The Future of Ethyl Acetate in Industry Evolution
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone significant evolution since its discovery in the early 19th century. Initially synthesized as a laboratory curiosity, it quickly found applications in various industries due to its unique properties. The evolution of ethyl acetate production and utilization can be traced through several key phases, each marked by technological advancements and expanding applications.
In the early stages, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid, a process that remained dominant for many decades. This method, while effective, was limited by the availability and cost of raw materials. The mid-20th century saw a shift towards more efficient production methods, including the Tishchenko reaction using acetaldehyde, which offered improved yields and reduced production costs.
The late 20th century brought about significant changes in ethyl acetate production with the introduction of catalytic processes. These innovations allowed for continuous production methods, greatly increasing efficiency and output. Notably, the development of heterogeneous catalysts enabled more environmentally friendly production routes, reducing waste and energy consumption.
In recent years, the focus has shifted towards sustainable production methods. Bio-based ethyl acetate, derived from renewable resources, has gained traction as industries seek to reduce their carbon footprint. This approach utilizes fermentation processes to produce ethanol and acetic acid from biomass, which are then combined to form ethyl acetate. While still in its early stages, this method shows promise for future large-scale adoption.
The application landscape of ethyl acetate has also evolved significantly. Initially used primarily as a solvent in paints and coatings, its versatility led to adoption in diverse sectors. The pharmaceutical industry began utilizing ethyl acetate in drug formulation and as an extraction solvent. In the food industry, it found use as a flavoring agent and in the production of artificial fruit essences.
The electronics industry has become a major consumer of high-purity ethyl acetate, particularly in the manufacture of flexible printed circuits and as a cleaning agent for precision components. This has driven the development of ultra-pure grades of ethyl acetate, with stringent quality control measures to meet the exacting standards of electronic manufacturing.
Looking ahead, the evolution of ethyl acetate is likely to continue along the lines of sustainability and specialized applications. Research into catalytic processes that can utilize waste carbon dioxide as a feedstock for ethyl acetate production represents a promising frontier. Additionally, the development of novel, high-performance materials incorporating ethyl acetate as a key component is expected to open new avenues in advanced manufacturing and materials science.
In the early stages, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid, a process that remained dominant for many decades. This method, while effective, was limited by the availability and cost of raw materials. The mid-20th century saw a shift towards more efficient production methods, including the Tishchenko reaction using acetaldehyde, which offered improved yields and reduced production costs.
The late 20th century brought about significant changes in ethyl acetate production with the introduction of catalytic processes. These innovations allowed for continuous production methods, greatly increasing efficiency and output. Notably, the development of heterogeneous catalysts enabled more environmentally friendly production routes, reducing waste and energy consumption.
In recent years, the focus has shifted towards sustainable production methods. Bio-based ethyl acetate, derived from renewable resources, has gained traction as industries seek to reduce their carbon footprint. This approach utilizes fermentation processes to produce ethanol and acetic acid from biomass, which are then combined to form ethyl acetate. While still in its early stages, this method shows promise for future large-scale adoption.
The application landscape of ethyl acetate has also evolved significantly. Initially used primarily as a solvent in paints and coatings, its versatility led to adoption in diverse sectors. The pharmaceutical industry began utilizing ethyl acetate in drug formulation and as an extraction solvent. In the food industry, it found use as a flavoring agent and in the production of artificial fruit essences.
The electronics industry has become a major consumer of high-purity ethyl acetate, particularly in the manufacture of flexible printed circuits and as a cleaning agent for precision components. This has driven the development of ultra-pure grades of ethyl acetate, with stringent quality control measures to meet the exacting standards of electronic manufacturing.
Looking ahead, the evolution of ethyl acetate is likely to continue along the lines of sustainability and specialized applications. Research into catalytic processes that can utilize waste carbon dioxide as a feedstock for ethyl acetate production represents a promising frontier. Additionally, the development of novel, high-performance materials incorporating ethyl acetate as a key component is expected to open new avenues in advanced manufacturing and materials science.
Market Demand Analysis
The market demand for ethyl acetate continues to show robust growth, driven by its versatile applications across various industries. As a key solvent and intermediate in the chemical industry, ethyl acetate's market is closely tied to the performance of end-use sectors such as paints and coatings, adhesives, pharmaceuticals, and food packaging.
In the paints and coatings industry, which represents a significant portion of ethyl acetate consumption, the demand is propelled by the expanding construction and automotive sectors. The growing emphasis on eco-friendly and low-VOC (volatile organic compound) formulations has further boosted the use of ethyl acetate as a preferred solvent. This trend is particularly evident in developing economies where rapid urbanization and infrastructure development are driving the demand for paints and coatings.
The adhesives industry is another major consumer of ethyl acetate, with increasing applications in packaging, woodworking, and consumer goods. The rise of e-commerce and the subsequent growth in packaging requirements have contributed to the sustained demand for ethyl acetate-based adhesives. Moreover, the shift towards water-based and solvent-based adhesives with lower environmental impact has positioned ethyl acetate favorably in this market segment.
In the pharmaceutical sector, ethyl acetate's role as a solvent in drug formulation and as an extraction medium in the production of active pharmaceutical ingredients (APIs) continues to drive demand. The global expansion of the pharmaceutical industry, coupled with the increasing focus on generic drugs and biosimilars, is expected to maintain a steady growth trajectory for ethyl acetate in this sector.
The food industry represents another significant market for ethyl acetate, particularly in flavor and fragrance applications. Its use as a food-grade solvent for the extraction of natural flavors and in the production of artificial flavors has seen consistent growth. The rising consumer preference for natural and clean-label products has further accentuated the importance of ethyl acetate in this sector.
Geographically, Asia-Pacific remains the largest and fastest-growing market for ethyl acetate, driven by the rapid industrialization in countries like China and India. The region's expanding manufacturing base, particularly in electronics, textiles, and packaging, continues to fuel the demand for ethyl acetate. North America and Europe, while mature markets, are witnessing steady growth due to the increasing adoption of bio-based ethyl acetate and stringent environmental regulations favoring its use over other solvents.
Looking ahead, the market demand for ethyl acetate is projected to maintain its growth trajectory, with emerging applications in biodegradable plastics and green solvents opening new avenues for expansion. The industry's focus on developing bio-based ethyl acetate from renewable sources is likely to reshape the market landscape, addressing sustainability concerns and potentially unlocking new market segments.
In the paints and coatings industry, which represents a significant portion of ethyl acetate consumption, the demand is propelled by the expanding construction and automotive sectors. The growing emphasis on eco-friendly and low-VOC (volatile organic compound) formulations has further boosted the use of ethyl acetate as a preferred solvent. This trend is particularly evident in developing economies where rapid urbanization and infrastructure development are driving the demand for paints and coatings.
The adhesives industry is another major consumer of ethyl acetate, with increasing applications in packaging, woodworking, and consumer goods. The rise of e-commerce and the subsequent growth in packaging requirements have contributed to the sustained demand for ethyl acetate-based adhesives. Moreover, the shift towards water-based and solvent-based adhesives with lower environmental impact has positioned ethyl acetate favorably in this market segment.
In the pharmaceutical sector, ethyl acetate's role as a solvent in drug formulation and as an extraction medium in the production of active pharmaceutical ingredients (APIs) continues to drive demand. The global expansion of the pharmaceutical industry, coupled with the increasing focus on generic drugs and biosimilars, is expected to maintain a steady growth trajectory for ethyl acetate in this sector.
The food industry represents another significant market for ethyl acetate, particularly in flavor and fragrance applications. Its use as a food-grade solvent for the extraction of natural flavors and in the production of artificial flavors has seen consistent growth. The rising consumer preference for natural and clean-label products has further accentuated the importance of ethyl acetate in this sector.
Geographically, Asia-Pacific remains the largest and fastest-growing market for ethyl acetate, driven by the rapid industrialization in countries like China and India. The region's expanding manufacturing base, particularly in electronics, textiles, and packaging, continues to fuel the demand for ethyl acetate. North America and Europe, while mature markets, are witnessing steady growth due to the increasing adoption of bio-based ethyl acetate and stringent environmental regulations favoring its use over other solvents.
Looking ahead, the market demand for ethyl acetate is projected to maintain its growth trajectory, with emerging applications in biodegradable plastics and green solvents opening new avenues for expansion. The industry's focus on developing bio-based ethyl acetate from renewable sources is likely to reshape the market landscape, addressing sustainability concerns and potentially unlocking new market segments.
Technical Challenges
The production and utilization of ethyl acetate face several technical challenges that need to be addressed for its sustainable future in industrial evolution. One of the primary concerns is the environmental impact of traditional manufacturing processes. The conventional method of producing ethyl acetate involves the esterification of ethanol and acetic acid, which often requires high temperatures and pressures, leading to significant energy consumption and greenhouse gas emissions.
Another challenge lies in the sourcing of raw materials. The production of ethyl acetate heavily relies on petroleum-based feedstocks, which are non-renewable and subject to price volatility. As global efforts to reduce dependency on fossil fuels intensify, finding alternative, sustainable sources for ethyl acetate production becomes crucial.
The purification process of ethyl acetate presents additional technical hurdles. Current methods often involve energy-intensive distillation techniques, which contribute to the overall environmental footprint of production. Developing more efficient separation and purification technologies is essential to improve the sustainability and cost-effectiveness of ethyl acetate manufacturing.
In terms of application, ethyl acetate faces challenges in certain industries due to its volatility and flammability. These properties can limit its use in some consumer products and industrial processes where safety concerns are paramount. Enhancing the stability and safety profile of ethyl acetate without compromising its desirable properties is a significant technical challenge.
The recyclability and end-of-life management of ethyl acetate-based products also pose technical difficulties. As circular economy principles gain traction, developing efficient recycling methods for ethyl acetate and its derivatives becomes increasingly important. This includes addressing issues related to the separation of ethyl acetate from complex mixtures and its potential degradation during recycling processes.
Furthermore, the development of bio-based alternatives to traditional ethyl acetate is an emerging area of research. While promising, these bio-derived versions face challenges in achieving comparable performance, scalability, and cost-effectiveness to their petroleum-based counterparts. Overcoming these hurdles requires significant advancements in biotechnology and process engineering.
Lastly, the optimization of ethyl acetate production and use in terms of energy efficiency presents ongoing technical challenges. This includes improving reactor designs, catalysts, and process control systems to minimize energy consumption and maximize yield. Additionally, integrating renewable energy sources into ethyl acetate production processes to reduce its carbon footprint is a complex technical endeavor that requires innovative solutions.
Another challenge lies in the sourcing of raw materials. The production of ethyl acetate heavily relies on petroleum-based feedstocks, which are non-renewable and subject to price volatility. As global efforts to reduce dependency on fossil fuels intensify, finding alternative, sustainable sources for ethyl acetate production becomes crucial.
The purification process of ethyl acetate presents additional technical hurdles. Current methods often involve energy-intensive distillation techniques, which contribute to the overall environmental footprint of production. Developing more efficient separation and purification technologies is essential to improve the sustainability and cost-effectiveness of ethyl acetate manufacturing.
In terms of application, ethyl acetate faces challenges in certain industries due to its volatility and flammability. These properties can limit its use in some consumer products and industrial processes where safety concerns are paramount. Enhancing the stability and safety profile of ethyl acetate without compromising its desirable properties is a significant technical challenge.
The recyclability and end-of-life management of ethyl acetate-based products also pose technical difficulties. As circular economy principles gain traction, developing efficient recycling methods for ethyl acetate and its derivatives becomes increasingly important. This includes addressing issues related to the separation of ethyl acetate from complex mixtures and its potential degradation during recycling processes.
Furthermore, the development of bio-based alternatives to traditional ethyl acetate is an emerging area of research. While promising, these bio-derived versions face challenges in achieving comparable performance, scalability, and cost-effectiveness to their petroleum-based counterparts. Overcoming these hurdles requires significant advancements in biotechnology and process engineering.
Lastly, the optimization of ethyl acetate production and use in terms of energy efficiency presents ongoing technical challenges. This includes improving reactor designs, catalysts, and process control systems to minimize energy consumption and maximize yield. Additionally, integrating renewable energy sources into ethyl acetate production processes to reduce its carbon footprint is a complex technical endeavor that requires innovative solutions.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of specific catalysts. These processes aim to improve the yield and purity of ethyl acetate, which is an important industrial solvent and chemical intermediate.- Production and purification of ethyl acetate: Various methods are employed for the production and purification of ethyl acetate, including esterification reactions, distillation processes, and the use of catalysts. These techniques aim to improve yield, purity, and efficiency in the manufacturing of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is widely used as a solvent and reagent in various chemical processes. It finds applications in extraction, synthesis, and as a reaction medium in different industries, including pharmaceuticals, polymers, and fine chemicals.
- Ethyl acetate in coating and adhesive formulations: Ethyl acetate is a key component in many coating and adhesive formulations. It is used as a solvent in paints, varnishes, and adhesives due to its favorable properties such as low toxicity, fast evaporation rate, and good solvency for many resins and polymers.
- Recovery and recycling of ethyl acetate: Processes for recovering and recycling ethyl acetate from industrial waste streams and spent solvents have been developed. These methods aim to reduce environmental impact and improve cost-effectiveness in industries that use large quantities of ethyl acetate.
- Ethyl acetate as a green solvent alternative: Ethyl acetate is being explored as a more environmentally friendly alternative to traditional solvents in various applications. Its relatively low toxicity, biodegradability, and favorable physical properties make it an attractive option for green chemistry initiatives and sustainable industrial processes.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes, including as a solvent for extractions, reactions, and formulations. It is particularly useful in the production of pharmaceuticals, coatings, and other specialty chemicals due to its favorable properties and relatively low toxicity.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications, such as in the preparation of polymer solutions, as a component in adhesive formulations, and in the production of various composite materials. Its use can affect the properties and performance of the resulting materials.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing more sustainable production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and volatility.Expand Specific Solutions05 Novel derivatives and modifications of ethyl acetate
Researchers are exploring novel derivatives and modifications of ethyl acetate to enhance its properties or create new compounds with unique characteristics. This includes the development of functionalized ethyl acetate derivatives for specific applications in various industries.Expand Specific Solutions
Key Industry Players
The ethyl acetate industry is in a mature growth phase, with a global market size expected to reach $4.3 billion by 2027. The technology is well-established, with major players like Celanese International Corp., Eastman Chemical Co., and China Petroleum & Chemical Corp. dominating the market. These companies have advanced production capabilities and extensive distribution networks. Research institutions such as Dalian Institute of Chemical Physics and universities like Tianjin University are actively involved in developing new applications and improving production processes. The industry is seeing increased focus on sustainable production methods, with companies like LanzaTech NZ, Inc. exploring bio-based ethyl acetate production. Overall, the competitive landscape is characterized by established players and ongoing innovation efforts.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using ethylene and acetic acid as raw materials. This method, known as the Celanese VA-Ethyl Acetate Process, offers significant advantages over traditional esterification routes. The process utilizes a proprietary catalyst system that enables high selectivity and yield, resulting in a more efficient and cost-effective production method[1]. Additionally, Celanese has implemented advanced process control systems and energy recovery techniques to further optimize the production process, reducing energy consumption and minimizing environmental impact[3].
Strengths: High efficiency, cost-effectiveness, and reduced environmental impact. Weaknesses: Dependence on ethylene availability and potential sensitivity to raw material price fluctuations.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel approach to ethyl acetate production through its Eastman Gasification Technology. This process involves the gasification of coal or biomass to produce syngas, which is then converted to ethanol and subsequently to ethyl acetate. The technology incorporates advanced catalysts and reactor designs to maximize conversion efficiency and product purity[2]. Eastman has also implemented a closed-loop system that recycles unreacted materials and byproducts, significantly reducing waste and improving overall process economics[4]. Furthermore, the company has invested in research to explore the use of renewable feedstocks, aiming to decrease the carbon footprint of ethyl acetate production[6].
Strengths: Versatility in feedstock usage, potential for carbon footprint reduction, and improved process economics. Weaknesses: High initial capital investment and complexity of the gasification process.
Innovative Formulations
INTEGRATED SYSTEM FOR THE PRODUCTION OF ETHYL ACETATE, ACETALDEHYDE, HYDROGEN AND ETHYLENE, INTEGRATED PROCESS FOR OBTAINING ETHYL ACETATE, ACETALDEHYDE, HYDROGEN AND ETHYLENE AND PRODUCTS OBTAINED THUS
PatentActiveBRPI1104013A2
Innovation
- An integrated system using a fixed-bed reactor with a calcined hydrotalcite-type catalyst for ethanol dehydrogenation and dehydration, followed by a series of distillation columns for efficient separation and product recovery, minimizing solvent use and energy consumption.
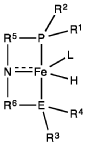
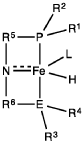
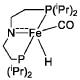
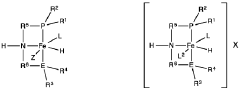
Homogeneous iron catalysts for the conversion of ethanol to ethyl acetate and hydrogen
PatentWO2019027965A1
Innovation
- A process utilizing a homogeneous iron catalyst with a tridentate pincer ligand for dehydrogenative coupling of ethanol at moderate temperatures, producing ethyl acetate efficiently and selectively, with iron loadings as low as 0.001 mol%, allowing for continuous operation and easy separation of ethyl acetate from the catalyst.
Environmental Impact
The environmental impact of ethyl acetate in industrial evolution is a critical consideration as industries strive for sustainability and regulatory compliance. Ethyl acetate, widely used as a solvent in various industries, has both positive and negative environmental implications that warrant careful examination.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. As a VOC, ethyl acetate contributes to the formation of ground-level ozone when released into the atmosphere. This can lead to air quality issues and potential health risks for both humans and ecosystems. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential, making it a preferable choice in some applications.
Water pollution is another environmental aspect to consider. While ethyl acetate is biodegradable and does not persist in aquatic environments for extended periods, its release in large quantities can still have short-term impacts on aquatic life. Proper handling and disposal practices are essential to mitigate these risks and prevent contamination of water sources.
On the positive side, ethyl acetate's biodegradability offers an advantage over many synthetic solvents. It breaks down relatively quickly in the environment, reducing long-term ecological impacts. Additionally, ethyl acetate can be produced from renewable resources, such as ethanol derived from biomass, potentially lowering its carbon footprint compared to petroleum-based alternatives.
The future of ethyl acetate in industry evolution is likely to be shaped by increasing environmental regulations and sustainability goals. Industries are exploring greener production methods, such as using bio-based feedstocks and implementing closed-loop recycling systems to minimize waste and emissions. These advancements could significantly reduce the environmental footprint of ethyl acetate production and use.
Furthermore, ongoing research into the development of more environmentally friendly alternatives may influence the future role of ethyl acetate in various industries. While ethyl acetate remains a versatile and relatively low-impact solvent, the push for even greener solutions may lead to the gradual replacement of ethyl acetate in some applications with bio-based or water-based alternatives.
As industries continue to evolve, the environmental impact of ethyl acetate will likely be subject to increased scrutiny and ongoing assessment. Balancing its utility with environmental concerns will be crucial in determining its long-term viability and role in sustainable industrial practices. This will require continued innovation in production methods, application techniques, and end-of-life management to ensure that ethyl acetate remains a responsible choice in the face of growing environmental challenges.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. As a VOC, ethyl acetate contributes to the formation of ground-level ozone when released into the atmosphere. This can lead to air quality issues and potential health risks for both humans and ecosystems. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential, making it a preferable choice in some applications.
Water pollution is another environmental aspect to consider. While ethyl acetate is biodegradable and does not persist in aquatic environments for extended periods, its release in large quantities can still have short-term impacts on aquatic life. Proper handling and disposal practices are essential to mitigate these risks and prevent contamination of water sources.
On the positive side, ethyl acetate's biodegradability offers an advantage over many synthetic solvents. It breaks down relatively quickly in the environment, reducing long-term ecological impacts. Additionally, ethyl acetate can be produced from renewable resources, such as ethanol derived from biomass, potentially lowering its carbon footprint compared to petroleum-based alternatives.
The future of ethyl acetate in industry evolution is likely to be shaped by increasing environmental regulations and sustainability goals. Industries are exploring greener production methods, such as using bio-based feedstocks and implementing closed-loop recycling systems to minimize waste and emissions. These advancements could significantly reduce the environmental footprint of ethyl acetate production and use.
Furthermore, ongoing research into the development of more environmentally friendly alternatives may influence the future role of ethyl acetate in various industries. While ethyl acetate remains a versatile and relatively low-impact solvent, the push for even greener solutions may lead to the gradual replacement of ethyl acetate in some applications with bio-based or water-based alternatives.
As industries continue to evolve, the environmental impact of ethyl acetate will likely be subject to increased scrutiny and ongoing assessment. Balancing its utility with environmental concerns will be crucial in determining its long-term viability and role in sustainable industrial practices. This will require continued innovation in production methods, application techniques, and end-of-life management to ensure that ethyl acetate remains a responsible choice in the face of growing environmental challenges.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate is evolving in response to growing environmental and health concerns. Governments worldwide are implementing stricter regulations on the production, use, and disposal of this chemical compound. In the United States, the Environmental Protection Agency (EPA) has set stringent guidelines for ethyl acetate emissions under the Clean Air Act, classifying it as a volatile organic compound (VOC). Industries using ethyl acetate must adhere to these regulations, implementing control technologies to reduce emissions and maintain air quality standards.
The European Union has also tightened its regulatory framework through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, manufacturers and importers of ethyl acetate must register the substance and provide detailed safety information. This has led to increased transparency and accountability in the ethyl acetate supply chain, influencing industry practices globally.
In Asia, countries like China and India are rapidly updating their chemical regulations to align with international standards. China's Decree No. 591, which governs the registration and management of new chemical substances, has implications for ethyl acetate production and import. Similarly, India's Chemical (Management and Safety) Rules are becoming more comprehensive, affecting the ethyl acetate industry's operations in the region.
The regulatory focus is not limited to production and use but extends to transportation and storage as well. The International Maritime Dangerous Goods (IMDG) Code classifies ethyl acetate as a flammable liquid, imposing specific requirements for its safe transport by sea. This classification has ripple effects on logistics and supply chain management for industries relying on ethyl acetate.
As sustainability becomes a central theme in global policy-making, regulations are increasingly emphasizing the need for greener alternatives and circular economy practices. This trend is pushing the ethyl acetate industry towards developing bio-based production methods and exploring recycling technologies. The EU's Circular Economy Action Plan, for instance, indirectly influences the ethyl acetate market by promoting sustainable product policies and waste reduction.
Occupational health and safety regulations are also becoming more stringent. Organizations such as the U.S. Occupational Safety and Health Administration (OSHA) have established permissible exposure limits for ethyl acetate in the workplace, necessitating improved ventilation systems and personal protective equipment in industrial settings.
The future regulatory landscape for ethyl acetate is likely to see further tightening of environmental standards, increased emphasis on worker safety, and a push towards sustainable practices. Industries will need to adapt to these evolving regulations, investing in cleaner technologies and exploring alternative production methods to ensure compliance and maintain market competitiveness.
The European Union has also tightened its regulatory framework through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, manufacturers and importers of ethyl acetate must register the substance and provide detailed safety information. This has led to increased transparency and accountability in the ethyl acetate supply chain, influencing industry practices globally.
In Asia, countries like China and India are rapidly updating their chemical regulations to align with international standards. China's Decree No. 591, which governs the registration and management of new chemical substances, has implications for ethyl acetate production and import. Similarly, India's Chemical (Management and Safety) Rules are becoming more comprehensive, affecting the ethyl acetate industry's operations in the region.
The regulatory focus is not limited to production and use but extends to transportation and storage as well. The International Maritime Dangerous Goods (IMDG) Code classifies ethyl acetate as a flammable liquid, imposing specific requirements for its safe transport by sea. This classification has ripple effects on logistics and supply chain management for industries relying on ethyl acetate.
As sustainability becomes a central theme in global policy-making, regulations are increasingly emphasizing the need for greener alternatives and circular economy practices. This trend is pushing the ethyl acetate industry towards developing bio-based production methods and exploring recycling technologies. The EU's Circular Economy Action Plan, for instance, indirectly influences the ethyl acetate market by promoting sustainable product policies and waste reduction.
Occupational health and safety regulations are also becoming more stringent. Organizations such as the U.S. Occupational Safety and Health Administration (OSHA) have established permissible exposure limits for ethyl acetate in the workplace, necessitating improved ventilation systems and personal protective equipment in industrial settings.
The future regulatory landscape for ethyl acetate is likely to see further tightening of environmental standards, increased emphasis on worker safety, and a push towards sustainable practices. Industries will need to adapt to these evolving regulations, investing in cleaner technologies and exploring alternative production methods to ensure compliance and maintain market competitiveness.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!