Cementing Ethyl Acetate’s Position in Industry Solutions
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone a remarkable evolution since its discovery in the early 19th century. Initially recognized as a naturally occurring compound in fruits and wines, it quickly gained attention for its unique properties and potential applications. The journey of ethyl acetate from a laboratory curiosity to an industrial staple is marked by significant milestones and technological advancements.
In the early stages of its industrial use, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This process, while effective, was limited in scale and efficiency. The growing demand for the compound in various industries prompted researchers and engineers to explore more advanced production methods. The introduction of the Tishchenko reaction in the mid-20th century marked a significant leap forward, allowing for the direct synthesis of ethyl acetate from acetaldehyde.
As the chemical industry evolved, so did the production techniques for ethyl acetate. The development of continuous flow reactors and improved catalysts in the latter half of the 20th century led to substantial increases in production capacity and efficiency. These advancements not only reduced production costs but also improved the purity of the final product, opening up new applications in high-tech industries.
The turn of the 21st century saw a renewed focus on sustainable production methods for ethyl acetate. Bioethanol-based processes emerged as a promising alternative to traditional petrochemical routes, aligning with global efforts to reduce carbon footprints. Concurrently, advancements in separation and purification technologies, such as reactive distillation and membrane-based processes, further refined the production and recovery of ethyl acetate.
Recent years have witnessed the integration of digital technologies and automation in ethyl acetate production. Industry 4.0 principles, including real-time process monitoring and predictive maintenance, have been applied to optimize production lines, enhancing both efficiency and product quality. Additionally, the development of novel catalysts and process intensification techniques continues to push the boundaries of what's possible in ethyl acetate synthesis.
Looking ahead, the evolution of ethyl acetate production is likely to focus on further improving sustainability and efficiency. Research into bio-based feedstocks, green chemistry principles, and circular economy models is expected to shape the future of ethyl acetate manufacturing. Moreover, the ongoing exploration of new applications, particularly in emerging fields like nanotechnology and advanced materials, promises to cement ethyl acetate's position as a versatile and indispensable industrial solvent for years to come.
In the early stages of its industrial use, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This process, while effective, was limited in scale and efficiency. The growing demand for the compound in various industries prompted researchers and engineers to explore more advanced production methods. The introduction of the Tishchenko reaction in the mid-20th century marked a significant leap forward, allowing for the direct synthesis of ethyl acetate from acetaldehyde.
As the chemical industry evolved, so did the production techniques for ethyl acetate. The development of continuous flow reactors and improved catalysts in the latter half of the 20th century led to substantial increases in production capacity and efficiency. These advancements not only reduced production costs but also improved the purity of the final product, opening up new applications in high-tech industries.
The turn of the 21st century saw a renewed focus on sustainable production methods for ethyl acetate. Bioethanol-based processes emerged as a promising alternative to traditional petrochemical routes, aligning with global efforts to reduce carbon footprints. Concurrently, advancements in separation and purification technologies, such as reactive distillation and membrane-based processes, further refined the production and recovery of ethyl acetate.
Recent years have witnessed the integration of digital technologies and automation in ethyl acetate production. Industry 4.0 principles, including real-time process monitoring and predictive maintenance, have been applied to optimize production lines, enhancing both efficiency and product quality. Additionally, the development of novel catalysts and process intensification techniques continues to push the boundaries of what's possible in ethyl acetate synthesis.
Looking ahead, the evolution of ethyl acetate production is likely to focus on further improving sustainability and efficiency. Research into bio-based feedstocks, green chemistry principles, and circular economy models is expected to shape the future of ethyl acetate manufacturing. Moreover, the ongoing exploration of new applications, particularly in emerging fields like nanotechnology and advanced materials, promises to cement ethyl acetate's position as a versatile and indispensable industrial solvent for years to come.
Market Demand Analysis
The market demand for ethyl acetate has been steadily growing, driven by its versatile applications across various industries. As a key solvent and intermediate in chemical processes, ethyl acetate's market is closely tied to the performance of end-use industries such as paints and coatings, adhesives, pharmaceuticals, and food packaging.
In the paints and coatings sector, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. The global paints and coatings market has been expanding, particularly in emerging economies, due to increased construction activities and automotive production. This growth directly translates to a higher demand for ethyl acetate as a solvent in these formulations.
The adhesives industry represents another significant market for ethyl acetate. With the rise of e-commerce and the subsequent increase in packaging needs, the demand for adhesives in the packaging sector has surged. Ethyl acetate's role as a solvent in adhesive formulations positions it to benefit from this trend.
In the pharmaceutical industry, ethyl acetate finds applications in the production of various drugs and as an extraction solvent. The global pharmaceutical market's steady growth, driven by factors such as an aging population and increased healthcare spending, contributes to the sustained demand for ethyl acetate in this sector.
The food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. As consumer preferences shift towards healthier and more diverse food options, the demand for natural and artificial flavors has increased, indirectly boosting the market for ethyl acetate.
Environmental regulations and sustainability concerns have also influenced the market dynamics for ethyl acetate. As a relatively low-toxicity solvent with good biodegradability, ethyl acetate has gained favor as a more environmentally friendly alternative to some other solvents. This has opened up new market opportunities, particularly in regions with stringent environmental regulations.
The Asia-Pacific region has emerged as a key growth driver for the ethyl acetate market. Rapid industrialization, urbanization, and economic growth in countries like China and India have led to increased consumption across various end-use industries. The region's expanding manufacturing sector, particularly in electronics and automotive, has further bolstered the demand for ethyl acetate.
Despite the positive market outlook, challenges such as raw material price volatility and competition from alternative solvents exist. The price fluctuations of ethanol and acetic acid, the primary raw materials for ethyl acetate production, can impact market dynamics. Additionally, ongoing research into bio-based alternatives and other sustainable solvents may influence future market trends.
In the paints and coatings sector, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. The global paints and coatings market has been expanding, particularly in emerging economies, due to increased construction activities and automotive production. This growth directly translates to a higher demand for ethyl acetate as a solvent in these formulations.
The adhesives industry represents another significant market for ethyl acetate. With the rise of e-commerce and the subsequent increase in packaging needs, the demand for adhesives in the packaging sector has surged. Ethyl acetate's role as a solvent in adhesive formulations positions it to benefit from this trend.
In the pharmaceutical industry, ethyl acetate finds applications in the production of various drugs and as an extraction solvent. The global pharmaceutical market's steady growth, driven by factors such as an aging population and increased healthcare spending, contributes to the sustained demand for ethyl acetate in this sector.
The food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. As consumer preferences shift towards healthier and more diverse food options, the demand for natural and artificial flavors has increased, indirectly boosting the market for ethyl acetate.
Environmental regulations and sustainability concerns have also influenced the market dynamics for ethyl acetate. As a relatively low-toxicity solvent with good biodegradability, ethyl acetate has gained favor as a more environmentally friendly alternative to some other solvents. This has opened up new market opportunities, particularly in regions with stringent environmental regulations.
The Asia-Pacific region has emerged as a key growth driver for the ethyl acetate market. Rapid industrialization, urbanization, and economic growth in countries like China and India have led to increased consumption across various end-use industries. The region's expanding manufacturing sector, particularly in electronics and automotive, has further bolstered the demand for ethyl acetate.
Despite the positive market outlook, challenges such as raw material price volatility and competition from alternative solvents exist. The price fluctuations of ethanol and acetic acid, the primary raw materials for ethyl acetate production, can impact market dynamics. Additionally, ongoing research into bio-based alternatives and other sustainable solvents may influence future market trends.
Technical Challenges
Despite its widespread use and established position in various industries, ethyl acetate faces several technical challenges that need to be addressed to cement its position as a preferred solvent and chemical intermediate. One of the primary challenges is the optimization of production processes to enhance efficiency and reduce costs. Current manufacturing methods, such as the esterification of ethanol and acetic acid, often require high energy inputs and produce unwanted by-products, leading to increased production costs and environmental concerns.
Another significant challenge lies in the purification of ethyl acetate. The presence of impurities, such as water and unreacted starting materials, can affect the quality and performance of the final product. Developing more efficient and cost-effective purification techniques, such as advanced distillation methods or membrane separation technologies, is crucial for maintaining high-quality standards and meeting stringent industry requirements.
The environmental impact of ethyl acetate production and use presents another technical hurdle. While ethyl acetate is considered less harmful than many other solvents, there is still a need to reduce emissions and improve overall sustainability. This includes developing greener production methods, implementing more effective recycling processes, and exploring bio-based alternatives to traditional petrochemical-derived ethyl acetate.
Stability and storage of ethyl acetate pose additional challenges, particularly in industrial applications. The compound is highly volatile and flammable, requiring careful handling and storage conditions. Improving stability through the development of novel stabilizers or packaging solutions could enhance safety and extend shelf life, making ethyl acetate more attractive for a wider range of applications.
In the realm of application development, there is an ongoing need to expand the versatility of ethyl acetate. While it is already used in numerous industries, from coatings to pharmaceuticals, researchers are continually exploring new potential uses. This includes investigating its role in emerging technologies such as 3D printing, advanced materials synthesis, and green chemistry applications. However, adapting ethyl acetate for these new applications often requires overcoming technical barriers related to compatibility, performance, and process integration.
Lastly, regulatory compliance presents a complex challenge for ethyl acetate manufacturers and users. As global regulations become increasingly stringent, particularly regarding volatile organic compounds (VOCs) and environmental protection, there is a pressing need to develop formulations and applications that meet these evolving standards while maintaining product efficacy. This may involve reformulating products, improving emission control technologies, or developing entirely new application methods that minimize environmental impact while maximizing the benefits of ethyl acetate.
Another significant challenge lies in the purification of ethyl acetate. The presence of impurities, such as water and unreacted starting materials, can affect the quality and performance of the final product. Developing more efficient and cost-effective purification techniques, such as advanced distillation methods or membrane separation technologies, is crucial for maintaining high-quality standards and meeting stringent industry requirements.
The environmental impact of ethyl acetate production and use presents another technical hurdle. While ethyl acetate is considered less harmful than many other solvents, there is still a need to reduce emissions and improve overall sustainability. This includes developing greener production methods, implementing more effective recycling processes, and exploring bio-based alternatives to traditional petrochemical-derived ethyl acetate.
Stability and storage of ethyl acetate pose additional challenges, particularly in industrial applications. The compound is highly volatile and flammable, requiring careful handling and storage conditions. Improving stability through the development of novel stabilizers or packaging solutions could enhance safety and extend shelf life, making ethyl acetate more attractive for a wider range of applications.
In the realm of application development, there is an ongoing need to expand the versatility of ethyl acetate. While it is already used in numerous industries, from coatings to pharmaceuticals, researchers are continually exploring new potential uses. This includes investigating its role in emerging technologies such as 3D printing, advanced materials synthesis, and green chemistry applications. However, adapting ethyl acetate for these new applications often requires overcoming technical barriers related to compatibility, performance, and process integration.
Lastly, regulatory compliance presents a complex challenge for ethyl acetate manufacturers and users. As global regulations become increasingly stringent, particularly regarding volatile organic compounds (VOCs) and environmental protection, there is a pressing need to develop formulations and applications that meet these evolving standards while maintaining product efficacy. This may involve reformulating products, improving emission control technologies, or developing entirely new application methods that minimize environmental impact while maximizing the benefits of ethyl acetate.
Current Applications
01 Production and purification of ethyl acetate
Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts or reactants to improve yield and purity.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes, including as a solvent in reactions, extractions, and separations. It plays a role in the production of other chemicals and materials, showcasing its versatility in industrial applications.
- Ethyl acetate in pharmaceutical and cosmetic formulations: Ethyl acetate is employed in the formulation of pharmaceutical and cosmetic products. It serves as a solvent or carrier for active ingredients, and its properties make it suitable for various topical and oral preparations.
- Environmental and safety considerations for ethyl acetate use: Research and development efforts focus on improving the environmental impact and safety of ethyl acetate use. This includes developing greener production methods, enhancing handling procedures, and exploring alternatives in certain applications to reduce potential risks.
- Novel applications and modifications of ethyl acetate: Innovative uses and modifications of ethyl acetate are being explored, including its incorporation into new materials, its role in energy-related applications, and potential modifications to enhance its properties for specific industrial needs.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in diverse chemical processes, including as a solvent, reagent, or intermediate in the production of other compounds. Its applications span across multiple industries such as pharmaceuticals, coatings, and electronics.Expand Specific Solutions03 Ethyl acetate in polymer and material science
The use of ethyl acetate in polymer synthesis, material processing, and as a component in various formulations is explored. This includes its role in creating specific material properties or as a processing aid in manufacturing.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental profile and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling safety.Expand Specific Solutions05 Novel applications and derivatives of ethyl acetate
Innovative uses of ethyl acetate and the development of its derivatives are explored. This includes its application in emerging technologies, novel chemical reactions, and the creation of new compounds based on ethyl acetate.Expand Specific Solutions
Industry Leaders
The ethyl acetate industry is in a mature growth phase, characterized by steady demand and established production processes. The global market size for ethyl acetate is estimated to be around $3-4 billion, with moderate annual growth. Technologically, the production of ethyl acetate is well-established, with major players like China Petroleum & Chemical Corp., Wacker Chemie AG, and Eastman Chemical Co. continuously optimizing processes for efficiency and sustainability. Emerging trends include bio-based ethyl acetate production, as seen in research efforts by institutions like Dalian Institute of Chemical Physics and Nanjing Tech University. Companies such as Nantong Baichuan New Material and Jiangsu Baichuan High-Tech New Materials are focusing on developing high-purity grades for specialized applications, indicating a shift towards value-added products in this mature market.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative process for ethyl acetate production using a reactive distillation technology. This method combines esterification and distillation in a single column, significantly improving efficiency and reducing energy consumption. The process utilizes a heterogeneous catalyst, typically a strong acid ion exchange resin, which allows for continuous operation and easier separation of the product[1][3]. Sinopec's approach achieves a conversion rate of over 99% and a selectivity of more than 99.5% for ethyl acetate[2]. The company has also implemented advanced process control systems and heat integration techniques to further optimize the production process, resulting in a 15-20% reduction in overall energy consumption compared to conventional methods[4].
Strengths: High conversion rate and selectivity, energy-efficient process, continuous operation. Weaknesses: Potential catalyst deactivation over time, higher initial capital investment for reactive distillation equipment.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed a novel approach to ethyl acetate production using its proprietary integrated vinyl acetate-ethylene (VAE) process. This method involves the direct addition of ethylene to acetic acid in the presence of a palladium-based catalyst, followed by hydrogenation to produce ethyl acetate[1]. The process achieves high yields of up to 95% and operates at lower temperatures (100-150°C) compared to traditional methods[2]. Wacker has also implemented advanced process control and optimization techniques, including real-time monitoring and predictive maintenance, to ensure consistent product quality and minimize downtime. The company's ethyl acetate production facilities are integrated with its VAE operations, allowing for efficient use of raw materials and energy[3].
Strengths: High yield, lower operating temperatures, integrated production process. Weaknesses: Dependence on ethylene availability, potential catalyst cost and complexity.
Key Patents Review
Solvent composition
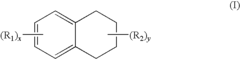
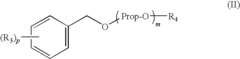
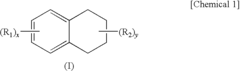
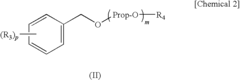
PatentInactiveUS7648651B2
Innovation
- A solvent composition combining a tetralin compound and a specific polyether compound, which enhances solute dissolution and substrate affinity while being safe and odorless, is developed, with specific formulations and usage ratios optimized for various applications.
ESTERS OF ACETALS PRODUCED FROM PURIFIED GLYCERIN FOR USE AND APPLICATIONS AS EMOLLIENTS, LUBRICANTS, PLASTICIZERS, SOLVENTS, COALESCENTS, HUMBECTANTS, POLYMERIZATION MONOMERS, ADDITIVES FOR BIOFUELS
PatentInactiveBR102013010477A2
Innovation
- Development of a new group of monoesters and diesters of acetals with multifunctional properties, combining ester function and cyclic ethers.
- Utilization of purified glycerin as a raw material, promoting sustainability and value-added use of a byproduct.
- Versatile application potential across multiple industries, including pesticides, paints, polymers, lubricants, cosmetics, and biofuels.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate plays a crucial role in shaping its position in industry solutions. As a widely used solvent and chemical intermediate, ethyl acetate is subject to various regulations across different regions and sectors.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). It is listed on the TSCA inventory and is subject to reporting requirements for manufacturers and importers. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace environments, ensuring worker safety.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register ethyl acetate with the European Chemicals Agency (ECHA) and provide safety data. The Classification, Labeling, and Packaging (CLP) regulation also applies, mandating proper hazard communication for ethyl acetate.
In the food industry, ethyl acetate is recognized as a Generally Recognized as Safe (GRAS) substance by the U.S. Food and Drug Administration (FDA). It is approved for use as a food additive and flavoring agent, subject to specific limitations. The European Food Safety Authority (EFSA) has also evaluated ethyl acetate and established acceptable daily intake levels.
Environmental regulations impact ethyl acetate's use and disposal. As a volatile organic compound (VOC), its emissions are regulated to control air pollution. Many countries have implemented VOC reduction strategies, influencing industrial processes involving ethyl acetate.
Transportation of ethyl acetate is governed by international agreements such as the United Nations Recommendations on the Transport of Dangerous Goods. These regulations specify packaging, labeling, and handling requirements for safe transportation.
As sustainability concerns grow, regulations promoting green chemistry and circular economy principles are emerging. This trend may influence future regulations on ethyl acetate, potentially encouraging the development of bio-based alternatives or improved recycling methods.
Compliance with these diverse regulations is essential for companies using ethyl acetate in their products or processes. Staying informed about regulatory changes and adapting to new requirements is crucial for maintaining ethyl acetate's position in industry solutions while ensuring safety and environmental protection.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). It is listed on the TSCA inventory and is subject to reporting requirements for manufacturers and importers. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace environments, ensuring worker safety.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register ethyl acetate with the European Chemicals Agency (ECHA) and provide safety data. The Classification, Labeling, and Packaging (CLP) regulation also applies, mandating proper hazard communication for ethyl acetate.
In the food industry, ethyl acetate is recognized as a Generally Recognized as Safe (GRAS) substance by the U.S. Food and Drug Administration (FDA). It is approved for use as a food additive and flavoring agent, subject to specific limitations. The European Food Safety Authority (EFSA) has also evaluated ethyl acetate and established acceptable daily intake levels.
Environmental regulations impact ethyl acetate's use and disposal. As a volatile organic compound (VOC), its emissions are regulated to control air pollution. Many countries have implemented VOC reduction strategies, influencing industrial processes involving ethyl acetate.
Transportation of ethyl acetate is governed by international agreements such as the United Nations Recommendations on the Transport of Dangerous Goods. These regulations specify packaging, labeling, and handling requirements for safe transportation.
As sustainability concerns grow, regulations promoting green chemistry and circular economy principles are emerging. This trend may influence future regulations on ethyl acetate, potentially encouraging the development of bio-based alternatives or improved recycling methods.
Compliance with these diverse regulations is essential for companies using ethyl acetate in their products or processes. Staying informed about regulatory changes and adapting to new requirements is crucial for maintaining ethyl acetate's position in industry solutions while ensuring safety and environmental protection.
Environmental Impact
The environmental impact of ethyl acetate production and usage is a critical consideration in cementing its position as an industry solution. As a volatile organic compound (VOC), ethyl acetate can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. However, compared to many other solvents, ethyl acetate has a relatively low toxicity and is considered biodegradable, which mitigates some of its environmental concerns.
In terms of production, the traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid has been optimized to reduce energy consumption and waste generation. Modern catalytic processes have significantly improved the efficiency and reduced the environmental footprint of ethyl acetate manufacturing. Additionally, the development of bio-based ethyl acetate production methods, utilizing renewable resources such as sugarcane or corn, offers a more sustainable alternative to petrochemical-derived ethyl acetate.
The use of ethyl acetate in various industries also presents environmental considerations. In the coatings and adhesives sector, ethyl acetate-based formulations are often preferred over more harmful solvents due to their lower ozone depletion potential and reduced impact on air quality. However, proper handling and disposal practices are essential to prevent soil and water contamination.
Recycling and recovery systems play a crucial role in minimizing the environmental impact of ethyl acetate. Many industrial processes now incorporate solvent recovery units, which capture and purify used ethyl acetate for reuse. This not only reduces waste but also decreases the demand for new production, thereby lowering the overall environmental footprint.
The pharmaceutical industry's use of ethyl acetate in drug manufacturing processes has led to increased scrutiny of its environmental impact. Stringent regulations and guidelines have been implemented to ensure proper waste management and emission control in pharmaceutical production facilities. Green chemistry initiatives are driving research into alternative solvents and more environmentally friendly synthesis routes that could potentially replace ethyl acetate in certain applications.
As global environmental regulations become more stringent, the industry is focusing on developing greener alternatives and improving the lifecycle assessment of ethyl acetate. This includes exploring bio-based feedstocks, enhancing production efficiencies, and implementing closed-loop systems to minimize emissions and waste. The ongoing efforts to reduce the environmental impact of ethyl acetate are crucial for maintaining its position as a preferred solvent in various industrial applications while meeting sustainability goals.
In terms of production, the traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid has been optimized to reduce energy consumption and waste generation. Modern catalytic processes have significantly improved the efficiency and reduced the environmental footprint of ethyl acetate manufacturing. Additionally, the development of bio-based ethyl acetate production methods, utilizing renewable resources such as sugarcane or corn, offers a more sustainable alternative to petrochemical-derived ethyl acetate.
The use of ethyl acetate in various industries also presents environmental considerations. In the coatings and adhesives sector, ethyl acetate-based formulations are often preferred over more harmful solvents due to their lower ozone depletion potential and reduced impact on air quality. However, proper handling and disposal practices are essential to prevent soil and water contamination.
Recycling and recovery systems play a crucial role in minimizing the environmental impact of ethyl acetate. Many industrial processes now incorporate solvent recovery units, which capture and purify used ethyl acetate for reuse. This not only reduces waste but also decreases the demand for new production, thereby lowering the overall environmental footprint.
The pharmaceutical industry's use of ethyl acetate in drug manufacturing processes has led to increased scrutiny of its environmental impact. Stringent regulations and guidelines have been implemented to ensure proper waste management and emission control in pharmaceutical production facilities. Green chemistry initiatives are driving research into alternative solvents and more environmentally friendly synthesis routes that could potentially replace ethyl acetate in certain applications.
As global environmental regulations become more stringent, the industry is focusing on developing greener alternatives and improving the lifecycle assessment of ethyl acetate. This includes exploring bio-based feedstocks, enhancing production efficiencies, and implementing closed-loop systems to minimize emissions and waste. The ongoing efforts to reduce the environmental impact of ethyl acetate are crucial for maintaining its position as a preferred solvent in various industrial applications while meeting sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!