between industry and academia to reduce the ecological footprint of ethyl acetate production.
BASF Corp.
Technical Solution: BASF has developed a bio-based production process for ethyl acetate using renewable feedstocks. Their approach involves fermenting sugars derived from agricultural waste to produce ethanol, which is then catalytically converted to ethyl acetate. This process reduces the carbon footprint by up to 50% compared to conventional petrochemical routes [1]. BASF's technology also incorporates energy-efficient distillation and membrane separation techniques, further minimizing environmental impact. The company has implemented this process at industrial scale, demonstrating its viability for large-scale production of more sustainable ethyl acetate [3].
Strengths: Significant reduction in carbon footprint, use of renewable resources, scalable to industrial production. Weaknesses: Dependent on availability and price fluctuations of agricultural feedstocks, may require modifications to existing production infrastructure.
Wacker Chemie AG
Technical Solution: Wacker Chemie has pioneered an innovative acetic acid esterification process for ethyl acetate production that substantially reduces energy consumption and improves yield. Their ACETOCELL® technology uses a highly selective heterogeneous catalyst and reactive distillation, combining reaction and separation steps in a single unit operation [2]. This integrated approach reduces the number of process steps, lowering both capital and operating costs. The process achieves near-complete conversion of raw materials, minimizing waste and improving atom economy. Wacker's method also operates at lower temperatures than conventional processes, further reducing energy requirements and associated emissions [4].
Strengths: High energy efficiency, improved yield, reduced waste generation. Weaknesses: May require significant initial investment for retrofitting existing plants, potential catalyst lifecycle management challenges.
Ethyl Acetate Green Chemistry
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
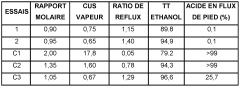
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Process for the production of ethyle acetate
PatentInactiveEP0339330A3
Innovation
- A process utilizing citric acid as a natural catalyst, combined with biologically produced ethanol and acetic acid, eliminates the need for mineral acids, employing a reactor system with specific heating and condensation steps to achieve high purity ethyl acetate production.
Environmental Regulations Impact
The impact of environmental regulations on the use of ethyl acetate as an eco-friendly solvent has been significant in recent years. As governments worldwide implement stricter environmental policies, industries are increasingly turning to more sustainable alternatives like ethyl acetate to reduce their ecological footprint.
One of the primary drivers for the adoption of ethyl acetate is the regulation of volatile organic compounds (VOCs). Many countries have established limits on VOC emissions, prompting manufacturers to seek low-VOC solvents. Ethyl acetate, with its lower volatility compared to traditional solvents, helps companies comply with these regulations while maintaining product performance.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has also played a crucial role in promoting the use of ethyl acetate. Under REACH, substances are evaluated for their potential risks to human health and the environment. Ethyl acetate's favorable environmental profile has positioned it as a preferred choice for many applications, as it meets the stringent REACH requirements.
In the United States, the Environmental Protection Agency (EPA) has implemented the Toxic Substances Control Act (TSCA), which regulates the production and use of chemicals. Ethyl acetate's classification as a low-toxicity substance under TSCA has further encouraged its adoption across various industries, from coatings and adhesives to pharmaceuticals and food processing.
The Montreal Protocol, an international treaty designed to protect the ozone layer, has indirectly boosted the use of ethyl acetate. As the protocol phased out ozone-depleting substances, many industries sought alternatives that were both effective and environmentally friendly. Ethyl acetate emerged as a suitable replacement in numerous applications, contributing to its increased market share.
Carbon footprint reduction initiatives, driven by global climate agreements such as the Paris Agreement, have also influenced the shift towards ethyl acetate. As companies strive to minimize their greenhouse gas emissions, the use of bio-based ethyl acetate, derived from renewable resources, has gained traction. This aligns with regulatory efforts to promote circular economy principles and reduce dependence on fossil-based raw materials.
The implementation of Extended Producer Responsibility (EPR) programs in various countries has further incentivized the use of environmentally friendly solvents like ethyl acetate. These programs hold manufacturers responsible for the entire lifecycle of their products, including disposal. By using ethyl acetate, which is biodegradable and less harmful to ecosystems, companies can more easily comply with EPR requirements and reduce their end-of-life environmental impact.
As environmental regulations continue to evolve and become more stringent, the role of ethyl acetate in reducing ecological footprints is likely to expand. Its ability to meet regulatory standards while offering performance benefits positions it as a key player in the transition towards more sustainable industrial practices.
One of the primary drivers for the adoption of ethyl acetate is the regulation of volatile organic compounds (VOCs). Many countries have established limits on VOC emissions, prompting manufacturers to seek low-VOC solvents. Ethyl acetate, with its lower volatility compared to traditional solvents, helps companies comply with these regulations while maintaining product performance.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has also played a crucial role in promoting the use of ethyl acetate. Under REACH, substances are evaluated for their potential risks to human health and the environment. Ethyl acetate's favorable environmental profile has positioned it as a preferred choice for many applications, as it meets the stringent REACH requirements.
In the United States, the Environmental Protection Agency (EPA) has implemented the Toxic Substances Control Act (TSCA), which regulates the production and use of chemicals. Ethyl acetate's classification as a low-toxicity substance under TSCA has further encouraged its adoption across various industries, from coatings and adhesives to pharmaceuticals and food processing.
The Montreal Protocol, an international treaty designed to protect the ozone layer, has indirectly boosted the use of ethyl acetate. As the protocol phased out ozone-depleting substances, many industries sought alternatives that were both effective and environmentally friendly. Ethyl acetate emerged as a suitable replacement in numerous applications, contributing to its increased market share.
Carbon footprint reduction initiatives, driven by global climate agreements such as the Paris Agreement, have also influenced the shift towards ethyl acetate. As companies strive to minimize their greenhouse gas emissions, the use of bio-based ethyl acetate, derived from renewable resources, has gained traction. This aligns with regulatory efforts to promote circular economy principles and reduce dependence on fossil-based raw materials.
The implementation of Extended Producer Responsibility (EPR) programs in various countries has further incentivized the use of environmentally friendly solvents like ethyl acetate. These programs hold manufacturers responsible for the entire lifecycle of their products, including disposal. By using ethyl acetate, which is biodegradable and less harmful to ecosystems, companies can more easily comply with EPR requirements and reduce their end-of-life environmental impact.
As environmental regulations continue to evolve and become more stringent, the role of ethyl acetate in reducing ecological footprints is likely to expand. Its ability to meet regulatory standards while offering performance benefits positions it as a key player in the transition towards more sustainable industrial practices.
Life Cycle Assessment Methods
Life Cycle Assessment (LCA) is a crucial methodology for evaluating the environmental impact of ethyl acetate throughout its entire lifecycle. This comprehensive approach considers all stages, from raw material extraction to production, use, and disposal. The ISO 14040 and 14044 standards provide a framework for conducting LCAs, ensuring consistency and reliability in the assessment process.
One key aspect of LCA for ethyl acetate is the inventory analysis, which involves quantifying all inputs and outputs associated with the product's lifecycle. This includes energy consumption, water usage, emissions, and waste generation. Data collection is critical, often requiring collaboration with suppliers and manufacturers to obtain accurate information.
The impact assessment phase of LCA translates the inventory data into potential environmental effects. For ethyl acetate, this may include global warming potential, acidification, eutrophication, and ozone depletion. Various impact assessment methods, such as ReCiPe or TRACI, can be employed to calculate these impacts, each with its own set of characterization factors and impact categories.
Sensitivity analysis is an essential component of LCA, allowing researchers to identify the most significant contributors to environmental impact. For ethyl acetate, this might reveal that the production phase or specific raw materials have the greatest influence on the overall ecological footprint. Such insights can guide targeted improvements in the production process or supply chain.
Comparative LCAs are particularly valuable when assessing ethyl acetate's ecological footprint. By comparing ethyl acetate with alternative solvents or production methods, researchers can determine its relative environmental performance. This approach helps identify opportunities for reducing the ecological footprint through process optimization or material substitution.
The interpretation phase of LCA synthesizes the results, drawing conclusions and making recommendations. For ethyl acetate, this might involve suggesting process improvements, exploring alternative feedstocks, or recommending changes in waste management practices. It's crucial to consider the limitations and uncertainties inherent in the LCA process during this phase.
As LCA methodologies continue to evolve, new approaches such as consequential LCA and social LCA are emerging. These methods can provide a more holistic view of ethyl acetate's impact, considering broader economic and social implications alongside environmental factors. Additionally, the integration of LCA with other tools, such as risk assessment or cost-benefit analysis, can offer a more comprehensive evaluation of ethyl acetate's sustainability profile.
One key aspect of LCA for ethyl acetate is the inventory analysis, which involves quantifying all inputs and outputs associated with the product's lifecycle. This includes energy consumption, water usage, emissions, and waste generation. Data collection is critical, often requiring collaboration with suppliers and manufacturers to obtain accurate information.
The impact assessment phase of LCA translates the inventory data into potential environmental effects. For ethyl acetate, this may include global warming potential, acidification, eutrophication, and ozone depletion. Various impact assessment methods, such as ReCiPe or TRACI, can be employed to calculate these impacts, each with its own set of characterization factors and impact categories.
Sensitivity analysis is an essential component of LCA, allowing researchers to identify the most significant contributors to environmental impact. For ethyl acetate, this might reveal that the production phase or specific raw materials have the greatest influence on the overall ecological footprint. Such insights can guide targeted improvements in the production process or supply chain.
Comparative LCAs are particularly valuable when assessing ethyl acetate's ecological footprint. By comparing ethyl acetate with alternative solvents or production methods, researchers can determine its relative environmental performance. This approach helps identify opportunities for reducing the ecological footprint through process optimization or material substitution.
The interpretation phase of LCA synthesizes the results, drawing conclusions and making recommendations. For ethyl acetate, this might involve suggesting process improvements, exploring alternative feedstocks, or recommending changes in waste management practices. It's crucial to consider the limitations and uncertainties inherent in the LCA process during this phase.
As LCA methodologies continue to evolve, new approaches such as consequential LCA and social LCA are emerging. These methods can provide a more holistic view of ethyl acetate's impact, considering broader economic and social implications alongside environmental factors. Additionally, the integration of LCA with other tools, such as risk assessment or cost-benefit analysis, can offer a more comprehensive evaluation of ethyl acetate's sustainability profile.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!