How Ethyl Acetate Contributes to Cutting‑Edge Techniques?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
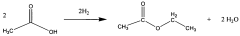
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has emerged as a crucial component in various cutting-edge techniques across multiple industries. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its unique properties, including low toxicity, high solvency, and rapid evaporation rate, have made it an indispensable ingredient in numerous advanced applications.
In the realm of materials science, ethyl acetate plays a pivotal role in the development of novel coatings and adhesives. Its excellent solvent properties enable the creation of high-performance, fast-drying formulations that find applications in electronics, automotive, and aerospace industries. The compound's ability to dissolve a wide range of polymers and resins has led to breakthroughs in the production of advanced composite materials with enhanced mechanical and thermal properties.
The pharmaceutical industry has also benefited significantly from ethyl acetate's contributions to cutting-edge techniques. Its use as a solvent in the extraction and purification of active pharmaceutical ingredients (APIs) has revolutionized drug manufacturing processes. The compound's low boiling point and high selectivity make it an ideal choice for liquid-liquid extraction and recrystallization procedures, enabling the production of high-purity pharmaceutical compounds with improved efficiency and yield.
In the field of nanotechnology, ethyl acetate has proven instrumental in the synthesis and modification of nanoparticles. Its controlled evaporation characteristics allow for precise manipulation of particle size and morphology during sol-gel processes and emulsion-based synthesis methods. This has led to advancements in the development of nanomaterials with tailored properties for applications in catalysis, drug delivery, and energy storage.
The electronics industry has harnessed ethyl acetate's properties in the fabrication of advanced semiconductor devices. Its use as a cleaning agent and photoresist solvent in photolithography processes has contributed to the miniaturization of electronic components and the development of high-density integrated circuits. The compound's low residue formation and compatibility with sensitive materials make it an essential component in the production of cutting-edge microelectronics.
Furthermore, ethyl acetate has found applications in green chemistry initiatives, aligning with the growing demand for sustainable and environmentally friendly processes. Its biodegradability and low toxicity make it an attractive alternative to more hazardous solvents in various industrial processes. This has led to the development of cleaner production methods and eco-friendly products across multiple sectors, including cosmetics, food processing, and agrochemicals.
In the realm of materials science, ethyl acetate plays a pivotal role in the development of novel coatings and adhesives. Its excellent solvent properties enable the creation of high-performance, fast-drying formulations that find applications in electronics, automotive, and aerospace industries. The compound's ability to dissolve a wide range of polymers and resins has led to breakthroughs in the production of advanced composite materials with enhanced mechanical and thermal properties.
The pharmaceutical industry has also benefited significantly from ethyl acetate's contributions to cutting-edge techniques. Its use as a solvent in the extraction and purification of active pharmaceutical ingredients (APIs) has revolutionized drug manufacturing processes. The compound's low boiling point and high selectivity make it an ideal choice for liquid-liquid extraction and recrystallization procedures, enabling the production of high-purity pharmaceutical compounds with improved efficiency and yield.
In the field of nanotechnology, ethyl acetate has proven instrumental in the synthesis and modification of nanoparticles. Its controlled evaporation characteristics allow for precise manipulation of particle size and morphology during sol-gel processes and emulsion-based synthesis methods. This has led to advancements in the development of nanomaterials with tailored properties for applications in catalysis, drug delivery, and energy storage.
The electronics industry has harnessed ethyl acetate's properties in the fabrication of advanced semiconductor devices. Its use as a cleaning agent and photoresist solvent in photolithography processes has contributed to the miniaturization of electronic components and the development of high-density integrated circuits. The compound's low residue formation and compatibility with sensitive materials make it an essential component in the production of cutting-edge microelectronics.
Furthermore, ethyl acetate has found applications in green chemistry initiatives, aligning with the growing demand for sustainable and environmentally friendly processes. Its biodegradability and low toxicity make it an attractive alternative to more hazardous solvents in various industrial processes. This has led to the development of cleaner production methods and eco-friendly products across multiple sectors, including cosmetics, food processing, and agrochemicals.
Market Demand Analysis
The market demand for ethyl acetate in cutting-edge techniques has been steadily growing, driven by its versatile applications across various industries. In the pharmaceutical sector, ethyl acetate plays a crucial role in the production of active pharmaceutical ingredients (APIs) and drug formulations. Its ability to act as an efficient solvent and extraction medium has made it indispensable in the development of novel drug delivery systems and controlled-release medications.
In the electronics industry, ethyl acetate has found increasing use in the manufacturing of advanced printed circuit boards (PCBs) and semiconductor devices. Its low toxicity and excellent solvency properties make it an ideal choice for cleaning and degreasing electronic components, contributing to the production of high-performance, miniaturized electronic devices.
The automotive sector has also seen a rise in ethyl acetate demand, particularly in the development of eco-friendly coatings and adhesives. As the industry shifts towards more sustainable practices, ethyl acetate's role in water-based and low-VOC formulations has become increasingly significant. This trend is expected to continue as environmental regulations become more stringent globally.
In the field of nanotechnology, ethyl acetate has emerged as a valuable component in the synthesis of nanoparticles and nanostructured materials. Its unique properties allow for precise control over particle size and morphology, enabling the creation of advanced materials with tailored properties for applications in energy storage, catalysis, and biomedical engineering.
The food and beverage industry has witnessed a growing demand for ethyl acetate in the production of natural and artificial flavors. Its use as a flavoring agent and solvent in the extraction of natural essences has expanded, driven by consumer preferences for clean-label products and natural ingredients.
Market analysts project that the global ethyl acetate market will experience substantial growth in the coming years. This growth is attributed to the increasing adoption of ethyl acetate in emerging technologies and the expansion of its application scope in existing industries. The Asia-Pacific region is expected to be a key driver of this growth, with rapid industrialization and increasing investments in research and development.
As sustainability becomes a central focus across industries, the demand for bio-based ethyl acetate is anticipated to rise. This shift towards renewable sources aligns with the global push for greener technologies and circular economy principles, potentially opening new market opportunities and driving innovation in production processes.
In the electronics industry, ethyl acetate has found increasing use in the manufacturing of advanced printed circuit boards (PCBs) and semiconductor devices. Its low toxicity and excellent solvency properties make it an ideal choice for cleaning and degreasing electronic components, contributing to the production of high-performance, miniaturized electronic devices.
The automotive sector has also seen a rise in ethyl acetate demand, particularly in the development of eco-friendly coatings and adhesives. As the industry shifts towards more sustainable practices, ethyl acetate's role in water-based and low-VOC formulations has become increasingly significant. This trend is expected to continue as environmental regulations become more stringent globally.
In the field of nanotechnology, ethyl acetate has emerged as a valuable component in the synthesis of nanoparticles and nanostructured materials. Its unique properties allow for precise control over particle size and morphology, enabling the creation of advanced materials with tailored properties for applications in energy storage, catalysis, and biomedical engineering.
The food and beverage industry has witnessed a growing demand for ethyl acetate in the production of natural and artificial flavors. Its use as a flavoring agent and solvent in the extraction of natural essences has expanded, driven by consumer preferences for clean-label products and natural ingredients.
Market analysts project that the global ethyl acetate market will experience substantial growth in the coming years. This growth is attributed to the increasing adoption of ethyl acetate in emerging technologies and the expansion of its application scope in existing industries. The Asia-Pacific region is expected to be a key driver of this growth, with rapid industrialization and increasing investments in research and development.
As sustainability becomes a central focus across industries, the demand for bio-based ethyl acetate is anticipated to rise. This shift towards renewable sources aligns with the global push for greener technologies and circular economy principles, potentially opening new market opportunities and driving innovation in production processes.
Technical Challenges
Despite the widespread use of ethyl acetate in various industries, its application in cutting-edge techniques faces several technical challenges. One of the primary obstacles is the optimization of ethyl acetate's properties for specific advanced applications. While its solvent properties are well-known, fine-tuning these characteristics for novel uses in nanotechnology, advanced materials, or biotechnology requires extensive research and development.
The volatility of ethyl acetate, while beneficial in many applications, poses challenges in certain cutting-edge techniques. For instance, in 3D printing and additive manufacturing, controlling the evaporation rate of ethyl acetate-based solutions is crucial for achieving precise structural integrity and surface finish. Developing methods to regulate this volatility without compromising other desirable properties remains a significant technical hurdle.
Another challenge lies in the purification and quality control of ethyl acetate for high-precision applications. As technologies advance, the demand for ultra-pure ethyl acetate increases, particularly in the semiconductor industry and in the production of advanced electronic components. Achieving and maintaining the required level of purity at scale presents both technical and economic challenges.
The environmental impact of ethyl acetate production and use is also a growing concern. While it is considered less harmful than many other solvents, developing greener synthesis routes and more efficient recycling methods is essential for its continued use in environmentally conscious cutting-edge applications. This challenge intersects with broader sustainability goals in advanced technologies.
In the field of drug delivery and controlled release systems, incorporating ethyl acetate into novel formulations presents unique challenges. Researchers must overcome issues related to stability, biocompatibility, and controlled degradation to harness ethyl acetate's potential in these advanced biomedical applications.
The integration of ethyl acetate into smart materials and responsive systems is another area of technical difficulty. Creating materials that can change properties in response to environmental stimuli, using ethyl acetate as a key component, requires innovative approaches in material science and chemical engineering.
Lastly, the scalability of ethyl acetate-based processes in cutting-edge technologies presents significant challenges. As laboratory-scale successes move towards industrial application, issues related to cost-effectiveness, process efficiency, and quality consistency at larger scales become prominent. Addressing these scaling challenges is crucial for the widespread adoption of ethyl acetate in advanced technological applications.
The volatility of ethyl acetate, while beneficial in many applications, poses challenges in certain cutting-edge techniques. For instance, in 3D printing and additive manufacturing, controlling the evaporation rate of ethyl acetate-based solutions is crucial for achieving precise structural integrity and surface finish. Developing methods to regulate this volatility without compromising other desirable properties remains a significant technical hurdle.
Another challenge lies in the purification and quality control of ethyl acetate for high-precision applications. As technologies advance, the demand for ultra-pure ethyl acetate increases, particularly in the semiconductor industry and in the production of advanced electronic components. Achieving and maintaining the required level of purity at scale presents both technical and economic challenges.
The environmental impact of ethyl acetate production and use is also a growing concern. While it is considered less harmful than many other solvents, developing greener synthesis routes and more efficient recycling methods is essential for its continued use in environmentally conscious cutting-edge applications. This challenge intersects with broader sustainability goals in advanced technologies.
In the field of drug delivery and controlled release systems, incorporating ethyl acetate into novel formulations presents unique challenges. Researchers must overcome issues related to stability, biocompatibility, and controlled degradation to harness ethyl acetate's potential in these advanced biomedical applications.
The integration of ethyl acetate into smart materials and responsive systems is another area of technical difficulty. Creating materials that can change properties in response to environmental stimuli, using ethyl acetate as a key component, requires innovative approaches in material science and chemical engineering.
Lastly, the scalability of ethyl acetate-based processes in cutting-edge technologies presents significant challenges. As laboratory-scale successes move towards industrial application, issues related to cost-effectiveness, process efficiency, and quality consistency at larger scales become prominent. Addressing these scaling challenges is crucial for the widespread adoption of ethyl acetate in advanced technological applications.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods and processes for producing and purifying ethyl acetate are described. These include distillation techniques, reactive distillation, and the use of specific catalysts to improve yield and purity. The processes aim to enhance efficiency and reduce energy consumption in ethyl acetate production.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also utilized in extraction processes and as a reaction medium in chemical synthesis.
- Ethyl acetate in polymer and material science: Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of various polymers and composites, as well as in the modification of material properties. The compound's solvent properties make it valuable in polymer processing and material fabrication.
- Environmental and safety considerations for ethyl acetate: Environmental and safety aspects related to ethyl acetate use and production are addressed. This includes methods for reducing emissions, improving handling safety, and developing more environmentally friendly production processes. Considerations for proper storage and disposal are also discussed.
- Novel synthesis routes and derivatives of ethyl acetate: Research into novel synthesis routes for ethyl acetate and its derivatives is ongoing. This includes the development of new catalysts, alternative feedstocks, and innovative reaction pathways. The creation of functionalized ethyl acetate derivatives for specific applications is also explored.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse manufacturing applications.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its solvent properties make it useful for isolating specific substances from mixtures, particularly in the pharmaceutical and food industries. Techniques involving ethyl acetate as an extraction solvent are described.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Methods for handling, storing, and disposing of ethyl acetate safely are discussed. These include techniques for reducing emissions, recovering ethyl acetate from waste streams, and ensuring worker safety when using the compound in industrial settings.Expand Specific Solutions05 Novel synthesis routes and modifications of ethyl acetate
Research into new synthesis routes and chemical modifications of ethyl acetate is presented. This includes the development of more efficient production methods, the creation of ethyl acetate derivatives, and the exploration of catalytic processes to enhance its properties or reactivity.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is in a mature growth stage, with a global market size estimated to reach $4.3 billion by 2026. The technology for ethyl acetate production is well-established, with major players like Celanese International Corp., Eastman Chemical Co., and SABIC Global Technologies BV dominating the industry. These companies have advanced manufacturing capabilities and extensive distribution networks. However, emerging players such as Resonac Corp. and Zhuhai Qianxin New Materials Co., Ltd. are introducing innovative production methods and applications, driving further market expansion. The competitive landscape is characterized by ongoing research and development efforts to improve production efficiency and explore new end-use applications in sectors like pharmaceuticals, packaging, and electronics.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using advanced catalysts and reactive distillation technology. This method allows for the direct esterification of ethanol and acetic acid in a single step, significantly improving efficiency and reducing energy consumption[1]. The company has also implemented a proprietary purification process that results in high-purity ethyl acetate suitable for demanding applications in electronics and pharmaceuticals[2]. Additionally, Celanese has invested in bio-based feedstock research, exploring the use of renewable ethanol sources to produce more sustainable ethyl acetate[3].
Strengths: High efficiency production, superior product purity, and progress in sustainable manufacturing. Weaknesses: Potential dependency on volatile raw material prices and competition from alternative solvents.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel approach to ethyl acetate production using its proprietary Eastman™ Gasification Technology. This process converts coal or natural gas into syngas, which is then used to produce acetic acid and subsequently ethyl acetate[4]. The company has also implemented advanced process control systems and predictive modeling to optimize production efficiency and product quality[5]. Eastman's research efforts have focused on enhancing the selectivity of catalysts used in ethyl acetate synthesis, resulting in higher yields and reduced byproduct formation[6].
Strengths: Versatile feedstock options, integrated production process, and advanced process control. Weaknesses: Potential environmental concerns with coal-based feedstock and high capital investment requirements.
Innovative EA Techniques
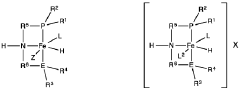
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentWO2010014145A2
Innovation
- A process utilizing a bimetallic catalyst supported on a suitable catalyst support, comprising metals like platinum, palladium, copper, and cobalt, which selectively hydrogenates acetic acid to ethyl acetate with high yield and selectivity, minimizing by-product formation.
Homogeneous iron catalysts for the conversion of ethanol to ethyl acetate and hydrogen
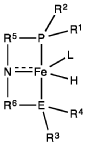
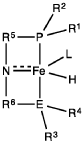
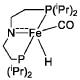
PatentWO2019027965A1
Innovation
- A process utilizing a homogeneous iron catalyst with a tridentate pincer ligand for dehydrogenative coupling of ethanol at moderate temperatures, producing ethyl acetate efficiently and selectively, with iron loadings as low as 0.001 mol%, allowing for continuous operation and easy separation of ethyl acetate from the catalyst.
Environmental Impact
Ethyl acetate, a widely used organic solvent and chemical intermediate, has significant environmental implications that must be carefully considered in its application to cutting-edge techniques. The production and use of ethyl acetate can have both positive and negative impacts on the environment, necessitating a comprehensive assessment of its lifecycle and potential consequences.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. As a VOC, ethyl acetate can contribute to the formation of ground-level ozone and smog when released into the atmosphere. This can lead to air quality issues, particularly in urban and industrial areas where its use is more prevalent. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential, making it a more environmentally friendly choice in certain applications.
The production of ethyl acetate also raises environmental considerations. Traditional manufacturing methods often rely on petrochemical feedstocks, which are derived from non-renewable resources and contribute to carbon emissions. However, recent advancements in bio-based production techniques offer a more sustainable alternative. These methods utilize renewable resources such as ethanol from fermented biomass, potentially reducing the carbon footprint associated with ethyl acetate production.
In terms of waste management, ethyl acetate presents both challenges and opportunities. Its high volatility means that it can easily evaporate, potentially leading to air pollution if not properly contained. However, this property also makes it easier to recover and recycle in industrial processes, reducing overall waste and environmental impact. Advanced recovery systems and closed-loop manufacturing processes can significantly mitigate the release of ethyl acetate into the environment.
Water pollution is another aspect to consider in the environmental impact of ethyl acetate. While it has low water solubility, accidental spills or improper disposal can still affect aquatic ecosystems. However, ethyl acetate's relatively rapid biodegradability in water and soil environments is a positive attribute, as it reduces long-term environmental persistence compared to more stable chemical compounds.
The use of ethyl acetate in cutting-edge techniques often involves replacing more harmful substances, which can lead to net positive environmental outcomes. For instance, in the electronics industry, ethyl acetate is being explored as a less toxic alternative to traditional solvents used in manufacturing processes. Similarly, in the pharmaceutical industry, it is employed in green chemistry applications, potentially reducing the environmental footprint of drug production.
As industries continue to adopt more sustainable practices, the role of ethyl acetate in environmentally conscious technologies is likely to expand. Research into its applications in bio-based materials, energy-efficient processes, and eco-friendly consumer products showcases its potential to contribute to a more sustainable industrial landscape. However, ongoing monitoring and assessment of its environmental impact remain crucial to ensure that its benefits outweigh any potential risks.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. As a VOC, ethyl acetate can contribute to the formation of ground-level ozone and smog when released into the atmosphere. This can lead to air quality issues, particularly in urban and industrial areas where its use is more prevalent. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential, making it a more environmentally friendly choice in certain applications.
The production of ethyl acetate also raises environmental considerations. Traditional manufacturing methods often rely on petrochemical feedstocks, which are derived from non-renewable resources and contribute to carbon emissions. However, recent advancements in bio-based production techniques offer a more sustainable alternative. These methods utilize renewable resources such as ethanol from fermented biomass, potentially reducing the carbon footprint associated with ethyl acetate production.
In terms of waste management, ethyl acetate presents both challenges and opportunities. Its high volatility means that it can easily evaporate, potentially leading to air pollution if not properly contained. However, this property also makes it easier to recover and recycle in industrial processes, reducing overall waste and environmental impact. Advanced recovery systems and closed-loop manufacturing processes can significantly mitigate the release of ethyl acetate into the environment.
Water pollution is another aspect to consider in the environmental impact of ethyl acetate. While it has low water solubility, accidental spills or improper disposal can still affect aquatic ecosystems. However, ethyl acetate's relatively rapid biodegradability in water and soil environments is a positive attribute, as it reduces long-term environmental persistence compared to more stable chemical compounds.
The use of ethyl acetate in cutting-edge techniques often involves replacing more harmful substances, which can lead to net positive environmental outcomes. For instance, in the electronics industry, ethyl acetate is being explored as a less toxic alternative to traditional solvents used in manufacturing processes. Similarly, in the pharmaceutical industry, it is employed in green chemistry applications, potentially reducing the environmental footprint of drug production.
As industries continue to adopt more sustainable practices, the role of ethyl acetate in environmentally conscious technologies is likely to expand. Research into its applications in bio-based materials, energy-efficient processes, and eco-friendly consumer products showcases its potential to contribute to a more sustainable industrial landscape. However, ongoing monitoring and assessment of its environmental impact remain crucial to ensure that its benefits outweigh any potential risks.
Regulatory Compliance
Regulatory compliance plays a crucial role in the use of ethyl acetate in cutting-edge techniques across various industries. As a widely used solvent and reagent, ethyl acetate is subject to numerous regulations and guidelines to ensure its safe and responsible application.
In the pharmaceutical industry, ethyl acetate's use is governed by strict regulations set forth by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulations focus on the purity and quality of ethyl acetate used in drug manufacturing processes, as well as its potential residual presence in final pharmaceutical products. Compliance with Good Manufacturing Practice (GMP) guidelines is essential when utilizing ethyl acetate in pharmaceutical applications.
The food industry also faces significant regulatory scrutiny regarding ethyl acetate usage. Food-grade ethyl acetate must meet stringent purity requirements established by regulatory bodies like the FDA and the European Food Safety Authority (EFSA). These agencies set maximum residue limits for ethyl acetate in food products and regulate its use as a food additive or processing aid.
Environmental regulations play a vital role in controlling the industrial use and disposal of ethyl acetate. The U.S. Environmental Protection Agency (EPA) classifies ethyl acetate as a volatile organic compound (VOC) and regulates its emissions under the Clean Air Act. Similarly, the European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes strict requirements on the registration, evaluation, and authorization of ethyl acetate use.
Occupational health and safety regulations are another critical aspect of ethyl acetate compliance. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) set exposure limits and safety guidelines for workers handling ethyl acetate. These regulations mandate proper ventilation, personal protective equipment, and safe handling procedures to minimize health risks associated with ethyl acetate exposure.
To ensure regulatory compliance, companies employing ethyl acetate in cutting-edge techniques must implement robust quality management systems and documentation practices. This includes maintaining detailed records of ethyl acetate sourcing, usage, and disposal, as well as conducting regular audits and assessments to verify adherence to applicable regulations.
As regulations continue to evolve, staying informed about changes in regulatory requirements is crucial for organizations utilizing ethyl acetate. Engaging with regulatory agencies, industry associations, and compliance experts can help companies navigate the complex landscape of ethyl acetate regulations and maintain compliance in their innovative applications.
In the pharmaceutical industry, ethyl acetate's use is governed by strict regulations set forth by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulations focus on the purity and quality of ethyl acetate used in drug manufacturing processes, as well as its potential residual presence in final pharmaceutical products. Compliance with Good Manufacturing Practice (GMP) guidelines is essential when utilizing ethyl acetate in pharmaceutical applications.
The food industry also faces significant regulatory scrutiny regarding ethyl acetate usage. Food-grade ethyl acetate must meet stringent purity requirements established by regulatory bodies like the FDA and the European Food Safety Authority (EFSA). These agencies set maximum residue limits for ethyl acetate in food products and regulate its use as a food additive or processing aid.
Environmental regulations play a vital role in controlling the industrial use and disposal of ethyl acetate. The U.S. Environmental Protection Agency (EPA) classifies ethyl acetate as a volatile organic compound (VOC) and regulates its emissions under the Clean Air Act. Similarly, the European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes strict requirements on the registration, evaluation, and authorization of ethyl acetate use.
Occupational health and safety regulations are another critical aspect of ethyl acetate compliance. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) set exposure limits and safety guidelines for workers handling ethyl acetate. These regulations mandate proper ventilation, personal protective equipment, and safe handling procedures to minimize health risks associated with ethyl acetate exposure.
To ensure regulatory compliance, companies employing ethyl acetate in cutting-edge techniques must implement robust quality management systems and documentation practices. This includes maintaining detailed records of ethyl acetate sourcing, usage, and disposal, as well as conducting regular audits and assessments to verify adherence to applicable regulations.
As regulations continue to evolve, staying informed about changes in regulatory requirements is crucial for organizations utilizing ethyl acetate. Engaging with regulatory agencies, industry associations, and compliance experts can help companies navigate the complex landscape of ethyl acetate regulations and maintain compliance in their innovative applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!