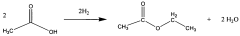
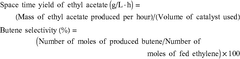How Ethyl Acetate Drives Research and Development Success?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate R&D Landscape
Ethyl acetate has emerged as a pivotal compound in the research and development landscape, driving innovation across multiple industries. This versatile organic solvent plays a crucial role in various scientific and industrial processes, contributing significantly to R&D success in fields such as pharmaceuticals, materials science, and chemical engineering.
In the pharmaceutical sector, ethyl acetate's unique properties make it an indispensable tool for drug discovery and development. Its ability to dissolve a wide range of organic compounds facilitates the extraction and purification of active pharmaceutical ingredients (APIs). Researchers leverage ethyl acetate's selective solubility to isolate target molecules from complex mixtures, streamlining the drug development process and accelerating the path to clinical trials.
The materials science field has also benefited greatly from ethyl acetate's versatility. Its low boiling point and rapid evaporation rate make it an ideal solvent for polymer synthesis and processing. This characteristic enables the development of advanced materials with tailored properties, such as high-performance coatings, adhesives, and specialty plastics. The compound's role in creating innovative materials has far-reaching implications for industries ranging from aerospace to consumer electronics.
In chemical engineering, ethyl acetate serves as a model compound for studying reaction kinetics and separation processes. Its well-understood properties make it an excellent choice for developing and optimizing industrial-scale processes. Researchers use ethyl acetate to validate theoretical models and design more efficient chemical reactors and separation units, ultimately leading to more sustainable and cost-effective manufacturing processes.
The environmental impact of chemical processes has become a critical concern in recent years, and ethyl acetate has played a significant role in developing greener alternatives. Its relatively low toxicity and biodegradability make it a preferred choice over more harmful solvents. This has spurred research into bio-based production methods for ethyl acetate, aligning with the principles of green chemistry and contributing to the development of more sustainable industrial practices.
Furthermore, ethyl acetate's role in analytical chemistry cannot be overstated. Its use as a mobile phase in chromatography techniques, such as high-performance liquid chromatography (HPLC), has enabled researchers to achieve high-resolution separation of complex mixtures. This capability is crucial for quality control in pharmaceutical manufacturing, environmental monitoring, and food safety analysis, driving advancements in analytical methodologies and instrumentation.
In the pharmaceutical sector, ethyl acetate's unique properties make it an indispensable tool for drug discovery and development. Its ability to dissolve a wide range of organic compounds facilitates the extraction and purification of active pharmaceutical ingredients (APIs). Researchers leverage ethyl acetate's selective solubility to isolate target molecules from complex mixtures, streamlining the drug development process and accelerating the path to clinical trials.
The materials science field has also benefited greatly from ethyl acetate's versatility. Its low boiling point and rapid evaporation rate make it an ideal solvent for polymer synthesis and processing. This characteristic enables the development of advanced materials with tailored properties, such as high-performance coatings, adhesives, and specialty plastics. The compound's role in creating innovative materials has far-reaching implications for industries ranging from aerospace to consumer electronics.
In chemical engineering, ethyl acetate serves as a model compound for studying reaction kinetics and separation processes. Its well-understood properties make it an excellent choice for developing and optimizing industrial-scale processes. Researchers use ethyl acetate to validate theoretical models and design more efficient chemical reactors and separation units, ultimately leading to more sustainable and cost-effective manufacturing processes.
The environmental impact of chemical processes has become a critical concern in recent years, and ethyl acetate has played a significant role in developing greener alternatives. Its relatively low toxicity and biodegradability make it a preferred choice over more harmful solvents. This has spurred research into bio-based production methods for ethyl acetate, aligning with the principles of green chemistry and contributing to the development of more sustainable industrial practices.
Furthermore, ethyl acetate's role in analytical chemistry cannot be overstated. Its use as a mobile phase in chromatography techniques, such as high-performance liquid chromatography (HPLC), has enabled researchers to achieve high-resolution separation of complex mixtures. This capability is crucial for quality control in pharmaceutical manufacturing, environmental monitoring, and food safety analysis, driving advancements in analytical methodologies and instrumentation.
Market Demand Analysis
The market demand for ethyl acetate has been steadily increasing due to its versatile applications across various industries. This compound plays a crucial role in research and development success, particularly in the pharmaceutical, food and beverage, and electronics sectors.
In the pharmaceutical industry, ethyl acetate is widely used as a solvent for drug synthesis and extraction processes. Its low toxicity and high solvency make it an ideal choice for researchers developing new medications and improving existing formulations. The growing emphasis on drug discovery and personalized medicine has further boosted the demand for ethyl acetate in pharmaceutical R&D.
The food and beverage industry also relies heavily on ethyl acetate for flavor extraction and as a food additive. Its ability to extract natural flavors from fruits and spices without altering their chemical composition has made it indispensable in the development of new food products and beverages. The rising consumer preference for natural and organic products has led to increased use of ethyl acetate in this sector.
In the electronics industry, ethyl acetate is utilized in the production of printed circuit boards and as a cleaning agent for electronic components. As the demand for smaller, more efficient electronic devices continues to grow, the need for high-purity ethyl acetate in R&D processes has also increased.
The global ethyl acetate market has shown significant growth in recent years, with a compound annual growth rate (CAGR) of around 6% from 2015 to 2020. This trend is expected to continue, driven by the expanding applications in various industries and the compound's eco-friendly nature compared to other solvents.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate, accounting for over 40% of the global market share. This is primarily due to the rapid industrialization and growth of manufacturing sectors in countries like China and India. North America and Europe follow, with steady demand from established pharmaceutical and food industries.
The increasing focus on sustainable and environmentally friendly products has also contributed to the growing demand for ethyl acetate. As a biodegradable solvent, it aligns well with the global push towards green chemistry and sustainable manufacturing practices. This has led to its increased adoption in R&D processes across various industries, replacing more harmful solvents.
However, the market demand for ethyl acetate is not without challenges. Price volatility of raw materials, such as ethanol and acetic acid, can impact the overall cost of ethyl acetate production. Additionally, stringent regulations regarding the use of volatile organic compounds (VOCs) in certain regions may affect its market growth in specific applications.
In the pharmaceutical industry, ethyl acetate is widely used as a solvent for drug synthesis and extraction processes. Its low toxicity and high solvency make it an ideal choice for researchers developing new medications and improving existing formulations. The growing emphasis on drug discovery and personalized medicine has further boosted the demand for ethyl acetate in pharmaceutical R&D.
The food and beverage industry also relies heavily on ethyl acetate for flavor extraction and as a food additive. Its ability to extract natural flavors from fruits and spices without altering their chemical composition has made it indispensable in the development of new food products and beverages. The rising consumer preference for natural and organic products has led to increased use of ethyl acetate in this sector.
In the electronics industry, ethyl acetate is utilized in the production of printed circuit boards and as a cleaning agent for electronic components. As the demand for smaller, more efficient electronic devices continues to grow, the need for high-purity ethyl acetate in R&D processes has also increased.
The global ethyl acetate market has shown significant growth in recent years, with a compound annual growth rate (CAGR) of around 6% from 2015 to 2020. This trend is expected to continue, driven by the expanding applications in various industries and the compound's eco-friendly nature compared to other solvents.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate, accounting for over 40% of the global market share. This is primarily due to the rapid industrialization and growth of manufacturing sectors in countries like China and India. North America and Europe follow, with steady demand from established pharmaceutical and food industries.
The increasing focus on sustainable and environmentally friendly products has also contributed to the growing demand for ethyl acetate. As a biodegradable solvent, it aligns well with the global push towards green chemistry and sustainable manufacturing practices. This has led to its increased adoption in R&D processes across various industries, replacing more harmful solvents.
However, the market demand for ethyl acetate is not without challenges. Price volatility of raw materials, such as ethanol and acetic acid, can impact the overall cost of ethyl acetate production. Additionally, stringent regulations regarding the use of volatile organic compounds (VOCs) in certain regions may affect its market growth in specific applications.
Technical Challenges
Ethyl acetate, a versatile organic compound, plays a crucial role in various research and development processes. However, its application faces several technical challenges that researchers and industries must address to maximize its potential.
One of the primary challenges is the optimization of ethyl acetate production processes. Current methods often involve energy-intensive reactions and complex purification steps, leading to high production costs and environmental concerns. Developing more efficient and sustainable production techniques remains a significant hurdle for researchers.
The stability of ethyl acetate under different conditions poses another challenge. Its tendency to hydrolyze in the presence of water or undergo decomposition at elevated temperatures limits its applicability in certain research environments. This instability can affect the reliability and reproducibility of experimental results, particularly in long-term studies or high-temperature applications.
Purification and quality control of ethyl acetate present additional technical difficulties. Trace impurities can significantly impact its performance in sensitive applications, such as in the pharmaceutical or electronics industries. Developing advanced purification methods and implementing stringent quality control measures are essential to ensure consistent and high-quality ethyl acetate for research purposes.
The handling and storage of ethyl acetate also pose challenges due to its volatile nature and flammability. Proper containment systems and safety protocols are necessary to prevent accidents and maintain the integrity of research materials. This requires ongoing improvements in storage technologies and safety equipment.
In the realm of analytical chemistry, the detection and quantification of ethyl acetate in complex matrices remain challenging. Developing more sensitive and selective analytical methods is crucial for accurate monitoring in various research applications, including environmental studies and quality control in manufacturing processes.
The environmental impact of ethyl acetate usage is another area of concern. While it is considered less harmful than many other solvents, its volatile organic compound (VOC) status necessitates the development of more environmentally friendly alternatives or improved recycling methods to reduce emissions and waste.
Lastly, the scalability of ethyl acetate-based processes from laboratory to industrial scale presents significant engineering challenges. Maintaining reaction efficiency, product quality, and safety standards during scale-up requires careful process design and optimization, often involving substantial research and development efforts.
Addressing these technical challenges is crucial for advancing the use of ethyl acetate in research and development. Overcoming these hurdles will not only enhance its effectiveness in current applications but also open up new possibilities for innovation across various scientific and industrial domains.
One of the primary challenges is the optimization of ethyl acetate production processes. Current methods often involve energy-intensive reactions and complex purification steps, leading to high production costs and environmental concerns. Developing more efficient and sustainable production techniques remains a significant hurdle for researchers.
The stability of ethyl acetate under different conditions poses another challenge. Its tendency to hydrolyze in the presence of water or undergo decomposition at elevated temperatures limits its applicability in certain research environments. This instability can affect the reliability and reproducibility of experimental results, particularly in long-term studies or high-temperature applications.
Purification and quality control of ethyl acetate present additional technical difficulties. Trace impurities can significantly impact its performance in sensitive applications, such as in the pharmaceutical or electronics industries. Developing advanced purification methods and implementing stringent quality control measures are essential to ensure consistent and high-quality ethyl acetate for research purposes.
The handling and storage of ethyl acetate also pose challenges due to its volatile nature and flammability. Proper containment systems and safety protocols are necessary to prevent accidents and maintain the integrity of research materials. This requires ongoing improvements in storage technologies and safety equipment.
In the realm of analytical chemistry, the detection and quantification of ethyl acetate in complex matrices remain challenging. Developing more sensitive and selective analytical methods is crucial for accurate monitoring in various research applications, including environmental studies and quality control in manufacturing processes.
The environmental impact of ethyl acetate usage is another area of concern. While it is considered less harmful than many other solvents, its volatile organic compound (VOC) status necessitates the development of more environmentally friendly alternatives or improved recycling methods to reduce emissions and waste.
Lastly, the scalability of ethyl acetate-based processes from laboratory to industrial scale presents significant engineering challenges. Maintaining reaction efficiency, product quality, and safety standards during scale-up requires careful process design and optimization, often involving substantial research and development efforts.
Addressing these technical challenges is crucial for advancing the use of ethyl acetate in research and development. Overcoming these hurdles will not only enhance its effectiveness in current applications but also open up new possibilities for innovation across various scientific and industrial domains.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques used in chemical and pharmaceutical industries.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and volatility.
- Novel applications and formulations of ethyl acetate: Innovative applications and formulations involving ethyl acetate are being developed. These include its use in new materials, coatings, adhesives, and specialty chemicals. Research is ongoing to explore potential uses in emerging technologies and industries.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in diverse chemical processes, such as solvent extraction, as a reaction medium, and in the production of other chemicals. Its properties make it suitable for use in various industries, including pharmaceuticals, coatings, and electronics.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications, including as a solvent for resins, in the production of composite materials, and in the development of novel materials with specific properties.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices.Expand Specific Solutions05 Analytical methods for ethyl acetate
Various analytical techniques and methods are employed for the detection, quantification, and characterization of ethyl acetate in different matrices. These methods are crucial for quality control, process monitoring, and research applications across industries.Expand Specific Solutions
Industry Key Players
The ethyl acetate market is in a mature growth stage, with a global market size estimated at over $3 billion. The technology for producing ethyl acetate is well-established, with major players like Celanese, Eastman Chemical, and SABIC dominating production. However, there is increasing focus on developing bio-based and sustainable production methods, as evidenced by research efforts at universities like the University of Florida and Rice University. Companies like Viridis Chemical are pioneering commercial-scale bio-based ethyl acetate production. The competitive landscape is characterized by large chemical manufacturers expanding capacity and exploring greener technologies to meet growing demand in sectors like coatings, pharmaceuticals, and flexible packaging.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary acetyl chain technology for ethyl acetate production, which integrates acetic acid and ethylene to produce ethyl acetate in a single step. This process offers significant advantages in terms of efficiency and cost-effectiveness[1]. The company has also invested in research to improve the purity of ethyl acetate, achieving levels up to 99.9%, which is crucial for high-end applications in electronics and pharmaceuticals[2]. Additionally, Celanese has implemented advanced catalytic systems that enhance selectivity and reduce byproduct formation, leading to a more sustainable production process with lower energy consumption and reduced waste[3].
Strengths: Highly efficient single-step production process, high-purity product capability, and improved sustainability. Weaknesses: Potential dependency on proprietary technology and raw material availability.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel esterification process for ethyl acetate production that utilizes a reactive distillation column. This innovative approach combines reaction and separation in a single unit operation, significantly reducing equipment costs and energy consumption[4]. The company has also implemented advanced process control systems that optimize reaction conditions in real-time, ensuring consistent product quality and maximizing yield[5]. Furthermore, Eastman has invested in research to develop bio-based ethyl acetate, using renewable feedstocks to create a more environmentally friendly product that meets growing market demand for sustainable chemicals[6].
Strengths: Innovative reactive distillation technology, advanced process control, and focus on sustainable production. Weaknesses: Potential higher initial investment costs for new technology implementation.
Key Innovations
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentWO2010014145A2
Innovation
- A process utilizing a bimetallic catalyst supported on a suitable catalyst support, comprising metals like platinum, palladium, copper, and cobalt, which selectively hydrogenates acetic acid to ethyl acetate with high yield and selectivity, minimizing by-product formation.
Method for producing ethyl acetate
PatentPendingUS20250002441A1
Innovation
- Controlling the palladium content in the catalyst within the range of 0.1 to 14 ppb by mass in a heteropolyacid or its salt supported on a carrier, such as silica, suppresses side reactions and ensures stable long-term operation.
Environmental Impact
Ethyl acetate, a widely used solvent in various industries, has significant environmental implications that must be considered in research and development processes. The production and use of ethyl acetate can impact air quality, water resources, and soil conditions, necessitating careful management and innovative solutions to mitigate its environmental footprint.
In terms of air quality, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and smog, potentially affecting human health and ecosystems. Research and development efforts have focused on reducing VOC emissions through improved manufacturing processes and the development of low-VOC or VOC-free alternatives.
Water pollution is another concern associated with ethyl acetate. Improper disposal or accidental spills can lead to contamination of water bodies, affecting aquatic life and potentially entering the human water supply. R&D initiatives have targeted the development of more efficient wastewater treatment technologies and the implementation of closed-loop systems to minimize the release of ethyl acetate into the environment.
Soil contamination can occur through spills or improper storage of ethyl acetate. This can lead to long-term environmental damage and pose risks to plant and animal life. Research efforts have focused on developing more effective containment methods and remediation techniques for contaminated soil.
The environmental impact of ethyl acetate extends to its production process as well. Traditional manufacturing methods often rely on petrochemical feedstocks, contributing to carbon emissions and resource depletion. R&D efforts have explored more sustainable production routes, including bio-based alternatives derived from renewable resources, which can significantly reduce the carbon footprint of ethyl acetate production.
Lifecycle assessment studies have been crucial in understanding the full environmental impact of ethyl acetate from cradle to grave. These assessments have driven research into more sustainable packaging, transportation, and end-of-life management strategies for ethyl acetate and products containing it.
As environmental regulations become increasingly stringent, the ethyl acetate industry has been compelled to innovate. This has led to the development of greener formulations, improved recycling technologies, and more efficient production processes. These advancements not only reduce environmental impact but also often result in cost savings and improved product performance.
The pursuit of environmental sustainability in ethyl acetate use has also spurred research into alternative solvents and technologies. This includes the exploration of supercritical fluids, ionic liquids, and other novel solvents that may offer similar functionality with reduced environmental impact.
In terms of air quality, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and smog, potentially affecting human health and ecosystems. Research and development efforts have focused on reducing VOC emissions through improved manufacturing processes and the development of low-VOC or VOC-free alternatives.
Water pollution is another concern associated with ethyl acetate. Improper disposal or accidental spills can lead to contamination of water bodies, affecting aquatic life and potentially entering the human water supply. R&D initiatives have targeted the development of more efficient wastewater treatment technologies and the implementation of closed-loop systems to minimize the release of ethyl acetate into the environment.
Soil contamination can occur through spills or improper storage of ethyl acetate. This can lead to long-term environmental damage and pose risks to plant and animal life. Research efforts have focused on developing more effective containment methods and remediation techniques for contaminated soil.
The environmental impact of ethyl acetate extends to its production process as well. Traditional manufacturing methods often rely on petrochemical feedstocks, contributing to carbon emissions and resource depletion. R&D efforts have explored more sustainable production routes, including bio-based alternatives derived from renewable resources, which can significantly reduce the carbon footprint of ethyl acetate production.
Lifecycle assessment studies have been crucial in understanding the full environmental impact of ethyl acetate from cradle to grave. These assessments have driven research into more sustainable packaging, transportation, and end-of-life management strategies for ethyl acetate and products containing it.
As environmental regulations become increasingly stringent, the ethyl acetate industry has been compelled to innovate. This has led to the development of greener formulations, improved recycling technologies, and more efficient production processes. These advancements not only reduce environmental impact but also often result in cost savings and improved product performance.
The pursuit of environmental sustainability in ethyl acetate use has also spurred research into alternative solvents and technologies. This includes the exploration of supercritical fluids, ionic liquids, and other novel solvents that may offer similar functionality with reduced environmental impact.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in shaping its use and impact on research and development success. As a widely used solvent in various industries, ethyl acetate is subject to a complex web of regulations that govern its production, handling, transportation, and application.
At the international level, organizations such as the World Health Organization (WHO) and the International Labour Organization (ILO) provide guidelines for the safe use of ethyl acetate in industrial settings. These guidelines often serve as a basis for national regulations and standards, ensuring a degree of global consistency in the approach to managing this chemical.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and importers of ethyl acetate, as well as guidelines for its disposal. Additionally, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers handling ethyl acetate in laboratory and industrial environments.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ethyl acetate. Under REACH, manufacturers and importers are required to register the substance and provide detailed safety information. This regulation ensures that the risks associated with ethyl acetate are properly assessed and managed throughout its lifecycle.
In the pharmaceutical industry, the use of ethyl acetate is governed by Good Manufacturing Practice (GMP) guidelines. These guidelines, enforced by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), ensure that ethyl acetate used in drug production meets strict quality and safety standards.
The regulatory landscape also extends to the transportation of ethyl acetate. International agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code provide specific requirements for the safe transport of this flammable liquid.
As research and development activities often involve cross-border collaborations, navigating the varying regulatory requirements across different jurisdictions can be challenging. Companies and research institutions must stay informed about the latest regulatory developments and ensure compliance to avoid potential legal and safety issues.
The regulatory framework surrounding ethyl acetate continues to evolve as new scientific data emerges and societal expectations regarding chemical safety change. This dynamic regulatory environment necessitates ongoing adaptation and innovation in the research and development processes that rely on ethyl acetate, driving the need for more efficient and sustainable practices in its use and management.
At the international level, organizations such as the World Health Organization (WHO) and the International Labour Organization (ILO) provide guidelines for the safe use of ethyl acetate in industrial settings. These guidelines often serve as a basis for national regulations and standards, ensuring a degree of global consistency in the approach to managing this chemical.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and importers of ethyl acetate, as well as guidelines for its disposal. Additionally, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers handling ethyl acetate in laboratory and industrial environments.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ethyl acetate. Under REACH, manufacturers and importers are required to register the substance and provide detailed safety information. This regulation ensures that the risks associated with ethyl acetate are properly assessed and managed throughout its lifecycle.
In the pharmaceutical industry, the use of ethyl acetate is governed by Good Manufacturing Practice (GMP) guidelines. These guidelines, enforced by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), ensure that ethyl acetate used in drug production meets strict quality and safety standards.
The regulatory landscape also extends to the transportation of ethyl acetate. International agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code provide specific requirements for the safe transport of this flammable liquid.
As research and development activities often involve cross-border collaborations, navigating the varying regulatory requirements across different jurisdictions can be challenging. Companies and research institutions must stay informed about the latest regulatory developments and ensure compliance to avoid potential legal and safety issues.
The regulatory framework surrounding ethyl acetate continues to evolve as new scientific data emerges and societal expectations regarding chemical safety change. This dynamic regulatory environment necessitates ongoing adaptation and innovation in the research and development processes that rely on ethyl acetate, driving the need for more efficient and sustainable practices in its use and management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!