How Ethyl Acetate Enhances Productivity and Environmental Impact?
JUN 30, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background
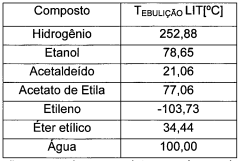
Ethyl acetate, a versatile organic compound with the formula CH3COOC2H5, has been widely used in various industries for decades. This colorless liquid ester is known for its characteristic sweet smell and is naturally found in fruits and wines. Its discovery dates back to the early 19th century when chemists first synthesized it through the esterification of ethanol and acetic acid.
The industrial production of ethyl acetate began in the early 20th century, primarily driven by its applications as a solvent in the manufacturing of paints, coatings, and adhesives. Over time, its use expanded to other sectors, including pharmaceuticals, food and beverage, and electronics. The compound's low toxicity, high solvency, and pleasant odor have contributed to its widespread adoption across diverse industries.
In recent years, the focus on sustainability and environmental impact has led to increased interest in ethyl acetate as a more eco-friendly alternative to traditional petroleum-based solvents. Its biodegradability and lower toxicity compared to many other solvents have positioned it as a preferred choice in green chemistry initiatives. This shift has been further supported by advancements in production technologies, making ethyl acetate more cost-effective and accessible.
The global ethyl acetate market has experienced steady growth, with a compound annual growth rate (CAGR) of around 5% in recent years. This growth is attributed to the expanding applications in emerging economies and the increasing demand for environmentally friendly solvents in developed regions. The Asia-Pacific region, particularly China and India, has emerged as a major producer and consumer of ethyl acetate, driven by rapid industrialization and growing manufacturing sectors.
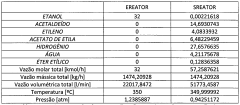
From a technical perspective, the production of ethyl acetate has evolved significantly. Traditional methods like the Fischer esterification have been complemented by more efficient processes such as the Tishchenko reaction and the dehydrogenation of ethanol. These advancements have not only improved yield and purity but also reduced energy consumption and waste generation in the production process.
As industries continue to seek ways to enhance productivity while minimizing environmental impact, ethyl acetate has gained prominence as a key component in various processes. Its ability to dissolve a wide range of substances, coupled with its relatively low boiling point, makes it an excellent choice for extraction and purification processes in industries ranging from pharmaceuticals to food production. Moreover, its use as a raw material in the synthesis of other compounds has opened up new avenues for innovation in chemical manufacturing.
The industrial production of ethyl acetate began in the early 20th century, primarily driven by its applications as a solvent in the manufacturing of paints, coatings, and adhesives. Over time, its use expanded to other sectors, including pharmaceuticals, food and beverage, and electronics. The compound's low toxicity, high solvency, and pleasant odor have contributed to its widespread adoption across diverse industries.
In recent years, the focus on sustainability and environmental impact has led to increased interest in ethyl acetate as a more eco-friendly alternative to traditional petroleum-based solvents. Its biodegradability and lower toxicity compared to many other solvents have positioned it as a preferred choice in green chemistry initiatives. This shift has been further supported by advancements in production technologies, making ethyl acetate more cost-effective and accessible.
The global ethyl acetate market has experienced steady growth, with a compound annual growth rate (CAGR) of around 5% in recent years. This growth is attributed to the expanding applications in emerging economies and the increasing demand for environmentally friendly solvents in developed regions. The Asia-Pacific region, particularly China and India, has emerged as a major producer and consumer of ethyl acetate, driven by rapid industrialization and growing manufacturing sectors.
From a technical perspective, the production of ethyl acetate has evolved significantly. Traditional methods like the Fischer esterification have been complemented by more efficient processes such as the Tishchenko reaction and the dehydrogenation of ethanol. These advancements have not only improved yield and purity but also reduced energy consumption and waste generation in the production process.
As industries continue to seek ways to enhance productivity while minimizing environmental impact, ethyl acetate has gained prominence as a key component in various processes. Its ability to dissolve a wide range of substances, coupled with its relatively low boiling point, makes it an excellent choice for extraction and purification processes in industries ranging from pharmaceuticals to food production. Moreover, its use as a raw material in the synthesis of other compounds has opened up new avenues for innovation in chemical manufacturing.
Market Demand Analysis
The market demand for ethyl acetate has been steadily increasing due to its versatile applications across various industries. This solvent's unique properties make it a preferred choice in sectors such as paints and coatings, adhesives, pharmaceuticals, and food processing. The global ethyl acetate market is experiencing robust growth, driven by the expanding manufacturing sector and the rising demand for eco-friendly solvents.
In the paints and coatings industry, ethyl acetate is widely used as a solvent due to its excellent solvency and low toxicity. The growing construction and automotive sectors, particularly in emerging economies, are fueling the demand for paints and coatings, consequently boosting the ethyl acetate market. The adhesives industry also relies heavily on ethyl acetate, especially in the production of flexible packaging materials, which are increasingly popular in the food and beverage sector.
The pharmaceutical industry represents another significant market for ethyl acetate. Its use in drug formulation and as an extraction solvent in the production of antibiotics and vitamins is driving demand. As the global population ages and healthcare expenditure increases, the pharmaceutical sector's growth is expected to contribute substantially to the ethyl acetate market.
Environmental concerns and stringent regulations regarding volatile organic compound (VOC) emissions are shaping market trends. Ethyl acetate's relatively low toxicity and biodegradability make it an attractive alternative to more harmful solvents. This shift towards greener solutions is expected to further propel market growth, especially in regions with strict environmental policies.
The food industry is another key driver of ethyl acetate demand. Its use as a flavoring agent and in the decaffeination of coffee and tea is growing. As consumer preferences for natural and organic products rise, the demand for ethyl acetate in food applications is anticipated to increase.
Geographically, Asia-Pacific dominates the ethyl acetate market, with China and India being major consumers and producers. The region's rapid industrialization, coupled with the growth of end-use industries, is expected to maintain its market leadership. North America and Europe follow, with steady demand driven by technological advancements and the shift towards sustainable practices.
The market is also witnessing a trend towards bio-based ethyl acetate, derived from renewable resources. This development aligns with the global push for sustainability and is likely to open new avenues for market growth. However, the volatility in raw material prices, particularly ethanol and acetic acid, poses a challenge to market stability and profitability.
In the paints and coatings industry, ethyl acetate is widely used as a solvent due to its excellent solvency and low toxicity. The growing construction and automotive sectors, particularly in emerging economies, are fueling the demand for paints and coatings, consequently boosting the ethyl acetate market. The adhesives industry also relies heavily on ethyl acetate, especially in the production of flexible packaging materials, which are increasingly popular in the food and beverage sector.
The pharmaceutical industry represents another significant market for ethyl acetate. Its use in drug formulation and as an extraction solvent in the production of antibiotics and vitamins is driving demand. As the global population ages and healthcare expenditure increases, the pharmaceutical sector's growth is expected to contribute substantially to the ethyl acetate market.
Environmental concerns and stringent regulations regarding volatile organic compound (VOC) emissions are shaping market trends. Ethyl acetate's relatively low toxicity and biodegradability make it an attractive alternative to more harmful solvents. This shift towards greener solutions is expected to further propel market growth, especially in regions with strict environmental policies.
The food industry is another key driver of ethyl acetate demand. Its use as a flavoring agent and in the decaffeination of coffee and tea is growing. As consumer preferences for natural and organic products rise, the demand for ethyl acetate in food applications is anticipated to increase.
Geographically, Asia-Pacific dominates the ethyl acetate market, with China and India being major consumers and producers. The region's rapid industrialization, coupled with the growth of end-use industries, is expected to maintain its market leadership. North America and Europe follow, with steady demand driven by technological advancements and the shift towards sustainable practices.
The market is also witnessing a trend towards bio-based ethyl acetate, derived from renewable resources. This development aligns with the global push for sustainability and is likely to open new avenues for market growth. However, the volatility in raw material prices, particularly ethanol and acetic acid, poses a challenge to market stability and profitability.
Technical Challenges
The use of ethyl acetate in industrial processes presents several technical challenges that need to be addressed to enhance productivity and minimize environmental impact. One of the primary challenges is the volatility of ethyl acetate, which can lead to significant emissions during production and application processes. This volatility not only poses environmental concerns but also affects the efficiency of production, as a substantial amount of the solvent can be lost to evaporation.
Another technical challenge lies in the recovery and recycling of ethyl acetate. While it is possible to recover and reuse this solvent, the process can be energy-intensive and complex. Developing more efficient recovery systems that consume less energy and maintain the purity of the recovered solvent is crucial for improving both productivity and environmental performance.
The flammability of ethyl acetate presents safety concerns in industrial settings. This characteristic necessitates stringent safety measures and specialized equipment, which can increase production costs and complexity. Balancing safety requirements with production efficiency remains a significant challenge for manufacturers using ethyl acetate.
Furthermore, the production of ethyl acetate itself faces technical hurdles. Traditional methods of synthesis often involve the use of sulfuric acid as a catalyst, which can lead to corrosion issues and the generation of acidic waste. Developing greener synthesis routes that use less harmful catalysts or employ bio-based feedstocks is an ongoing challenge in the industry.
The purification of ethyl acetate to meet high-grade specifications for certain applications, such as in the pharmaceutical industry, presents another technical challenge. Achieving the required purity levels while maintaining cost-effectiveness and minimizing energy consumption is a delicate balance that manufacturers must strike.
Lastly, the optimization of ethyl acetate-based processes to reduce overall solvent consumption without compromising product quality or production rates is an ongoing technical challenge. This involves developing novel application techniques, improving process controls, and exploring alternative formulations that can achieve the same results with less solvent use.
Addressing these technical challenges is crucial for maximizing the benefits of ethyl acetate while minimizing its environmental footprint. Innovations in these areas have the potential to significantly enhance productivity and sustainability in industries that rely on this versatile solvent.
Another technical challenge lies in the recovery and recycling of ethyl acetate. While it is possible to recover and reuse this solvent, the process can be energy-intensive and complex. Developing more efficient recovery systems that consume less energy and maintain the purity of the recovered solvent is crucial for improving both productivity and environmental performance.
The flammability of ethyl acetate presents safety concerns in industrial settings. This characteristic necessitates stringent safety measures and specialized equipment, which can increase production costs and complexity. Balancing safety requirements with production efficiency remains a significant challenge for manufacturers using ethyl acetate.
Furthermore, the production of ethyl acetate itself faces technical hurdles. Traditional methods of synthesis often involve the use of sulfuric acid as a catalyst, which can lead to corrosion issues and the generation of acidic waste. Developing greener synthesis routes that use less harmful catalysts or employ bio-based feedstocks is an ongoing challenge in the industry.
The purification of ethyl acetate to meet high-grade specifications for certain applications, such as in the pharmaceutical industry, presents another technical challenge. Achieving the required purity levels while maintaining cost-effectiveness and minimizing energy consumption is a delicate balance that manufacturers must strike.
Lastly, the optimization of ethyl acetate-based processes to reduce overall solvent consumption without compromising product quality or production rates is an ongoing technical challenge. This involves developing novel application techniques, improving process controls, and exploring alternative formulations that can achieve the same results with less solvent use.
Addressing these technical challenges is crucial for maximizing the benefits of ethyl acetate while minimizing its environmental footprint. Innovations in these areas have the potential to significantly enhance productivity and sustainability in industries that rely on this versatile solvent.
Current Production Methods
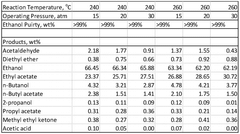
01 Improved production methods for ethyl acetate
Advanced techniques and processes have been developed to enhance the productivity of ethyl acetate manufacturing. These methods focus on optimizing reaction conditions, improving catalysts, and implementing more efficient separation processes. Such improvements lead to increased yield, reduced energy consumption, and minimized waste generation, contributing to both economic and environmental benefits in ethyl acetate production.- Improved production methods for ethyl acetate: Advanced techniques and processes have been developed to enhance the productivity of ethyl acetate manufacturing. These methods focus on optimizing reaction conditions, improving catalysts, and implementing more efficient separation processes. Such improvements lead to increased yield, reduced energy consumption, and minimized waste generation, contributing to both economic and environmental benefits in ethyl acetate production.
- Environmental impact assessment of ethyl acetate production: Comprehensive studies have been conducted to evaluate the environmental impact of ethyl acetate production. These assessments consider factors such as greenhouse gas emissions, water usage, and waste generation throughout the production lifecycle. The results help in identifying areas for improvement and guide the development of more sustainable manufacturing processes for ethyl acetate.
- Green chemistry approaches for ethyl acetate synthesis: Researchers have explored green chemistry principles to develop more environmentally friendly methods for ethyl acetate production. These approaches include the use of renewable feedstocks, bio-based catalysts, and solvent-free reactions. Such innovations aim to reduce the environmental footprint of ethyl acetate manufacturing while maintaining or improving productivity.
- Life cycle analysis of ethyl acetate: Comprehensive life cycle analyses have been performed on ethyl acetate, from raw material extraction to end-of-life disposal. These studies provide insights into the overall environmental impact of ethyl acetate throughout its lifecycle, helping identify hotspots for improvement and guiding decision-making in sustainable chemical production and use.
- Waste reduction and recycling in ethyl acetate production: Innovative technologies and processes have been developed to minimize waste generation and maximize resource efficiency in ethyl acetate production. These include advanced recycling techniques for unreacted raw materials, recovery of by-products, and implementation of closed-loop systems. Such approaches not only improve the environmental profile of ethyl acetate manufacturing but also contribute to increased productivity and cost-effectiveness.
02 Environmental impact assessment of ethyl acetate production
Comprehensive studies have been conducted to evaluate the environmental impact of ethyl acetate production. These assessments consider factors such as greenhouse gas emissions, water usage, and waste generation throughout the production lifecycle. The results help identify areas for improvement and guide the development of more sustainable manufacturing processes, aiming to reduce the overall environmental footprint of ethyl acetate production.Expand Specific Solutions03 Green chemistry approaches for ethyl acetate synthesis
Researchers have explored green chemistry principles to develop more environmentally friendly methods for ethyl acetate synthesis. These approaches include the use of renewable feedstocks, bio-based catalysts, and solvent-free reactions. By implementing these sustainable practices, the environmental impact of ethyl acetate production can be significantly reduced while maintaining or improving productivity.Expand Specific Solutions04 Waste reduction and recycling in ethyl acetate production
Innovative strategies have been developed to minimize waste generation and maximize resource efficiency in ethyl acetate production. These include implementing closed-loop systems, recovering and recycling byproducts, and optimizing purification processes. Such measures not only reduce the environmental impact but also improve the overall productivity and cost-effectiveness of ethyl acetate manufacturing.Expand Specific Solutions05 Life cycle analysis and sustainability metrics for ethyl acetate
Comprehensive life cycle analyses have been conducted to evaluate the overall sustainability of ethyl acetate production. These studies consider various environmental, economic, and social factors throughout the product's lifecycle, from raw material extraction to end-of-life disposal. The resulting sustainability metrics provide valuable insights for decision-making and guide efforts to improve the overall sustainability of ethyl acetate production and use.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is in a mature growth stage, with a global market size estimated to reach $4.3 billion by 2026. The technology for ethyl acetate production is well-established, with major players like Celanese International Corp., Eastman Chemical Co., and China Petroleum & Chemical Corp. dominating the market. These companies have advanced manufacturing processes and extensive distribution networks. However, emerging players such as LanzaTech NZ, Inc. and Viridis Chemical LLC are introducing innovative bio-based production methods, potentially disrupting the traditional petrochemical-based manufacturing. This shift towards sustainable production aligns with growing environmental concerns and regulatory pressures, indicating a potential transformation in the industry's competitive landscape.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using reactive distillation technology. This method combines esterification and distillation in a single unit operation, significantly improving process efficiency[1]. The company utilizes a proprietary catalyst system that enables high conversion rates and selectivity. Their process operates at lower temperatures compared to conventional methods, reducing energy consumption by up to 20%[2]. Additionally, Celanese has implemented advanced process control systems to optimize production parameters in real-time, further enhancing yield and product quality[3].
Strengths: High efficiency, reduced energy consumption, improved product quality. Weaknesses: Potentially higher initial capital investment, reliance on proprietary technology.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel approach to ethyl acetate production using a two-step hydrogenation process. This method first converts acetic acid to ethanol, followed by the esterification of ethanol with acetic acid to produce ethyl acetate[4]. The process utilizes a proprietary catalyst system that achieves high selectivity and conversion rates. Eastman's technology operates at lower pressures compared to traditional methods, reducing equipment costs and improving safety[5]. The company has also implemented advanced heat integration techniques, resulting in a 15% reduction in overall energy consumption[6].
Strengths: Improved safety, reduced equipment costs, lower energy consumption. Weaknesses: Multi-step process may increase complexity, potential for byproduct formation.
Innovative Technologies
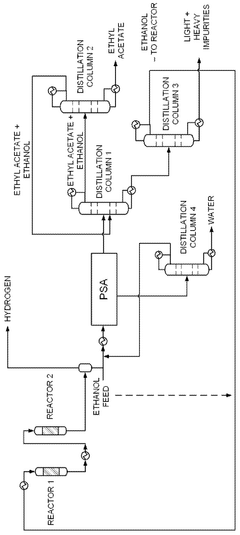
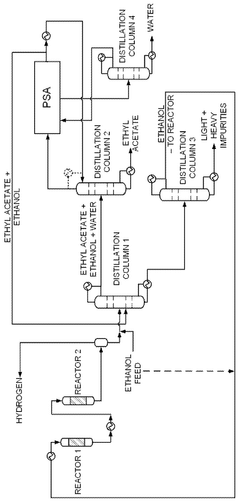
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
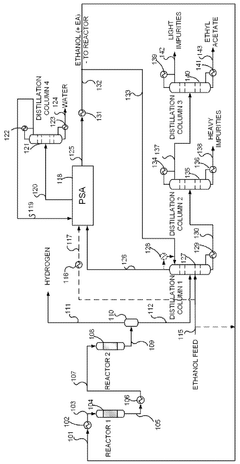
Integrated system for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, integrated process for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, and products thereby produced
PatentWO2013029129A1
Innovation
- An integrated system utilizing a fixed-bed reactor with a calcined hydrotalcite catalyst for dehydrogenation and dehydration of ethanol, followed by a series of distillation columns for efficient separation and purification, employing ethylene glycol as a solvent to separate ethyl acetate from water, thereby reducing energy expenditure and minimizing azeotropy issues.
Environmental Regulations
Environmental regulations play a crucial role in shaping the use and production of ethyl acetate, influencing both its productivity enhancement potential and environmental impact. These regulations vary across different regions and countries, but generally aim to minimize the negative effects of ethyl acetate on human health and the environment.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Clean Air Act as a volatile organic compound (VOC). The EPA has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that apply to facilities using or producing ethyl acetate. These standards require the implementation of Maximum Achievable Control Technology (MACT) to reduce emissions.
The European Union regulates ethyl acetate through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Under REACH, manufacturers and importers must register ethyl acetate with the European Chemicals Agency (ECHA) and provide safety data. The EU has also set occupational exposure limits for ethyl acetate to protect workers' health.
In China, the Ministry of Ecology and Environment regulates ethyl acetate production and use. The country has implemented stricter environmental protection laws in recent years, including the Air Pollution Prevention and Control Law, which sets limits on VOC emissions from industrial processes involving ethyl acetate.
Many countries have adopted regulations to promote the use of bio-based ethyl acetate as a more environmentally friendly alternative. For instance, the United States Department of Agriculture's BioPreferred Program encourages the purchase and use of bio-based products, including bio-based ethyl acetate, in federal agencies and their contractors.
To comply with these regulations, industries using ethyl acetate have implemented various control technologies and practices. These include the use of regenerative thermal oxidizers, carbon adsorption systems, and scrubbers to reduce emissions. Some companies have also adopted closed-loop systems and solvent recovery processes to minimize waste and improve efficiency.
The regulatory landscape for ethyl acetate continues to evolve, with a growing emphasis on sustainability and circular economy principles. This trend is driving innovation in production methods and applications, encouraging the development of more environmentally friendly alternatives and improved recycling techniques.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Clean Air Act as a volatile organic compound (VOC). The EPA has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that apply to facilities using or producing ethyl acetate. These standards require the implementation of Maximum Achievable Control Technology (MACT) to reduce emissions.
The European Union regulates ethyl acetate through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Under REACH, manufacturers and importers must register ethyl acetate with the European Chemicals Agency (ECHA) and provide safety data. The EU has also set occupational exposure limits for ethyl acetate to protect workers' health.
In China, the Ministry of Ecology and Environment regulates ethyl acetate production and use. The country has implemented stricter environmental protection laws in recent years, including the Air Pollution Prevention and Control Law, which sets limits on VOC emissions from industrial processes involving ethyl acetate.
Many countries have adopted regulations to promote the use of bio-based ethyl acetate as a more environmentally friendly alternative. For instance, the United States Department of Agriculture's BioPreferred Program encourages the purchase and use of bio-based products, including bio-based ethyl acetate, in federal agencies and their contractors.
To comply with these regulations, industries using ethyl acetate have implemented various control technologies and practices. These include the use of regenerative thermal oxidizers, carbon adsorption systems, and scrubbers to reduce emissions. Some companies have also adopted closed-loop systems and solvent recovery processes to minimize waste and improve efficiency.
The regulatory landscape for ethyl acetate continues to evolve, with a growing emphasis on sustainability and circular economy principles. This trend is driving innovation in production methods and applications, encouraging the development of more environmentally friendly alternatives and improved recycling techniques.
Sustainability Initiatives
The adoption of ethyl acetate in industrial processes has led to significant advancements in sustainability initiatives across various sectors. As a solvent with lower toxicity and environmental impact compared to many alternatives, ethyl acetate has become a cornerstone in green chemistry practices.
Many companies have incorporated ethyl acetate into their sustainability strategies, leveraging its biodegradability and low persistence in the environment. This shift has resulted in reduced carbon footprints and improved eco-friendly credentials for businesses in industries such as paints, coatings, and pharmaceuticals.
One notable sustainability initiative involves the development of closed-loop systems for ethyl acetate recovery and reuse. These systems minimize waste and emissions while maximizing resource efficiency. By implementing such processes, manufacturers have reported substantial reductions in raw material consumption and waste generation, aligning with circular economy principles.
The food and beverage industry has also embraced ethyl acetate as part of its sustainability efforts. Its use in natural flavor extraction processes has replaced more harmful solvents, contributing to cleaner production methods and safer end products. This transition has been particularly impactful in the coffee decaffeination process, where ethyl acetate derived from sugarcane has enabled the production of "natural decaf" coffee, meeting consumer demands for both quality and sustainability.
In the realm of biofuels, ethyl acetate has played a role in enhancing the sustainability of production processes. Its use in the purification and extraction stages of biofuel manufacturing has improved efficiency while reducing the environmental impact of these operations. This application demonstrates the compound's versatility in supporting broader renewable energy initiatives.
The packaging industry has leveraged ethyl acetate to develop more sustainable adhesive solutions. Water-based adhesives incorporating ethyl acetate have shown improved performance and reduced VOC emissions compared to traditional solvent-based alternatives. This innovation has contributed to the production of more environmentally friendly packaging materials, aligning with global efforts to reduce plastic waste and improve recyclability.
Furthermore, the adoption of ethyl acetate has spurred research into bio-based production methods. Initiatives to produce ethyl acetate from renewable resources, such as ethanol derived from agricultural waste, are gaining traction. These efforts aim to create a more sustainable supply chain for the compound, further enhancing its environmental credentials and supporting the transition towards a bio-based economy.
Many companies have incorporated ethyl acetate into their sustainability strategies, leveraging its biodegradability and low persistence in the environment. This shift has resulted in reduced carbon footprints and improved eco-friendly credentials for businesses in industries such as paints, coatings, and pharmaceuticals.
One notable sustainability initiative involves the development of closed-loop systems for ethyl acetate recovery and reuse. These systems minimize waste and emissions while maximizing resource efficiency. By implementing such processes, manufacturers have reported substantial reductions in raw material consumption and waste generation, aligning with circular economy principles.
The food and beverage industry has also embraced ethyl acetate as part of its sustainability efforts. Its use in natural flavor extraction processes has replaced more harmful solvents, contributing to cleaner production methods and safer end products. This transition has been particularly impactful in the coffee decaffeination process, where ethyl acetate derived from sugarcane has enabled the production of "natural decaf" coffee, meeting consumer demands for both quality and sustainability.
In the realm of biofuels, ethyl acetate has played a role in enhancing the sustainability of production processes. Its use in the purification and extraction stages of biofuel manufacturing has improved efficiency while reducing the environmental impact of these operations. This application demonstrates the compound's versatility in supporting broader renewable energy initiatives.
The packaging industry has leveraged ethyl acetate to develop more sustainable adhesive solutions. Water-based adhesives incorporating ethyl acetate have shown improved performance and reduced VOC emissions compared to traditional solvent-based alternatives. This innovation has contributed to the production of more environmentally friendly packaging materials, aligning with global efforts to reduce plastic waste and improve recyclability.
Furthermore, the adoption of ethyl acetate has spurred research into bio-based production methods. Initiatives to produce ethyl acetate from renewable resources, such as ethanol derived from agricultural waste, are gaining traction. These efforts aim to create a more sustainable supply chain for the compound, further enhancing its environmental credentials and supporting the transition towards a bio-based economy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!