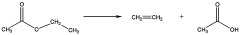
Exploring the Paradigm Shifts with Ethyl Acetate Use
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone significant evolution since its discovery in the early 19th century. Initially synthesized as a laboratory curiosity, it quickly found applications in various industries due to its unique properties. The evolution of ethyl acetate can be traced through several key phases, each marked by technological advancements and expanding applications.
In the early stages, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This process, while effective, was limited in scale and efficiency. The industrial revolution brought about new production methods, including the Tishchenko reaction, which allowed for larger-scale manufacturing. This shift in production capability led to the widespread use of ethyl acetate in the chemical industry.
The mid-20th century saw a paradigm shift in ethyl acetate production with the introduction of catalytic processes. These innovations significantly improved yield and purity, making ethyl acetate more accessible and cost-effective. The development of continuous flow reactors further revolutionized production, allowing for greater control over reaction conditions and product quality.
As environmental concerns grew in the latter part of the 20th century, the evolution of ethyl acetate production took a sustainable turn. Research focused on developing greener synthesis routes, including the use of bio-based feedstocks and enzymatic processes. These advancements not only reduced the environmental impact but also opened up new applications in eco-friendly products.
The digital age brought about another significant shift in ethyl acetate evolution. Advanced process control systems and real-time monitoring technologies enabled manufacturers to optimize production, reduce waste, and improve overall efficiency. This digital transformation also facilitated the development of custom-grade ethyl acetate for specific applications, expanding its use in high-tech industries.
Recent years have seen a focus on circular economy principles in ethyl acetate production. Innovations in recycling and recovery technologies have emerged, allowing for the reuse of ethyl acetate in various processes. This shift towards a more sustainable lifecycle has been driven by both environmental regulations and market demands for greener chemical products.
Looking ahead, the evolution of ethyl acetate is likely to continue along the path of sustainability and technological integration. Emerging trends include the development of bio-based ethyl acetate from renewable sources, advanced purification techniques for ultra-high purity grades, and the exploration of novel applications in fields such as nanotechnology and advanced materials science.
In the early stages, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This process, while effective, was limited in scale and efficiency. The industrial revolution brought about new production methods, including the Tishchenko reaction, which allowed for larger-scale manufacturing. This shift in production capability led to the widespread use of ethyl acetate in the chemical industry.
The mid-20th century saw a paradigm shift in ethyl acetate production with the introduction of catalytic processes. These innovations significantly improved yield and purity, making ethyl acetate more accessible and cost-effective. The development of continuous flow reactors further revolutionized production, allowing for greater control over reaction conditions and product quality.
As environmental concerns grew in the latter part of the 20th century, the evolution of ethyl acetate production took a sustainable turn. Research focused on developing greener synthesis routes, including the use of bio-based feedstocks and enzymatic processes. These advancements not only reduced the environmental impact but also opened up new applications in eco-friendly products.
The digital age brought about another significant shift in ethyl acetate evolution. Advanced process control systems and real-time monitoring technologies enabled manufacturers to optimize production, reduce waste, and improve overall efficiency. This digital transformation also facilitated the development of custom-grade ethyl acetate for specific applications, expanding its use in high-tech industries.
Recent years have seen a focus on circular economy principles in ethyl acetate production. Innovations in recycling and recovery technologies have emerged, allowing for the reuse of ethyl acetate in various processes. This shift towards a more sustainable lifecycle has been driven by both environmental regulations and market demands for greener chemical products.
Looking ahead, the evolution of ethyl acetate is likely to continue along the path of sustainability and technological integration. Emerging trends include the development of bio-based ethyl acetate from renewable sources, advanced purification techniques for ultra-high purity grades, and the exploration of novel applications in fields such as nanotechnology and advanced materials science.
Market Demand Analysis
The market demand for ethyl acetate has been experiencing significant growth and transformation in recent years. This versatile organic compound, widely used as a solvent and in various industrial applications, has seen a surge in demand across multiple sectors. The global ethyl acetate market size was valued at approximately 3.5 billion USD in 2020 and is projected to reach over 5 billion USD by 2027, growing at a CAGR of around 5.5% during the forecast period.
One of the primary drivers of this market growth is the increasing demand from the packaging industry, particularly in flexible packaging applications. Ethyl acetate's excellent solvent properties make it an essential component in the production of flexible packaging materials, which are gaining popularity due to their lightweight nature and reduced environmental impact compared to traditional rigid packaging.
The automotive industry has also emerged as a significant consumer of ethyl acetate, primarily in the production of automotive paints and coatings. As the global automotive sector continues to expand, especially in emerging economies, the demand for ethyl acetate in this segment is expected to rise steadily. Additionally, the growing trend towards eco-friendly and low-VOC (Volatile Organic Compound) coatings has further boosted the use of ethyl acetate as a preferred solvent in automotive applications.
In the pharmaceutical sector, ethyl acetate plays a crucial role in the production of various drugs and active pharmaceutical ingredients (APIs). The ongoing expansion of the pharmaceutical industry, driven by factors such as increasing healthcare expenditure and the growing prevalence of chronic diseases, is expected to contribute significantly to the demand for ethyl acetate in the coming years.
The food and beverage industry represents another key market for ethyl acetate, where it is used as a flavoring agent and in the production of artificial fruit essences. With the rising consumer preference for natural and organic products, there is a growing demand for ethyl acetate derived from sustainable sources, such as bio-based feedstocks.
Geographically, Asia-Pacific has emerged as the largest and fastest-growing market for ethyl acetate, driven by rapid industrialization, urbanization, and the expansion of end-use industries in countries like China and India. North America and Europe also remain significant markets, with steady demand from established industries and increasing focus on sustainable production methods.
However, the market faces challenges such as volatility in raw material prices and environmental concerns associated with the production and use of ethyl acetate. These factors are driving research and development efforts towards more sustainable production methods and bio-based alternatives, which are expected to shape the future landscape of the ethyl acetate market.
One of the primary drivers of this market growth is the increasing demand from the packaging industry, particularly in flexible packaging applications. Ethyl acetate's excellent solvent properties make it an essential component in the production of flexible packaging materials, which are gaining popularity due to their lightweight nature and reduced environmental impact compared to traditional rigid packaging.
The automotive industry has also emerged as a significant consumer of ethyl acetate, primarily in the production of automotive paints and coatings. As the global automotive sector continues to expand, especially in emerging economies, the demand for ethyl acetate in this segment is expected to rise steadily. Additionally, the growing trend towards eco-friendly and low-VOC (Volatile Organic Compound) coatings has further boosted the use of ethyl acetate as a preferred solvent in automotive applications.
In the pharmaceutical sector, ethyl acetate plays a crucial role in the production of various drugs and active pharmaceutical ingredients (APIs). The ongoing expansion of the pharmaceutical industry, driven by factors such as increasing healthcare expenditure and the growing prevalence of chronic diseases, is expected to contribute significantly to the demand for ethyl acetate in the coming years.
The food and beverage industry represents another key market for ethyl acetate, where it is used as a flavoring agent and in the production of artificial fruit essences. With the rising consumer preference for natural and organic products, there is a growing demand for ethyl acetate derived from sustainable sources, such as bio-based feedstocks.
Geographically, Asia-Pacific has emerged as the largest and fastest-growing market for ethyl acetate, driven by rapid industrialization, urbanization, and the expansion of end-use industries in countries like China and India. North America and Europe also remain significant markets, with steady demand from established industries and increasing focus on sustainable production methods.
However, the market faces challenges such as volatility in raw material prices and environmental concerns associated with the production and use of ethyl acetate. These factors are driving research and development efforts towards more sustainable production methods and bio-based alternatives, which are expected to shape the future landscape of the ethyl acetate market.
Technical Challenges
The use of ethyl acetate has faced several technical challenges that have hindered its widespread adoption and optimal utilization. One of the primary obstacles is the compound's high volatility, which leads to significant evaporation losses during storage, handling, and application processes. This characteristic not only results in economic inefficiencies but also raises environmental concerns due to the release of volatile organic compounds (VOCs) into the atmosphere.
Another major challenge lies in the production process of ethyl acetate. Traditional methods often involve energy-intensive reactions and the use of sulfuric acid as a catalyst, which can lead to equipment corrosion and generate substantial amounts of waste. The need for more sustainable and cost-effective production routes has become increasingly apparent, driving research into alternative catalysts and process optimizations.
The purification of ethyl acetate presents additional technical hurdles. The formation of azeotropes with water and other solvents complicates the separation process, necessitating complex distillation techniques or the use of entrainers. These purification challenges can impact the final product quality and increase production costs, limiting the economic viability of ethyl acetate in certain applications.
Furthermore, the flammability of ethyl acetate poses significant safety concerns in industrial settings. Its low flash point requires stringent safety measures and specialized handling equipment, which can be costly to implement and maintain. This aspect has been a deterrent for some industries, particularly those with strict safety regulations or limited resources for infrastructure upgrades.
In recent years, there has been growing interest in developing bio-based routes for ethyl acetate production. However, these methods face their own set of challenges, including feedstock availability, process scalability, and product consistency. The transition from petrochemical-based to bio-based production requires substantial research and development efforts to overcome these hurdles and achieve commercial viability.
The application of ethyl acetate in advanced materials and emerging technologies has also revealed limitations in its performance characteristics. For instance, in the field of electronics, the solvent's relatively high boiling point can lead to residue issues in precision cleaning applications. Similarly, in pharmaceutical formulations, the compound's stability and compatibility with certain active ingredients have been areas of concern, necessitating the development of novel formulation strategies.
Addressing these technical challenges requires a multifaceted approach, combining innovations in chemical engineering, materials science, and process technology. Ongoing research efforts are focused on developing more efficient catalysts, exploring novel reaction pathways, and designing advanced separation techniques to overcome the current limitations of ethyl acetate use and production.
Another major challenge lies in the production process of ethyl acetate. Traditional methods often involve energy-intensive reactions and the use of sulfuric acid as a catalyst, which can lead to equipment corrosion and generate substantial amounts of waste. The need for more sustainable and cost-effective production routes has become increasingly apparent, driving research into alternative catalysts and process optimizations.
The purification of ethyl acetate presents additional technical hurdles. The formation of azeotropes with water and other solvents complicates the separation process, necessitating complex distillation techniques or the use of entrainers. These purification challenges can impact the final product quality and increase production costs, limiting the economic viability of ethyl acetate in certain applications.
Furthermore, the flammability of ethyl acetate poses significant safety concerns in industrial settings. Its low flash point requires stringent safety measures and specialized handling equipment, which can be costly to implement and maintain. This aspect has been a deterrent for some industries, particularly those with strict safety regulations or limited resources for infrastructure upgrades.
In recent years, there has been growing interest in developing bio-based routes for ethyl acetate production. However, these methods face their own set of challenges, including feedstock availability, process scalability, and product consistency. The transition from petrochemical-based to bio-based production requires substantial research and development efforts to overcome these hurdles and achieve commercial viability.
The application of ethyl acetate in advanced materials and emerging technologies has also revealed limitations in its performance characteristics. For instance, in the field of electronics, the solvent's relatively high boiling point can lead to residue issues in precision cleaning applications. Similarly, in pharmaceutical formulations, the compound's stability and compatibility with certain active ingredients have been areas of concern, necessitating the development of novel formulation strategies.
Addressing these technical challenges requires a multifaceted approach, combining innovations in chemical engineering, materials science, and process technology. Ongoing research efforts are focused on developing more efficient catalysts, exploring novel reaction pathways, and designing advanced separation techniques to overcome the current limitations of ethyl acetate use and production.
Current Applications
01 Production and purification of ethyl acetate
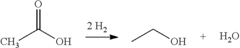
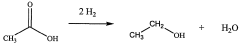
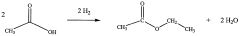
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from its precursors, typically ethanol and acetic acid.- Production and purification of ethyl acetate: Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts or reactants to improve yield and purity.
- Applications of ethyl acetate in industrial processes: Ethyl acetate is utilized in diverse industrial applications, including as a solvent in chemical reactions, as a component in coatings and adhesives, and in the production of various materials such as plastics and textiles.
- Ethyl acetate in pharmaceutical and cosmetic formulations: The use of ethyl acetate in pharmaceutical and cosmetic products is explored, including its role as a solvent for active ingredients, as an excipient in drug formulations, and as a component in personal care products.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices.
- Novel synthesis routes and derivatives of ethyl acetate: Innovative approaches to synthesizing ethyl acetate and creating new derivatives are presented. These include the development of new catalysts, alternative feedstocks, and the exploration of ethyl acetate-based compounds with enhanced properties for specific applications.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. Its use in extraction processes and as a reaction medium is also highlighted in several patents.Expand Specific Solutions03 Ethyl acetate in formulations and compositions
Ethyl acetate is incorporated into various formulations and compositions for different purposes. These include its use in adhesives, coatings, inks, and personal care products. The patents describe specific formulations where ethyl acetate serves as a key ingredient or solvent.Expand Specific Solutions04 Recovery and recycling of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes are described. These include techniques for separating ethyl acetate from mixtures, purifying recovered ethyl acetate, and reusing it in various applications. The focus is on improving efficiency and reducing waste in industrial processes involving ethyl acetate.Expand Specific Solutions05 Ethyl acetate in analytical and testing methods
Ethyl acetate is used in various analytical and testing methods. This includes its application in chromatography, spectroscopy, and other analytical techniques. The patents describe specific methods where ethyl acetate plays a role in sample preparation, analysis, or as a reference compound.Expand Specific Solutions
Industry Leaders
The market for ethyl acetate is in a mature stage, with a global market size estimated to be over $3 billion. The technology for its production and use is well-established, with major players like Celanese International Corp., China Petroleum & Chemical Corp., and Eastman Chemical Co. dominating the industry. These companies have developed advanced processes for ethyl acetate synthesis, focusing on improving efficiency and sustainability. The competitive landscape is characterized by ongoing research and development efforts to enhance production methods and explore new applications. Emerging trends include the development of bio-based ethyl acetate and its increasing use in eco-friendly products, driven by growing environmental concerns and regulatory pressures.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using ethylene and acetic acid as raw materials. This method, known as the Celanese VA-Ethyl Acetate Process, offers significant advantages over traditional esterification routes. The process utilizes a unique catalyst system that allows for high selectivity and yield, with reported ethyl acetate production capacities of up to 300,000 metric tons per year [1]. Celanese's technology also incorporates advanced process control and energy integration, resulting in reduced energy consumption and improved overall efficiency [2]. The company has implemented this technology in several of its global production facilities, demonstrating its scalability and commercial viability [3].
Strengths: High selectivity and yield, large-scale production capability, energy-efficient process. Weaknesses: Reliance on ethylene as a feedstock, which may be subject to price volatility.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant strides in ethyl acetate production through its innovative catalytic distillation technology. This process combines reaction and separation in a single unit, leading to improved conversion rates and reduced energy consumption. Sinopec's approach utilizes a proprietary catalyst that enhances selectivity and reduces side reactions [4]. The company has reported achieving ethyl acetate yields of over 99% with this technology [5]. Additionally, Sinopec has integrated this process with its existing petrochemical infrastructure, allowing for efficient use of feedstocks and optimized supply chain management. The company has also focused on developing bio-based routes for ethyl acetate production, exploring the use of renewable resources as part of its sustainability initiatives [6].
Strengths: High conversion rates, energy efficiency, integration with existing infrastructure. Weaknesses: Potential dependence on fossil fuel-based feedstocks, though mitigated by bio-based research.
Key Innovations
Palladium Catalysts for Making Ethanol from Acetic Acid
PatentInactiveUS20110282110A1
Innovation
- A catalyst comprising palladium and chromium on a support material like silica, titania, or alumina, which is designed for high selectivity and stability, allowing for the direct formation of ethanol from acetic acid through vapor-phase hydrogenation at elevated temperatures.
Processes for making ethanol or ethyl acetate from acetic acid using bimetallic catalysts
PatentWO2011056248A2
Innovation
- The use of bimetallic catalysts with specific molar ratios of metals such as platinum and tin, or rhenium and palladium, in combination with support modifiers, to control the selectivity of ethanol or ethyl acetate production during the hydrogenation of acetic acid, allowing for tailored product ratios through adjustments in catalyst composition and operating conditions.
Environmental Impact
The environmental impact of ethyl acetate use has become a critical consideration in recent years, as industries and policymakers increasingly focus on sustainable practices. Ethyl acetate, a widely used solvent in various industries, presents both challenges and opportunities in terms of its environmental footprint.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. As a VOC, ethyl acetate contributes to the formation of ground-level ozone when released into the atmosphere. This can lead to air quality issues, particularly in urban areas with high industrial activity. However, compared to many other solvents, ethyl acetate has a relatively low ozone formation potential, making it a preferable choice in many applications.
Water pollution is another area of environmental concern. While ethyl acetate is not highly toxic to aquatic life, its release into water systems can still have detrimental effects on ecosystems. Proper handling and disposal practices are crucial to mitigate these risks. Fortunately, ethyl acetate's high biodegradability in both water and soil environments helps to reduce its long-term environmental impact.
From a lifecycle perspective, the production of ethyl acetate has seen significant improvements in recent years. Many manufacturers have adopted more environmentally friendly production methods, such as using bioethanol as a feedstock instead of petrochemical-derived ethanol. This shift not only reduces the carbon footprint of ethyl acetate production but also aligns with circular economy principles by utilizing renewable resources.
The end-of-life considerations for ethyl acetate are generally favorable from an environmental standpoint. Its high volatility means that much of the solvent evaporates during use, reducing the amount that needs to be disposed of as waste. Additionally, advanced recovery and recycling technologies have been developed, allowing for the efficient capture and reuse of ethyl acetate in industrial processes. This not only reduces waste but also minimizes the need for new production, further lowering the overall environmental impact.
In terms of human health and safety, ethyl acetate presents a relatively low risk compared to many other industrial solvents. It has low acute toxicity and is not classified as a carcinogen or mutagen. However, proper ventilation and personal protective equipment are still necessary to prevent potential respiratory irritation and other occupational health issues.
Looking towards the future, ongoing research is focused on further improving the environmental profile of ethyl acetate. This includes exploring bio-based production methods, enhancing recycling technologies, and developing more efficient application techniques to minimize emissions. As industries continue to seek more sustainable alternatives, the role of ethyl acetate in various applications is likely to evolve, with a growing emphasis on its relatively favorable environmental characteristics compared to more harmful solvents.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. As a VOC, ethyl acetate contributes to the formation of ground-level ozone when released into the atmosphere. This can lead to air quality issues, particularly in urban areas with high industrial activity. However, compared to many other solvents, ethyl acetate has a relatively low ozone formation potential, making it a preferable choice in many applications.
Water pollution is another area of environmental concern. While ethyl acetate is not highly toxic to aquatic life, its release into water systems can still have detrimental effects on ecosystems. Proper handling and disposal practices are crucial to mitigate these risks. Fortunately, ethyl acetate's high biodegradability in both water and soil environments helps to reduce its long-term environmental impact.
From a lifecycle perspective, the production of ethyl acetate has seen significant improvements in recent years. Many manufacturers have adopted more environmentally friendly production methods, such as using bioethanol as a feedstock instead of petrochemical-derived ethanol. This shift not only reduces the carbon footprint of ethyl acetate production but also aligns with circular economy principles by utilizing renewable resources.
The end-of-life considerations for ethyl acetate are generally favorable from an environmental standpoint. Its high volatility means that much of the solvent evaporates during use, reducing the amount that needs to be disposed of as waste. Additionally, advanced recovery and recycling technologies have been developed, allowing for the efficient capture and reuse of ethyl acetate in industrial processes. This not only reduces waste but also minimizes the need for new production, further lowering the overall environmental impact.
In terms of human health and safety, ethyl acetate presents a relatively low risk compared to many other industrial solvents. It has low acute toxicity and is not classified as a carcinogen or mutagen. However, proper ventilation and personal protective equipment are still necessary to prevent potential respiratory irritation and other occupational health issues.
Looking towards the future, ongoing research is focused on further improving the environmental profile of ethyl acetate. This includes exploring bio-based production methods, enhancing recycling technologies, and developing more efficient application techniques to minimize emissions. As industries continue to seek more sustainable alternatives, the role of ethyl acetate in various applications is likely to evolve, with a growing emphasis on its relatively favorable environmental characteristics compared to more harmful solvents.
Regulatory Framework
The regulatory framework surrounding ethyl acetate use has undergone significant changes in recent years, reflecting growing concerns about environmental impact and worker safety. These shifts have led to a paradigm change in how industries approach the use and handling of this versatile solvent.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the World Health Organization (WHO) have established guidelines for the safe use and disposal of ethyl acetate. These guidelines have been instrumental in shaping national policies and regulations across the globe.
In the United States, the Environmental Protection Agency (EPA) has tightened regulations on volatile organic compounds (VOCs), including ethyl acetate, under the Clean Air Act. This has resulted in stricter emission controls and reporting requirements for industries using ethyl acetate in their processes. The Occupational Safety and Health Administration (OSHA) has also updated its standards for workplace exposure limits, necessitating improved ventilation systems and personal protective equipment in facilities where ethyl acetate is used.
The European Union has implemented the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to assess and manage the risks associated with ethyl acetate and other chemicals. This has led to more comprehensive safety data sheets and risk assessments for ethyl acetate use across various industries.
In Asia, countries like China and India have also strengthened their regulatory frameworks. China's Ministry of Ecology and Environment has introduced stricter environmental protection laws, impacting the production and use of ethyl acetate. India's Central Pollution Control Board has similarly tightened regulations on industrial solvents, including ethyl acetate, to address air and water pollution concerns.
These regulatory changes have spurred innovation in green chemistry and sustainable manufacturing practices. Industries are now exploring bio-based alternatives to ethyl acetate and developing closed-loop systems to minimize emissions and waste. The push for sustainability has also led to increased research into recycling and recovery methods for ethyl acetate, aligning with circular economy principles.
The evolving regulatory landscape has necessitated significant investments in compliance and technology upgrades across industries. While these changes have posed challenges, they have also opened new opportunities for companies that can adapt quickly and innovate in response to the shifting paradigm of ethyl acetate use.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the World Health Organization (WHO) have established guidelines for the safe use and disposal of ethyl acetate. These guidelines have been instrumental in shaping national policies and regulations across the globe.
In the United States, the Environmental Protection Agency (EPA) has tightened regulations on volatile organic compounds (VOCs), including ethyl acetate, under the Clean Air Act. This has resulted in stricter emission controls and reporting requirements for industries using ethyl acetate in their processes. The Occupational Safety and Health Administration (OSHA) has also updated its standards for workplace exposure limits, necessitating improved ventilation systems and personal protective equipment in facilities where ethyl acetate is used.
The European Union has implemented the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to assess and manage the risks associated with ethyl acetate and other chemicals. This has led to more comprehensive safety data sheets and risk assessments for ethyl acetate use across various industries.
In Asia, countries like China and India have also strengthened their regulatory frameworks. China's Ministry of Ecology and Environment has introduced stricter environmental protection laws, impacting the production and use of ethyl acetate. India's Central Pollution Control Board has similarly tightened regulations on industrial solvents, including ethyl acetate, to address air and water pollution concerns.
These regulatory changes have spurred innovation in green chemistry and sustainable manufacturing practices. Industries are now exploring bio-based alternatives to ethyl acetate and developing closed-loop systems to minimize emissions and waste. The push for sustainability has also led to increased research into recycling and recovery methods for ethyl acetate, aligning with circular economy principles.
The evolving regulatory landscape has necessitated significant investments in compliance and technology upgrades across industries. While these changes have posed challenges, they have also opened new opportunities for companies that can adapt quickly and innovate in response to the shifting paradigm of ethyl acetate use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!