Charting the Industrial Influence of Ethyl Acetate
JUN 30, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has become an indispensable component in various industrial applications. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its widespread use can be attributed to its unique properties, including low toxicity, high solvency, and rapid evaporation rate.
In the chemical industry, ethyl acetate serves as a crucial solvent for a wide range of substances, making it an essential ingredient in the production of paints, coatings, and adhesives. Its ability to dissolve both polar and non-polar compounds makes it particularly valuable in these applications. The automotive and construction sectors heavily rely on ethyl acetate-based products for their finishing and bonding processes.
The pharmaceutical industry also benefits significantly from ethyl acetate's properties. It is employed in the extraction and purification of various drugs and as a reagent in the synthesis of complex pharmaceutical compounds. Its low toxicity profile makes it a preferred choice in many medicinal formulations, where safety is paramount.
In the food and beverage industry, ethyl acetate plays a dual role. Naturally occurring in fruits and wines, it contributes to their characteristic flavors. Additionally, it is used as an artificial flavoring agent to enhance or replicate fruit flavors in processed foods and beverages. Its application extends to the decaffeination of coffee and tea, where it acts as an efficient extraction solvent.
The electronics industry utilizes ethyl acetate in the production of circuit boards and semiconductor devices. Its excellent cleaning properties make it ideal for removing flux residues and other contaminants during the manufacturing process. This application has become increasingly important with the rapid growth of the electronics sector and the demand for high-performance, miniaturized devices.
Environmental considerations have also influenced the use of ethyl acetate. As a volatile organic compound (VOC), its emissions are regulated in many jurisdictions. However, compared to other solvents, it is considered relatively environmentally friendly due to its biodegradability and low ozone depletion potential. This has led to its increased adoption as a replacement for more harmful solvents in various industrial processes.
The global market for ethyl acetate continues to expand, driven by growing demand across multiple industries. Its production is closely tied to the availability and price of its precursors, ethanol and acetic acid. As industries evolve and new applications emerge, the influence of ethyl acetate in the industrial landscape is expected to persist and potentially grow, underscoring its significance as a key chemical compound in modern manufacturing and processing.
In the chemical industry, ethyl acetate serves as a crucial solvent for a wide range of substances, making it an essential ingredient in the production of paints, coatings, and adhesives. Its ability to dissolve both polar and non-polar compounds makes it particularly valuable in these applications. The automotive and construction sectors heavily rely on ethyl acetate-based products for their finishing and bonding processes.
The pharmaceutical industry also benefits significantly from ethyl acetate's properties. It is employed in the extraction and purification of various drugs and as a reagent in the synthesis of complex pharmaceutical compounds. Its low toxicity profile makes it a preferred choice in many medicinal formulations, where safety is paramount.
In the food and beverage industry, ethyl acetate plays a dual role. Naturally occurring in fruits and wines, it contributes to their characteristic flavors. Additionally, it is used as an artificial flavoring agent to enhance or replicate fruit flavors in processed foods and beverages. Its application extends to the decaffeination of coffee and tea, where it acts as an efficient extraction solvent.
The electronics industry utilizes ethyl acetate in the production of circuit boards and semiconductor devices. Its excellent cleaning properties make it ideal for removing flux residues and other contaminants during the manufacturing process. This application has become increasingly important with the rapid growth of the electronics sector and the demand for high-performance, miniaturized devices.
Environmental considerations have also influenced the use of ethyl acetate. As a volatile organic compound (VOC), its emissions are regulated in many jurisdictions. However, compared to other solvents, it is considered relatively environmentally friendly due to its biodegradability and low ozone depletion potential. This has led to its increased adoption as a replacement for more harmful solvents in various industrial processes.
The global market for ethyl acetate continues to expand, driven by growing demand across multiple industries. Its production is closely tied to the availability and price of its precursors, ethanol and acetic acid. As industries evolve and new applications emerge, the influence of ethyl acetate in the industrial landscape is expected to persist and potentially grow, underscoring its significance as a key chemical compound in modern manufacturing and processing.
Market Demand Analysis
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. As a key solvent and intermediate in chemical processes, ethyl acetate's demand is closely tied to the performance of end-use sectors such as paints and coatings, adhesives, pharmaceuticals, and food and beverages.
In the paints and coatings industry, ethyl acetate serves as an essential solvent due to its excellent solvency properties and low toxicity. The construction boom in emerging economies and the increasing demand for eco-friendly coatings have been significant factors propelling the market growth. The automotive sector, another major consumer of paints and coatings, has also contributed to the rising demand for ethyl acetate.
The adhesives industry represents another substantial market for ethyl acetate. With the growth in packaging, woodworking, and construction industries, the demand for adhesives has surged, consequently boosting ethyl acetate consumption. The trend towards water-based adhesives has somewhat tempered growth in this segment, but ethyl acetate remains a crucial component in many formulations.
In the pharmaceutical sector, ethyl acetate finds applications as a solvent in drug formulation and as an intermediate in the synthesis of various active pharmaceutical ingredients. The expanding pharmaceutical industry, particularly in developing countries, has been a key driver for ethyl acetate demand in this sector.
The food and beverage industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. With changing consumer preferences and the growing popularity of decaffeinated beverages, this application has seen steady growth.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, driven by rapid industrialization, urbanization, and the presence of major end-use industries in countries like China and India. North America and Europe follow, with mature markets characterized by steady demand and a focus on sustainable and bio-based alternatives.
The market dynamics are also influenced by raw material prices, particularly those of ethanol and acetic acid. Fluctuations in these prices can significantly impact the production costs and, consequently, the market growth of ethyl acetate.
Looking ahead, the ethyl acetate market is expected to continue its growth trajectory, albeit with some challenges. The increasing focus on environmental regulations and the shift towards green solvents may pose challenges to traditional ethyl acetate producers. However, opportunities lie in the development of bio-based ethyl acetate, which aligns with the growing demand for sustainable chemical solutions across industries.
In the paints and coatings industry, ethyl acetate serves as an essential solvent due to its excellent solvency properties and low toxicity. The construction boom in emerging economies and the increasing demand for eco-friendly coatings have been significant factors propelling the market growth. The automotive sector, another major consumer of paints and coatings, has also contributed to the rising demand for ethyl acetate.
The adhesives industry represents another substantial market for ethyl acetate. With the growth in packaging, woodworking, and construction industries, the demand for adhesives has surged, consequently boosting ethyl acetate consumption. The trend towards water-based adhesives has somewhat tempered growth in this segment, but ethyl acetate remains a crucial component in many formulations.
In the pharmaceutical sector, ethyl acetate finds applications as a solvent in drug formulation and as an intermediate in the synthesis of various active pharmaceutical ingredients. The expanding pharmaceutical industry, particularly in developing countries, has been a key driver for ethyl acetate demand in this sector.
The food and beverage industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. With changing consumer preferences and the growing popularity of decaffeinated beverages, this application has seen steady growth.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, driven by rapid industrialization, urbanization, and the presence of major end-use industries in countries like China and India. North America and Europe follow, with mature markets characterized by steady demand and a focus on sustainable and bio-based alternatives.
The market dynamics are also influenced by raw material prices, particularly those of ethanol and acetic acid. Fluctuations in these prices can significantly impact the production costs and, consequently, the market growth of ethyl acetate.
Looking ahead, the ethyl acetate market is expected to continue its growth trajectory, albeit with some challenges. The increasing focus on environmental regulations and the shift towards green solvents may pose challenges to traditional ethyl acetate producers. However, opportunities lie in the development of bio-based ethyl acetate, which aligns with the growing demand for sustainable chemical solutions across industries.
Technical Challenges
The production and utilization of ethyl acetate face several technical challenges that impact its industrial influence. One of the primary issues is the optimization of the manufacturing process. Traditional methods often involve energy-intensive distillation steps, leading to high production costs and environmental concerns. Developing more efficient and sustainable production techniques remains a significant hurdle for the industry.
Another challenge lies in the purification of ethyl acetate. The presence of impurities can significantly affect the quality and performance of the final product. Achieving high-purity ethyl acetate consistently and cost-effectively is crucial for meeting stringent industry standards across various applications. This necessitates continuous improvement in separation and purification technologies.
The storage and transportation of ethyl acetate also present technical difficulties. Its volatile nature and flammability require specialized handling and storage solutions to ensure safety and prevent product loss. Developing advanced containment systems and implementing robust safety protocols are ongoing challenges for manufacturers and distributors.
Environmental concerns pose another set of technical challenges. As regulations become more stringent, there is a growing need to reduce emissions and waste associated with ethyl acetate production and use. This includes developing greener synthesis routes, implementing more effective recycling processes, and finding ways to minimize the release of volatile organic compounds (VOCs) during various industrial applications.
The search for alternative raw materials is an additional technical challenge. Currently, ethyl acetate is primarily produced from petrochemical sources. Finding renewable and sustainable feedstocks that can be economically viable at industrial scales is a key area of research and development. This involves exploring bio-based routes and developing new catalysts and processes to enable efficient conversion from alternative sources.
Lastly, the adaptation of ethyl acetate for emerging applications presents its own set of challenges. As new industries and technologies evolve, there is a constant need to tailor the properties and performance of ethyl acetate to meet specific requirements. This may involve developing new formulations, improving stability under various conditions, or enhancing compatibility with other materials. The ability to innovate and customize ethyl acetate for diverse and evolving applications is crucial for expanding its industrial influence.
Another challenge lies in the purification of ethyl acetate. The presence of impurities can significantly affect the quality and performance of the final product. Achieving high-purity ethyl acetate consistently and cost-effectively is crucial for meeting stringent industry standards across various applications. This necessitates continuous improvement in separation and purification technologies.
The storage and transportation of ethyl acetate also present technical difficulties. Its volatile nature and flammability require specialized handling and storage solutions to ensure safety and prevent product loss. Developing advanced containment systems and implementing robust safety protocols are ongoing challenges for manufacturers and distributors.
Environmental concerns pose another set of technical challenges. As regulations become more stringent, there is a growing need to reduce emissions and waste associated with ethyl acetate production and use. This includes developing greener synthesis routes, implementing more effective recycling processes, and finding ways to minimize the release of volatile organic compounds (VOCs) during various industrial applications.
The search for alternative raw materials is an additional technical challenge. Currently, ethyl acetate is primarily produced from petrochemical sources. Finding renewable and sustainable feedstocks that can be economically viable at industrial scales is a key area of research and development. This involves exploring bio-based routes and developing new catalysts and processes to enable efficient conversion from alternative sources.
Lastly, the adaptation of ethyl acetate for emerging applications presents its own set of challenges. As new industries and technologies evolve, there is a constant need to tailor the properties and performance of ethyl acetate to meet specific requirements. This may involve developing new formulations, improving stability under various conditions, or enhancing compatibility with other materials. The ability to innovate and customize ethyl acetate for diverse and evolving applications is crucial for expanding its industrial influence.
Current Manufacturing
01 Production and purification of ethyl acetate
Various methods and processes for producing and purifying ethyl acetate are described. These include distillation techniques, reactive distillation, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate while minimizing byproducts and energy consumption.- Production and purification of ethyl acetate: Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and separation processes to obtain high-purity ethyl acetate. The methods aim to improve yield, efficiency, and product quality in industrial-scale production.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and applications. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in industries such as coatings, adhesives, and electronics manufacturing.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. It is used as a solvent to isolate and purify substances from mixtures, particularly in the pharmaceutical and food industries. The compound's properties make it suitable for liquid-liquid extraction and other separation techniques.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and volatility.
- Novel derivatives and modifications of ethyl acetate: Researchers are exploring novel derivatives and modifications of ethyl acetate to enhance its properties or create new compounds with improved characteristics. This includes developing new reaction pathways, synthesizing ethyl acetate-based materials, and investigating potential applications in emerging technologies.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and applications. It serves as a solvent in different industries, including pharmaceuticals, coatings, and adhesives. The compound is also used in extraction processes and as a reactant in organic synthesis reactions.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of various polymers, as a solvent for resins, and in the development of coatings and films. The compound's properties make it suitable for use in material processing and modification techniques.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, improving waste management, and enhancing safety measures in handling and storage of the compound.Expand Specific Solutions05 Novel synthesis routes and derivatives of ethyl acetate
Innovative approaches to synthesizing ethyl acetate and creating new derivatives are explored. These include the development of new catalysts, alternative feedstocks, and novel reaction pathways. The research aims to improve efficiency, reduce costs, and expand the range of applications for ethyl acetate and its derivatives.Expand Specific Solutions
Key Industry Players
The ethyl acetate industry is in a mature stage, characterized by established production processes and a stable market. The global market size for ethyl acetate is substantial, driven by its widespread use in various industries such as coatings, adhesives, and pharmaceuticals. Technologically, the production of ethyl acetate is well-developed, with major players like Celanese International Corp., Eastman Chemical Co., and BP Chemicals Ltd. leading the field. These companies have refined their manufacturing processes over decades, achieving high efficiency and product quality. Emerging players such as Viridis Chemical LLC are introducing innovative, bio-based production methods, indicating potential for future growth and sustainability in the industry.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using advanced catalysts and reactive distillation technology. This method combines esterification and distillation in a single unit, improving efficiency and reducing energy consumption. The company's VAntage® ethyl acetate technology achieves yields of over 99% and can produce up to 300,000 metric tons per year [1][3]. Celanese has also implemented a bio-based route using renewable ethanol as a feedstock, aligning with sustainability trends in the chemical industry [2].
Strengths: High yield, energy-efficient process, large-scale production capability, and bio-based options. Weaknesses: Dependence on volatile raw material prices and potential competition from alternative solvents.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a proprietary technology for ethyl acetate production using a two-step process. First, ethanol is dehydrogenated to acetaldehyde using a copper-based catalyst. The acetaldehyde is then reacted with additional ethanol in the presence of an acid catalyst to form ethyl acetate. This process achieves high selectivity and can utilize both petrochemical and bio-based feedstocks [4]. Eastman has also invested in circular economy solutions, exploring ways to recycle ethyl acetate and other solvents to reduce environmental impact [5].
Strengths: Flexible feedstock options, high selectivity process, and commitment to sustainability. Weaknesses: Multi-step process may have higher operational complexity compared to single-step alternatives.
Innovative Applications
Process for the production of esters
PatentWO2012162321A2
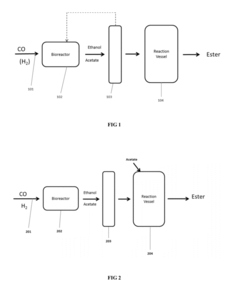
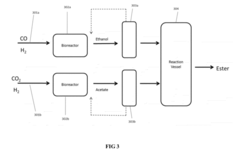
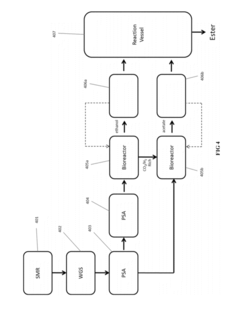
Innovation
- A process involving anaerobic microbial fermentation of CO to produce ethanol and acetic acid, followed by an esterification reaction to form ethyl acetate, which can be optimized through continuous removal of water and use of reactive distillation to enhance conversion rates.
Process for the Production of Esters
PatentActiveUS20120301934A1
Innovation
- Anaerobic microbial fermentation of CO to produce ethanol and acetic acid, followed by an esterification reaction to form ethyl acetate, utilizing a continuous process that includes bioreactors and reactive distillation to optimize product yield.
Environmental Impact
The environmental impact of ethyl acetate production and usage is a critical consideration in assessing its industrial influence. As a widely used solvent and chemical intermediate, ethyl acetate's lifecycle has significant implications for air, water, and soil quality.
In terms of air pollution, the production of ethyl acetate can release volatile organic compounds (VOCs) into the atmosphere. These emissions contribute to the formation of ground-level ozone and photochemical smog, potentially impacting local air quality and human health. However, compared to some other solvents, ethyl acetate has a relatively low ozone depletion potential and global warming potential.
Water pollution is another concern associated with ethyl acetate. Industrial effluents containing this compound can contaminate surface and groundwater if not properly treated. While ethyl acetate is biodegradable and has low persistence in aquatic environments, high concentrations can still pose risks to aquatic ecosystems and drinking water sources.
Soil contamination from ethyl acetate spills or improper disposal is generally less severe due to its high volatility and biodegradability. However, large-scale releases can still impact soil microorganisms and potentially leach into groundwater.
The production process of ethyl acetate also has environmental implications. Traditional methods often involve the use of sulfuric acid as a catalyst, which can lead to the generation of acidic waste streams. More environmentally friendly production routes, such as reactive distillation or the use of solid acid catalysts, are being developed to mitigate these impacts.
In recent years, there has been a growing focus on developing bio-based ethyl acetate production methods. These processes utilize renewable feedstocks and can potentially reduce the overall carbon footprint of ethyl acetate production. However, the environmental benefits of bio-based routes must be carefully evaluated against potential land-use changes and competition with food crops.
Waste management and recycling of ethyl acetate are crucial aspects of minimizing its environmental impact. Many industries have implemented solvent recovery systems to reclaim and reuse ethyl acetate, reducing both waste generation and raw material consumption. Additionally, end-of-life considerations for products containing ethyl acetate, such as adhesives and coatings, are becoming increasingly important in the context of circular economy principles.
In terms of air pollution, the production of ethyl acetate can release volatile organic compounds (VOCs) into the atmosphere. These emissions contribute to the formation of ground-level ozone and photochemical smog, potentially impacting local air quality and human health. However, compared to some other solvents, ethyl acetate has a relatively low ozone depletion potential and global warming potential.
Water pollution is another concern associated with ethyl acetate. Industrial effluents containing this compound can contaminate surface and groundwater if not properly treated. While ethyl acetate is biodegradable and has low persistence in aquatic environments, high concentrations can still pose risks to aquatic ecosystems and drinking water sources.
Soil contamination from ethyl acetate spills or improper disposal is generally less severe due to its high volatility and biodegradability. However, large-scale releases can still impact soil microorganisms and potentially leach into groundwater.
The production process of ethyl acetate also has environmental implications. Traditional methods often involve the use of sulfuric acid as a catalyst, which can lead to the generation of acidic waste streams. More environmentally friendly production routes, such as reactive distillation or the use of solid acid catalysts, are being developed to mitigate these impacts.
In recent years, there has been a growing focus on developing bio-based ethyl acetate production methods. These processes utilize renewable feedstocks and can potentially reduce the overall carbon footprint of ethyl acetate production. However, the environmental benefits of bio-based routes must be carefully evaluated against potential land-use changes and competition with food crops.
Waste management and recycling of ethyl acetate are crucial aspects of minimizing its environmental impact. Many industries have implemented solvent recovery systems to reclaim and reuse ethyl acetate, reducing both waste generation and raw material consumption. Additionally, end-of-life considerations for products containing ethyl acetate, such as adhesives and coatings, are becoming increasingly important in the context of circular economy principles.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in shaping its industrial influence and applications. Globally, the production, use, and handling of ethyl acetate are subject to various regulations and standards set by governmental bodies and international organizations.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, which means it has been assessed for potential risks to human health and the environment. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in the workplace, ensuring worker safety during its handling and use.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register ethyl acetate with the European Chemicals Agency (ECHA) and provide safety data sheets detailing its properties, hazards, and safe handling procedures.
In Asia, countries like China and Japan have their own regulatory frameworks for chemical substances. China's Ministry of Ecology and Environment oversees the registration and management of new chemical substances, including ethyl acetate, through the Measures for Environmental Management of New Chemical Substances. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of chemical substances, including ethyl acetate.
The transportation of ethyl acetate is subject to international regulations such as the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines provide a framework for the safe transport of hazardous materials, including ethyl acetate, across different modes of transportation.
Industry-specific regulations also impact the use of ethyl acetate. In the food and beverage industry, for example, ethyl acetate is regulated as a food additive by agencies such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). These agencies set limits on its use in food products and establish guidelines for its application as a processing aid or flavoring agent.
The pharmaceutical industry faces stringent regulations regarding the use of ethyl acetate in drug manufacturing processes. Regulatory bodies like the FDA and the European Medicines Agency (EMA) provide guidelines on the acceptable levels of residual solvents, including ethyl acetate, in pharmaceutical products.
As environmental concerns grow, regulations are evolving to address the potential impact of ethyl acetate on air quality and ozone depletion. Many countries have implemented volatile organic compound (VOC) regulations that affect the use of ethyl acetate in paints, coatings, and other consumer products.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, which means it has been assessed for potential risks to human health and the environment. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in the workplace, ensuring worker safety during its handling and use.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register ethyl acetate with the European Chemicals Agency (ECHA) and provide safety data sheets detailing its properties, hazards, and safe handling procedures.
In Asia, countries like China and Japan have their own regulatory frameworks for chemical substances. China's Ministry of Ecology and Environment oversees the registration and management of new chemical substances, including ethyl acetate, through the Measures for Environmental Management of New Chemical Substances. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of chemical substances, including ethyl acetate.
The transportation of ethyl acetate is subject to international regulations such as the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines provide a framework for the safe transport of hazardous materials, including ethyl acetate, across different modes of transportation.
Industry-specific regulations also impact the use of ethyl acetate. In the food and beverage industry, for example, ethyl acetate is regulated as a food additive by agencies such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). These agencies set limits on its use in food products and establish guidelines for its application as a processing aid or flavoring agent.
The pharmaceutical industry faces stringent regulations regarding the use of ethyl acetate in drug manufacturing processes. Regulatory bodies like the FDA and the European Medicines Agency (EMA) provide guidelines on the acceptable levels of residual solvents, including ethyl acetate, in pharmaceutical products.
As environmental concerns grow, regulations are evolving to address the potential impact of ethyl acetate on air quality and ozone depletion. Many countries have implemented volatile organic compound (VOC) regulations that affect the use of ethyl acetate in paints, coatings, and other consumer products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!