Key Contributions of Ethyl Acetate to Industry Supremacy
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
The evolution of ethyl acetate as a key industrial chemical can be traced back to the early 19th century when it was first synthesized. Initially, its production was limited and primarily for laboratory use. However, as industrial processes advanced, ethyl acetate's potential as a versatile solvent and reagent became increasingly apparent.
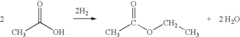
In the early 20th century, the development of large-scale production methods marked a significant milestone in ethyl acetate's evolution. The Fischer esterification process, involving the reaction of ethanol and acetic acid in the presence of a catalyst, became the primary method of production. This allowed for increased availability and reduced costs, paving the way for broader industrial applications.
The mid-20th century saw a surge in ethyl acetate's use across various industries. Its low toxicity and pleasant fruity odor made it an attractive choice for the food and beverage industry, where it found applications as a flavoring agent and in the production of artificial fruit essences. Simultaneously, the paint and coatings industry began to recognize its value as a fast-evaporating solvent, leading to its incorporation in numerous formulations.
The latter half of the 20th century witnessed further refinements in production techniques. The introduction of continuous flow reactors and more efficient catalysts significantly improved yield and purity while reducing energy consumption. These advancements not only made ethyl acetate more economically viable but also aligned with growing environmental concerns.
In recent decades, the evolution of ethyl acetate has been characterized by a focus on sustainability and green chemistry principles. Research efforts have been directed towards developing bio-based production methods, utilizing renewable feedstocks instead of petrochemical sources. Enzymatic processes and fermentation techniques have emerged as promising alternatives, offering potential for reduced environmental impact and enhanced product quality.
The digital age has also influenced ethyl acetate's evolution, with computer modeling and simulation tools enabling more precise control over production processes and facilitating the development of novel applications. This has led to optimized formulations in industries such as pharmaceuticals and electronics, where ethyl acetate plays crucial roles in drug delivery systems and semiconductor manufacturing.
As we move further into the 21st century, the evolution of ethyl acetate continues to be driven by technological advancements and changing market demands. Its role in emerging fields such as 3D printing and nanotechnology is being explored, opening up new avenues for innovation and industrial supremacy. The ongoing research into more efficient and sustainable production methods ensures that ethyl acetate will remain a key player in various industrial sectors for years to come.
In the early 20th century, the development of large-scale production methods marked a significant milestone in ethyl acetate's evolution. The Fischer esterification process, involving the reaction of ethanol and acetic acid in the presence of a catalyst, became the primary method of production. This allowed for increased availability and reduced costs, paving the way for broader industrial applications.
The mid-20th century saw a surge in ethyl acetate's use across various industries. Its low toxicity and pleasant fruity odor made it an attractive choice for the food and beverage industry, where it found applications as a flavoring agent and in the production of artificial fruit essences. Simultaneously, the paint and coatings industry began to recognize its value as a fast-evaporating solvent, leading to its incorporation in numerous formulations.
The latter half of the 20th century witnessed further refinements in production techniques. The introduction of continuous flow reactors and more efficient catalysts significantly improved yield and purity while reducing energy consumption. These advancements not only made ethyl acetate more economically viable but also aligned with growing environmental concerns.
In recent decades, the evolution of ethyl acetate has been characterized by a focus on sustainability and green chemistry principles. Research efforts have been directed towards developing bio-based production methods, utilizing renewable feedstocks instead of petrochemical sources. Enzymatic processes and fermentation techniques have emerged as promising alternatives, offering potential for reduced environmental impact and enhanced product quality.
The digital age has also influenced ethyl acetate's evolution, with computer modeling and simulation tools enabling more precise control over production processes and facilitating the development of novel applications. This has led to optimized formulations in industries such as pharmaceuticals and electronics, where ethyl acetate plays crucial roles in drug delivery systems and semiconductor manufacturing.
As we move further into the 21st century, the evolution of ethyl acetate continues to be driven by technological advancements and changing market demands. Its role in emerging fields such as 3D printing and nanotechnology is being explored, opening up new avenues for innovation and industrial supremacy. The ongoing research into more efficient and sustainable production methods ensures that ethyl acetate will remain a key player in various industrial sectors for years to come.
Market Demand Analysis
The market demand for ethyl acetate has been steadily growing, driven by its versatile applications across various industries. This compound plays a crucial role in the production of paints, coatings, adhesives, and pharmaceuticals, making it an indispensable component in manufacturing processes.
In the paints and coatings industry, ethyl acetate serves as an excellent solvent due to its low toxicity and high solvency power. The increasing construction activities worldwide, coupled with the growing automotive sector, have significantly boosted the demand for paints and coatings, consequently driving the market for ethyl acetate.
The adhesives industry has also witnessed a surge in ethyl acetate consumption. As packaging requirements become more sophisticated and the e-commerce sector expands, the need for high-performance adhesives has escalated. Ethyl acetate's ability to provide strong bonding properties while maintaining environmental friendliness has made it a preferred choice in this sector.
In the pharmaceutical industry, ethyl acetate finds extensive use as a solvent in the production of various drugs and active pharmaceutical ingredients (APIs). The global healthcare market's growth, particularly in emerging economies, has led to an increased demand for pharmaceuticals, thereby positively impacting the ethyl acetate market.
The food and beverage industry represents another significant market for ethyl acetate. Its application as a flavoring agent and in the production of artificial fruit essences has gained traction. The rising consumer preference for processed and convenience foods has further amplified the demand in this sector.
The global push towards sustainability and eco-friendly products has also influenced the ethyl acetate market. As industries seek alternatives to more harmful solvents, ethyl acetate's relatively low environmental impact has positioned it favorably in the market. This trend is expected to continue, potentially opening new avenues for ethyl acetate applications in green technologies.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate, primarily due to the rapid industrialization in countries like China and India. The region's booming manufacturing sector, particularly in electronics and automotive industries, has significantly contributed to the increased demand for ethyl acetate.
Despite the positive market outlook, challenges such as price volatility of raw materials and the availability of substitutes pose potential risks to market growth. However, ongoing research and development efforts aimed at improving production efficiency and exploring new applications are expected to mitigate these challenges and sustain market growth in the long term.
In the paints and coatings industry, ethyl acetate serves as an excellent solvent due to its low toxicity and high solvency power. The increasing construction activities worldwide, coupled with the growing automotive sector, have significantly boosted the demand for paints and coatings, consequently driving the market for ethyl acetate.
The adhesives industry has also witnessed a surge in ethyl acetate consumption. As packaging requirements become more sophisticated and the e-commerce sector expands, the need for high-performance adhesives has escalated. Ethyl acetate's ability to provide strong bonding properties while maintaining environmental friendliness has made it a preferred choice in this sector.
In the pharmaceutical industry, ethyl acetate finds extensive use as a solvent in the production of various drugs and active pharmaceutical ingredients (APIs). The global healthcare market's growth, particularly in emerging economies, has led to an increased demand for pharmaceuticals, thereby positively impacting the ethyl acetate market.
The food and beverage industry represents another significant market for ethyl acetate. Its application as a flavoring agent and in the production of artificial fruit essences has gained traction. The rising consumer preference for processed and convenience foods has further amplified the demand in this sector.
The global push towards sustainability and eco-friendly products has also influenced the ethyl acetate market. As industries seek alternatives to more harmful solvents, ethyl acetate's relatively low environmental impact has positioned it favorably in the market. This trend is expected to continue, potentially opening new avenues for ethyl acetate applications in green technologies.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate, primarily due to the rapid industrialization in countries like China and India. The region's booming manufacturing sector, particularly in electronics and automotive industries, has significantly contributed to the increased demand for ethyl acetate.
Despite the positive market outlook, challenges such as price volatility of raw materials and the availability of substitutes pose potential risks to market growth. However, ongoing research and development efforts aimed at improving production efficiency and exploring new applications are expected to mitigate these challenges and sustain market growth in the long term.
Technical Challenges
Despite its widespread use and significant contributions to various industries, ethyl acetate faces several technical challenges that hinder its full potential and industry supremacy. One of the primary concerns is the environmental impact associated with its production and use. Traditional manufacturing processes often involve the use of petrochemical feedstocks, which contribute to carbon emissions and environmental degradation. Developing more sustainable and eco-friendly production methods remains a significant challenge for the industry.
Another technical hurdle is the optimization of ethyl acetate's synthesis process. While current methods are well-established, there is a constant need for improving yield, reducing energy consumption, and minimizing waste generation. Achieving higher purity levels without increasing production costs also presents a considerable challenge, especially for applications in the pharmaceutical and electronics industries that demand ultra-pure ethyl acetate.
The volatility of ethyl acetate poses challenges in terms of storage, transportation, and handling. Its low boiling point and high vapor pressure make it prone to evaporation, leading to potential product loss and safety concerns. Developing advanced containment systems and improving stability during storage and transport are ongoing technical challenges that require innovative solutions.
In the realm of application, ethyl acetate's performance in certain conditions needs enhancement. For instance, its limited resistance to hydrolysis in aqueous environments restricts its use in some water-based formulations. Improving its stability and compatibility with various substrates and other chemicals is crucial for expanding its application range and maintaining industry supremacy.
The recovery and recycling of ethyl acetate present another set of technical challenges. In many industrial processes, ethyl acetate is used as a solvent and needs to be recovered for reuse. Developing efficient and cost-effective recovery methods, particularly for complex mixtures or contaminated streams, remains a significant area of research and development.
Lastly, the industry faces challenges in scaling up production to meet growing global demand while maintaining quality and cost-effectiveness. As new applications emerge and existing markets expand, there is a need for innovative reactor designs, process intensification techniques, and advanced control systems to enhance production capacity and efficiency.
Another technical hurdle is the optimization of ethyl acetate's synthesis process. While current methods are well-established, there is a constant need for improving yield, reducing energy consumption, and minimizing waste generation. Achieving higher purity levels without increasing production costs also presents a considerable challenge, especially for applications in the pharmaceutical and electronics industries that demand ultra-pure ethyl acetate.
The volatility of ethyl acetate poses challenges in terms of storage, transportation, and handling. Its low boiling point and high vapor pressure make it prone to evaporation, leading to potential product loss and safety concerns. Developing advanced containment systems and improving stability during storage and transport are ongoing technical challenges that require innovative solutions.
In the realm of application, ethyl acetate's performance in certain conditions needs enhancement. For instance, its limited resistance to hydrolysis in aqueous environments restricts its use in some water-based formulations. Improving its stability and compatibility with various substrates and other chemicals is crucial for expanding its application range and maintaining industry supremacy.
The recovery and recycling of ethyl acetate present another set of technical challenges. In many industrial processes, ethyl acetate is used as a solvent and needs to be recovered for reuse. Developing efficient and cost-effective recovery methods, particularly for complex mixtures or contaminated streams, remains a significant area of research and development.
Lastly, the industry faces challenges in scaling up production to meet growing global demand while maintaining quality and cost-effectiveness. As new applications emerge and existing markets expand, there is a need for innovative reactor designs, process intensification techniques, and advanced control systems to enhance production capacity and efficiency.
Current Manufacturing
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and separation methods. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in diverse chemical processes, such as solvent extraction, as a reaction medium, and in the production of other chemicals. Its properties make it suitable for use in various industrial applications and manufacturing processes.
- Ethyl acetate in pharmaceutical and cosmetic formulations: Ethyl acetate is employed in the formulation of pharmaceutical and cosmetic products. It serves as a solvent, excipient, or ingredient in various preparations, contributing to the effectiveness and stability of these products.
- Environmental and safety considerations for ethyl acetate use: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, enhancing handling procedures, and implementing proper disposal techniques.
- Novel applications and modifications of ethyl acetate: Innovative uses and modifications of ethyl acetate are explored, including its incorporation into new materials, its role in advanced chemical synthesis, and potential applications in emerging technologies. These developments aim to expand the utility of ethyl acetate in various industries.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. The versatility of ethyl acetate in different chemical reactions and its role in industrial processes are highlighted.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its use as a solvent in liquid-liquid extraction, chromatography, and other separation techniques is described. The effectiveness of ethyl acetate in isolating specific compounds from complex mixtures is emphasized.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The environmental impact and safety aspects of ethyl acetate production and use are addressed. This includes methods for reducing emissions, improving process safety, and developing more sustainable production techniques. Strategies for handling, storing, and disposing of ethyl acetate in an environmentally friendly manner are also discussed.Expand Specific Solutions05 Novel applications and formulations of ethyl acetate
Innovative uses and formulations of ethyl acetate are explored. These include its application in new materials, coatings, and specialty chemicals. The development of ethyl acetate-based products with enhanced properties or novel functionalities is described, showcasing the compound's potential in emerging technologies and industries.Expand Specific Solutions
Industry Leaders
The ethyl acetate industry is in a mature stage, characterized by established production processes and a stable market. The global market size is estimated to be around $3 billion, with steady growth projected due to increasing demand in various applications. Technologically, the industry is well-developed, with major players like Celanese International Corp., China Petroleum & Chemical Corp., and Eastman Chemical Co. focusing on process optimization and capacity expansion. Research institutions such as Tianjin University and the Council of Scientific & Industrial Research are contributing to incremental improvements in catalysts and production methods. While the technology is mature, ongoing efforts in sustainability and bio-based production, led by companies like LanzaTech NZ, Inc. and Gevo, Inc., are driving innovation in the sector.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary acetyls technology platform for ethyl acetate production, which utilizes advanced catalysts and process optimization. Their method involves the direct esterification of ethanol with acetic acid, achieving high yields and selectivity. The process operates at lower temperatures and pressures compared to traditional methods, resulting in reduced energy consumption and improved sustainability[1][3]. Celanese has also implemented an integrated production approach, where ethyl acetate is produced as part of a larger acetyls complex, allowing for efficient use of raw materials and energy[2].
Strengths: High efficiency, lower energy consumption, and integrated production. Weaknesses: Dependence on ethanol and acetic acid availability, potential sensitivity to raw material price fluctuations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative ethyl acetate production process using coal-based acetic acid and bio-ethanol. This approach combines traditional petrochemical feedstocks with renewable resources, aligning with sustainability goals. Sinopec's process employs advanced heterogeneous catalysts that enhance reaction rates and selectivity[4]. The company has also implemented heat integration and energy recovery systems to improve overall process efficiency. Additionally, Sinopec has invested in large-scale production facilities, allowing for economies of scale and reduced production costs[5].
Strengths: Integration of renewable resources, advanced catalysis, and large-scale production capabilities. Weaknesses: Reliance on coal-based feedstock may face environmental scrutiny, potential challenges in bio-ethanol supply chain.
Innovative Patents
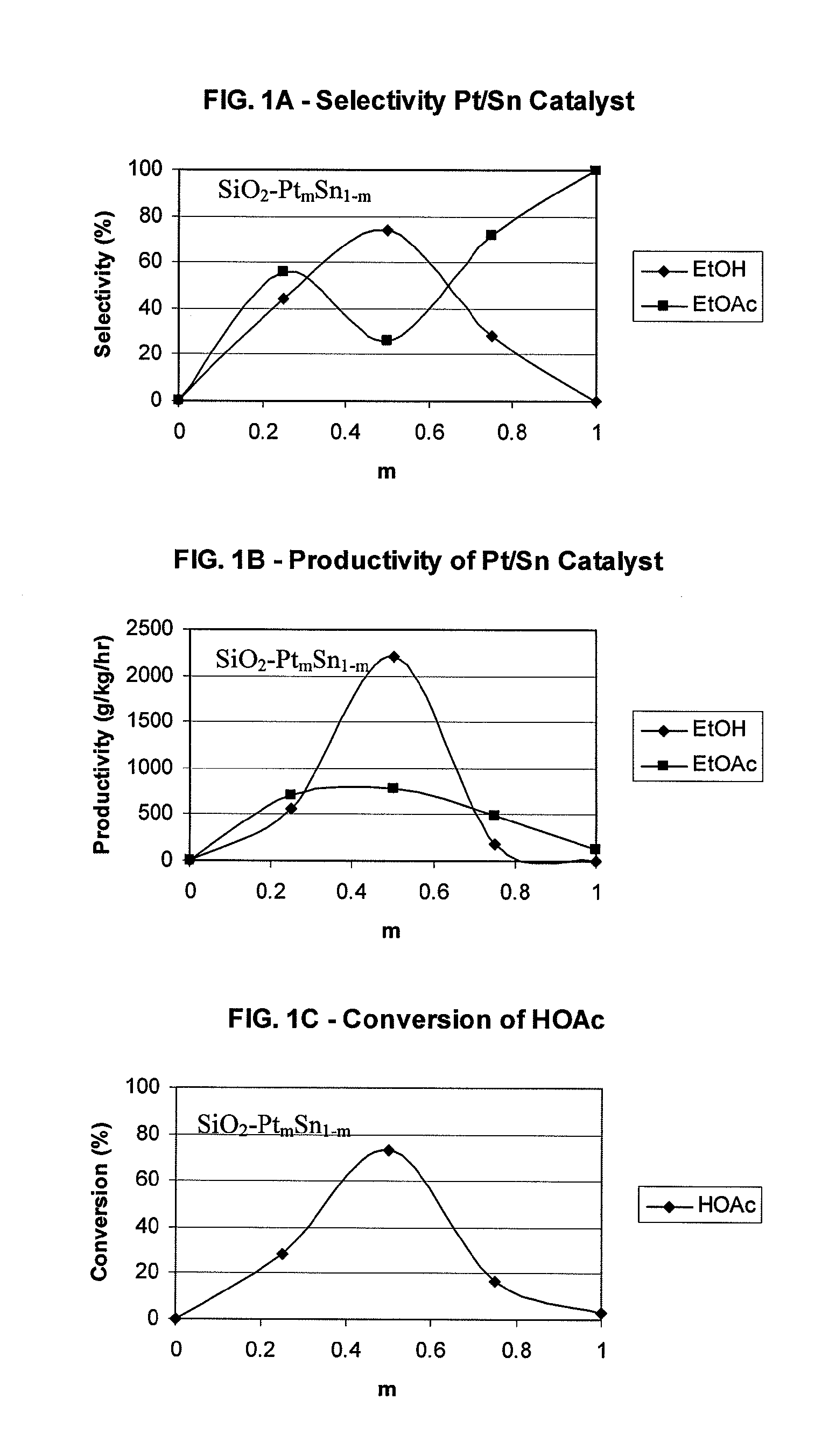
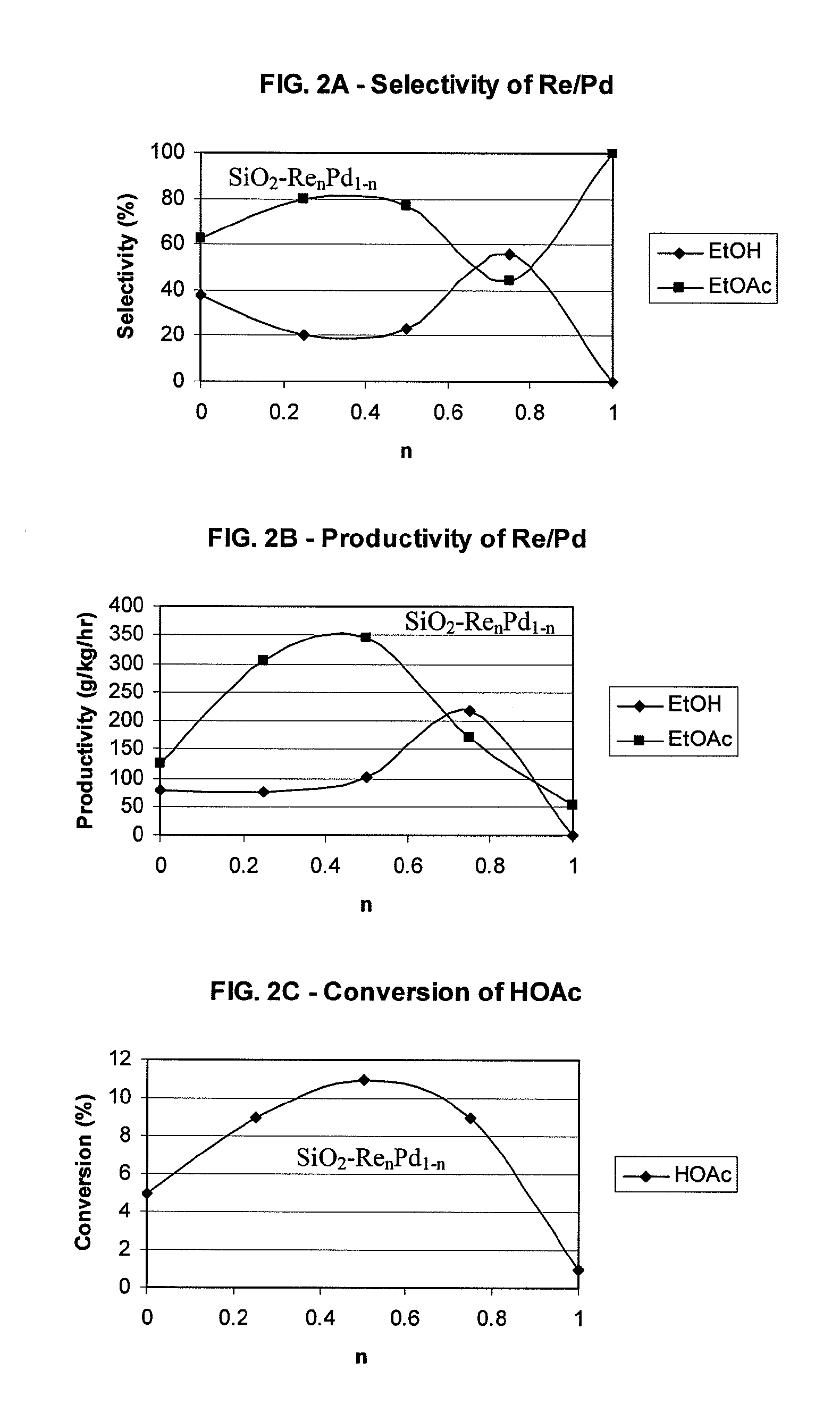
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentWO2010014145A2
Innovation
- A process utilizing a bimetallic catalyst supported on a suitable catalyst support, comprising metals like platinum, palladium, copper, and cobalt, which selectively hydrogenates acetic acid to ethyl acetate with high yield and selectivity, minimizing by-product formation.
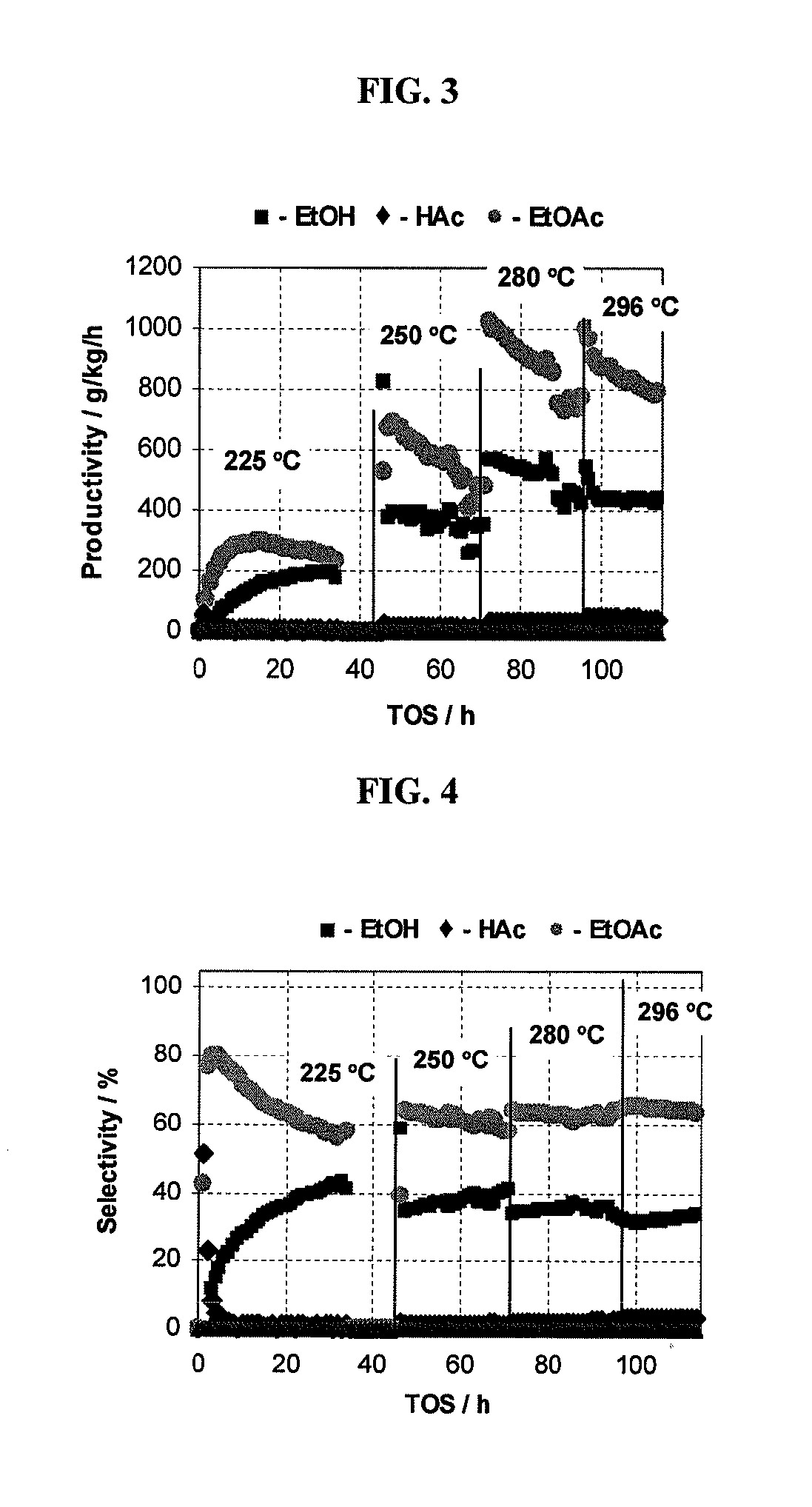
Processes for making ethyl acetate from acetic acid
PatentInactiveUS20100197959A1
Innovation
- A process utilizing catalysts comprising metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which are effective in hydrogenating acetic acid to produce ethyl acetate with high selectivity and minimizing by-product formation.
Environmental Impact
The environmental impact of ethyl acetate production and usage is a critical consideration in assessing its contributions to industry supremacy. While ethyl acetate offers numerous benefits in various industrial applications, its production and disposal processes can have significant environmental implications.
The manufacturing of ethyl acetate primarily involves the esterification of ethanol with acetic acid, a process that requires energy input and generates waste products. The energy consumption during production contributes to greenhouse gas emissions, particularly when fossil fuels are used as the primary energy source. However, advancements in green chemistry and sustainable manufacturing practices have led to more energy-efficient production methods, reducing the overall carbon footprint of ethyl acetate production.
Water pollution is another environmental concern associated with ethyl acetate. Improper disposal or accidental spills can lead to contamination of water bodies, potentially harming aquatic ecosystems. To mitigate this risk, stringent regulations and waste management protocols have been implemented in many countries, ensuring proper handling and disposal of ethyl acetate and its byproducts.
Air quality is also affected by ethyl acetate emissions during production and use. As a volatile organic compound (VOC), ethyl acetate can contribute to the formation of ground-level ozone and smog when released into the atmosphere. This has led to the development and adoption of advanced emission control technologies in industrial settings, such as regenerative thermal oxidizers and carbon adsorption systems, which significantly reduce VOC emissions.
Despite these challenges, ethyl acetate presents several environmental advantages compared to alternative solvents. It is biodegradable and has a relatively low toxicity profile, making it a more environmentally friendly option in many applications. Furthermore, its ability to replace more harmful solvents in various industries contributes to overall environmental improvement.
The recycling and recovery of ethyl acetate have become increasingly important in minimizing its environmental impact. Advanced distillation and membrane separation techniques allow for the efficient recovery and reuse of ethyl acetate in industrial processes, reducing waste and the need for new production. This circular approach not only decreases the environmental footprint but also improves cost-effectiveness for industries.
As sustainability becomes a key focus in industrial operations, ongoing research and development efforts are directed towards enhancing the environmental profile of ethyl acetate. This includes exploring bio-based production methods using renewable feedstocks, which could significantly reduce the reliance on petrochemical sources and further minimize the environmental impact of ethyl acetate production.
The manufacturing of ethyl acetate primarily involves the esterification of ethanol with acetic acid, a process that requires energy input and generates waste products. The energy consumption during production contributes to greenhouse gas emissions, particularly when fossil fuels are used as the primary energy source. However, advancements in green chemistry and sustainable manufacturing practices have led to more energy-efficient production methods, reducing the overall carbon footprint of ethyl acetate production.
Water pollution is another environmental concern associated with ethyl acetate. Improper disposal or accidental spills can lead to contamination of water bodies, potentially harming aquatic ecosystems. To mitigate this risk, stringent regulations and waste management protocols have been implemented in many countries, ensuring proper handling and disposal of ethyl acetate and its byproducts.
Air quality is also affected by ethyl acetate emissions during production and use. As a volatile organic compound (VOC), ethyl acetate can contribute to the formation of ground-level ozone and smog when released into the atmosphere. This has led to the development and adoption of advanced emission control technologies in industrial settings, such as regenerative thermal oxidizers and carbon adsorption systems, which significantly reduce VOC emissions.
Despite these challenges, ethyl acetate presents several environmental advantages compared to alternative solvents. It is biodegradable and has a relatively low toxicity profile, making it a more environmentally friendly option in many applications. Furthermore, its ability to replace more harmful solvents in various industries contributes to overall environmental improvement.
The recycling and recovery of ethyl acetate have become increasingly important in minimizing its environmental impact. Advanced distillation and membrane separation techniques allow for the efficient recovery and reuse of ethyl acetate in industrial processes, reducing waste and the need for new production. This circular approach not only decreases the environmental footprint but also improves cost-effectiveness for industries.
As sustainability becomes a key focus in industrial operations, ongoing research and development efforts are directed towards enhancing the environmental profile of ethyl acetate. This includes exploring bio-based production methods using renewable feedstocks, which could significantly reduce the reliance on petrochemical sources and further minimize the environmental impact of ethyl acetate production.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate plays a crucial role in shaping its industrial supremacy. Governments and international bodies have established comprehensive frameworks to ensure the safe production, handling, and use of this versatile chemical compound. In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA), which mandates reporting, record-keeping, and testing requirements. The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits to protect workers in industrial settings.
In the European Union, ethyl acetate falls under the purview of the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. This legislation aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances. Manufacturers and importers are required to gather information on the properties of their chemical substances and register the information in a central database managed by the European Chemicals Agency (ECHA).
The transportation of ethyl acetate is subject to strict regulations due to its flammable nature. The International Air Transport Association (IATA) and the International Maritime Organization (IMO) have established guidelines for the safe transport of dangerous goods, including ethyl acetate. These regulations cover aspects such as packaging, labeling, and documentation requirements.
In the food industry, ethyl acetate's use as a flavoring agent is regulated by the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). Both agencies have classified ethyl acetate as Generally Recognized as Safe (GRAS) when used in accordance with good manufacturing practices. However, specific limits on its concentration in food products are enforced to ensure consumer safety.
Environmental regulations also impact the industrial use of ethyl acetate. Many countries have implemented volatile organic compound (VOC) emission standards to reduce air pollution. As ethyl acetate is a VOC, industries using this solvent must comply with these regulations, often necessitating the implementation of emission control technologies or process modifications.
The pharmaceutical industry faces additional regulatory scrutiny when using ethyl acetate in drug manufacturing processes. Good Manufacturing Practice (GMP) guidelines, enforced by agencies such as the FDA and the European Medicines Agency (EMA), require stringent quality control measures and documentation of solvent use in pharmaceutical production.
As global awareness of environmental and health issues continues to grow, the regulatory landscape for ethyl acetate is likely to evolve. Industries relying on this compound must stay abreast of regulatory changes and adapt their practices accordingly to maintain compliance and sustain their competitive edge in the market.
In the European Union, ethyl acetate falls under the purview of the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. This legislation aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances. Manufacturers and importers are required to gather information on the properties of their chemical substances and register the information in a central database managed by the European Chemicals Agency (ECHA).
The transportation of ethyl acetate is subject to strict regulations due to its flammable nature. The International Air Transport Association (IATA) and the International Maritime Organization (IMO) have established guidelines for the safe transport of dangerous goods, including ethyl acetate. These regulations cover aspects such as packaging, labeling, and documentation requirements.
In the food industry, ethyl acetate's use as a flavoring agent is regulated by the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). Both agencies have classified ethyl acetate as Generally Recognized as Safe (GRAS) when used in accordance with good manufacturing practices. However, specific limits on its concentration in food products are enforced to ensure consumer safety.
Environmental regulations also impact the industrial use of ethyl acetate. Many countries have implemented volatile organic compound (VOC) emission standards to reduce air pollution. As ethyl acetate is a VOC, industries using this solvent must comply with these regulations, often necessitating the implementation of emission control technologies or process modifications.
The pharmaceutical industry faces additional regulatory scrutiny when using ethyl acetate in drug manufacturing processes. Good Manufacturing Practice (GMP) guidelines, enforced by agencies such as the FDA and the European Medicines Agency (EMA), require stringent quality control measures and documentation of solvent use in pharmaceutical production.
As global awareness of environmental and health issues continues to grow, the regulatory landscape for ethyl acetate is likely to evolve. Industries relying on this compound must stay abreast of regulatory changes and adapt their practices accordingly to maintain compliance and sustain their competitive edge in the market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!