How Ethyl Acetate Leverages for Sustainable Success?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has gained significant attention in recent years due to its potential for sustainable applications. This colorless liquid ester, characterized by its fruity odor, is widely used across various industries, including pharmaceuticals, food and beverages, cosmetics, and manufacturing.
The compound is primarily produced through the esterification of ethanol and acetic acid, a process that can be carried out using both petrochemical and bio-based feedstocks. This dual-source production capability positions ethyl acetate as a bridge between traditional chemical manufacturing and more sustainable, bio-based alternatives.
In the context of sustainable success, ethyl acetate offers several advantages. Its low toxicity and biodegradability make it an environmentally friendly alternative to many conventional solvents. This property aligns well with the growing global emphasis on green chemistry and sustainable industrial practices. Furthermore, the potential for bio-based production routes opens up opportunities for reducing reliance on fossil fuel-derived raw materials.
The versatility of ethyl acetate contributes significantly to its sustainable profile. In the pharmaceutical industry, it serves as a crucial solvent in the production of various drugs and active ingredients. The food and beverage sector utilizes it as a flavoring agent and in the decaffeination of coffee and tea. In cosmetics, it finds application in nail polish removers and perfumes. The manufacturing industry employs it as a solvent for paints, coatings, and adhesives.
Recent technological advancements have focused on improving the efficiency and sustainability of ethyl acetate production. Innovations in catalysis and process intensification have led to more energy-efficient manufacturing methods. Additionally, research into bio-based production pathways, such as the use of agricultural waste as feedstock, is paving the way for a more circular and sustainable supply chain.
The global market for ethyl acetate has been steadily growing, driven by increasing demand across various end-use industries. This growth is further bolstered by the compound's potential in emerging applications, such as in the production of biodegradable plastics and as a green solvent in the electronics industry. The shift towards sustainability in chemical manufacturing has positioned ethyl acetate as a key player in the transition to more environmentally friendly industrial processes.
As industries worldwide strive to reduce their environmental footprint and meet stringent regulatory requirements, ethyl acetate's role in sustainable success is likely to expand. Its unique combination of versatility, relatively low environmental impact, and potential for bio-based production makes it a valuable asset in the pursuit of more sustainable chemical and manufacturing practices.
The compound is primarily produced through the esterification of ethanol and acetic acid, a process that can be carried out using both petrochemical and bio-based feedstocks. This dual-source production capability positions ethyl acetate as a bridge between traditional chemical manufacturing and more sustainable, bio-based alternatives.
In the context of sustainable success, ethyl acetate offers several advantages. Its low toxicity and biodegradability make it an environmentally friendly alternative to many conventional solvents. This property aligns well with the growing global emphasis on green chemistry and sustainable industrial practices. Furthermore, the potential for bio-based production routes opens up opportunities for reducing reliance on fossil fuel-derived raw materials.
The versatility of ethyl acetate contributes significantly to its sustainable profile. In the pharmaceutical industry, it serves as a crucial solvent in the production of various drugs and active ingredients. The food and beverage sector utilizes it as a flavoring agent and in the decaffeination of coffee and tea. In cosmetics, it finds application in nail polish removers and perfumes. The manufacturing industry employs it as a solvent for paints, coatings, and adhesives.
Recent technological advancements have focused on improving the efficiency and sustainability of ethyl acetate production. Innovations in catalysis and process intensification have led to more energy-efficient manufacturing methods. Additionally, research into bio-based production pathways, such as the use of agricultural waste as feedstock, is paving the way for a more circular and sustainable supply chain.
The global market for ethyl acetate has been steadily growing, driven by increasing demand across various end-use industries. This growth is further bolstered by the compound's potential in emerging applications, such as in the production of biodegradable plastics and as a green solvent in the electronics industry. The shift towards sustainability in chemical manufacturing has positioned ethyl acetate as a key player in the transition to more environmentally friendly industrial processes.
As industries worldwide strive to reduce their environmental footprint and meet stringent regulatory requirements, ethyl acetate's role in sustainable success is likely to expand. Its unique combination of versatility, relatively low environmental impact, and potential for bio-based production makes it a valuable asset in the pursuit of more sustainable chemical and manufacturing practices.
Market Demand Analysis
The market demand for ethyl acetate has been steadily growing, driven by its versatile applications across various industries and its potential for sustainable practices. As a key solvent and reagent in the chemical industry, ethyl acetate's market is closely tied to the performance of end-use sectors such as paints and coatings, adhesives, pharmaceuticals, and food and beverages.
In the paints and coatings industry, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. The global paints and coatings market has been expanding, particularly in emerging economies, due to increased construction activities and growing automotive production. This trend directly contributes to the rising demand for ethyl acetate.
The adhesives sector represents another significant market for ethyl acetate. With the growth of packaging industries and the increasing use of adhesives in construction and woodworking, the demand for ethyl acetate-based adhesives continues to rise. The solvent's low toxicity and biodegradability make it an attractive option for manufacturers looking to meet stringent environmental regulations.
In the pharmaceutical industry, ethyl acetate plays a crucial role in the production of various drugs and active pharmaceutical ingredients. The global pharmaceutical market's steady growth, driven by an aging population and increased healthcare spending, indirectly boosts the demand for ethyl acetate.
The food and beverage industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. As consumer preferences shift towards natural and organic products, the demand for ethyl acetate derived from sustainable sources is expected to increase.
From a sustainability perspective, ethyl acetate offers several advantages. It can be produced from renewable resources such as ethanol derived from biomass, aligning with the growing trend towards bio-based chemicals. This aspect is particularly appealing to industries seeking to reduce their carbon footprint and meet sustainability goals.
The market for ethyl acetate is also influenced by regulatory factors. Stringent environmental regulations in many countries are driving the adoption of eco-friendly solvents, positioning ethyl acetate as a preferred alternative to more harmful chemicals. This regulatory push is expected to further stimulate market growth in the coming years.
Geographically, Asia-Pacific remains the largest market for ethyl acetate, driven by rapid industrialization and urbanization in countries like China and India. North America and Europe follow, with steady demand from established industries and a growing focus on sustainable chemical solutions.
In the paints and coatings industry, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. The global paints and coatings market has been expanding, particularly in emerging economies, due to increased construction activities and growing automotive production. This trend directly contributes to the rising demand for ethyl acetate.
The adhesives sector represents another significant market for ethyl acetate. With the growth of packaging industries and the increasing use of adhesives in construction and woodworking, the demand for ethyl acetate-based adhesives continues to rise. The solvent's low toxicity and biodegradability make it an attractive option for manufacturers looking to meet stringent environmental regulations.
In the pharmaceutical industry, ethyl acetate plays a crucial role in the production of various drugs and active pharmaceutical ingredients. The global pharmaceutical market's steady growth, driven by an aging population and increased healthcare spending, indirectly boosts the demand for ethyl acetate.
The food and beverage industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. As consumer preferences shift towards natural and organic products, the demand for ethyl acetate derived from sustainable sources is expected to increase.
From a sustainability perspective, ethyl acetate offers several advantages. It can be produced from renewable resources such as ethanol derived from biomass, aligning with the growing trend towards bio-based chemicals. This aspect is particularly appealing to industries seeking to reduce their carbon footprint and meet sustainability goals.
The market for ethyl acetate is also influenced by regulatory factors. Stringent environmental regulations in many countries are driving the adoption of eco-friendly solvents, positioning ethyl acetate as a preferred alternative to more harmful chemicals. This regulatory push is expected to further stimulate market growth in the coming years.
Geographically, Asia-Pacific remains the largest market for ethyl acetate, driven by rapid industrialization and urbanization in countries like China and India. North America and Europe follow, with steady demand from established industries and a growing focus on sustainable chemical solutions.
Technical Challenges
The utilization of ethyl acetate for sustainable success faces several technical challenges that require innovative solutions. One of the primary obstacles is the energy-intensive production process, which traditionally relies on petrochemical feedstocks. This not only contributes to greenhouse gas emissions but also raises concerns about long-term resource availability and price volatility.
Another significant challenge lies in the purification and recovery of ethyl acetate from reaction mixtures and waste streams. Current separation techniques often involve energy-intensive distillation processes, which can be both costly and environmentally unfriendly. Developing more efficient and sustainable separation methods is crucial for improving the overall sustainability profile of ethyl acetate production and use.
The stability and shelf life of ethyl acetate-based products present additional technical hurdles. As a volatile organic compound, ethyl acetate can evaporate quickly, leading to product degradation and potential environmental concerns. Formulating stable solutions that maintain the desired properties while minimizing evaporation and emissions is an ongoing challenge for manufacturers across various industries.
Furthermore, the biodegradability of ethyl acetate and its derivatives in different environmental conditions remains a concern. While ethyl acetate is generally considered biodegradable, its breakdown products and their potential environmental impacts require further investigation. Developing strategies to enhance biodegradability and ensure complete decomposition into harmless substances is essential for truly sustainable applications.
The scalability of green production methods for ethyl acetate is another technical challenge. While laboratory-scale processes using renewable feedstocks have shown promise, translating these into economically viable industrial-scale operations presents significant engineering and economic challenges. Optimizing reaction conditions, improving catalyst efficiency, and designing robust process equipment are critical areas requiring further research and development.
Lastly, the integration of ethyl acetate into circular economy models poses technical difficulties. Developing efficient recycling and upcycling processes for ethyl acetate-containing products, as well as creating closed-loop systems for its production and use, are complex challenges that require interdisciplinary approaches and innovative technologies.
Addressing these technical challenges is crucial for leveraging ethyl acetate's potential in sustainable applications. Overcoming these hurdles will not only enhance the environmental profile of ethyl acetate but also open up new opportunities for its use in green chemistry and sustainable product development across various industries.
Another significant challenge lies in the purification and recovery of ethyl acetate from reaction mixtures and waste streams. Current separation techniques often involve energy-intensive distillation processes, which can be both costly and environmentally unfriendly. Developing more efficient and sustainable separation methods is crucial for improving the overall sustainability profile of ethyl acetate production and use.
The stability and shelf life of ethyl acetate-based products present additional technical hurdles. As a volatile organic compound, ethyl acetate can evaporate quickly, leading to product degradation and potential environmental concerns. Formulating stable solutions that maintain the desired properties while minimizing evaporation and emissions is an ongoing challenge for manufacturers across various industries.
Furthermore, the biodegradability of ethyl acetate and its derivatives in different environmental conditions remains a concern. While ethyl acetate is generally considered biodegradable, its breakdown products and their potential environmental impacts require further investigation. Developing strategies to enhance biodegradability and ensure complete decomposition into harmless substances is essential for truly sustainable applications.
The scalability of green production methods for ethyl acetate is another technical challenge. While laboratory-scale processes using renewable feedstocks have shown promise, translating these into economically viable industrial-scale operations presents significant engineering and economic challenges. Optimizing reaction conditions, improving catalyst efficiency, and designing robust process equipment are critical areas requiring further research and development.
Lastly, the integration of ethyl acetate into circular economy models poses technical difficulties. Developing efficient recycling and upcycling processes for ethyl acetate-containing products, as well as creating closed-loop systems for its production and use, are complex challenges that require interdisciplinary approaches and innovative technologies.
Addressing these technical challenges is crucial for leveraging ethyl acetate's potential in sustainable applications. Overcoming these hurdles will not only enhance the environmental profile of ethyl acetate but also open up new opportunities for its use in green chemistry and sustainable product development across various industries.
Current Applications
01 Sustainable production methods for ethyl acetate
Innovative approaches to produce ethyl acetate in a more environmentally friendly manner, potentially using renewable resources or optimizing existing processes to reduce energy consumption and waste. These methods aim to improve the sustainability of ethyl acetate production while maintaining or enhancing product quality.- Sustainable production methods for ethyl acetate: Innovative approaches to produce ethyl acetate in a more environmentally friendly manner, focusing on reducing carbon footprint and utilizing renewable resources. These methods may include bio-based feedstocks, green chemistry principles, and energy-efficient processes to enhance sustainability in ethyl acetate manufacturing.
- Applications of sustainable ethyl acetate in various industries: Exploration of new and improved applications for sustainably produced ethyl acetate across different sectors, such as pharmaceuticals, cosmetics, and electronics. This includes developing formulations and processes that leverage the eco-friendly properties of sustainable ethyl acetate to create more environmentally responsible products.
- Recycling and circular economy strategies for ethyl acetate: Implementation of recycling technologies and circular economy principles to minimize waste and maximize the lifecycle of ethyl acetate. This involves developing efficient recovery and purification methods, as well as creating closed-loop systems for ethyl acetate use in industrial processes.
- Optimization of ethyl acetate production efficiency: Advancements in process engineering and technology to improve the efficiency of ethyl acetate production. This includes developing catalysts, reactor designs, and separation techniques that enhance yield, reduce energy consumption, and minimize byproduct formation, contributing to overall sustainability.
- Life cycle assessment and sustainability metrics for ethyl acetate: Development and application of comprehensive life cycle assessment tools and sustainability metrics specific to ethyl acetate production and use. These tools help quantify environmental impacts, identify areas for improvement, and guide decision-making towards more sustainable practices in the ethyl acetate industry.
02 Applications of ethyl acetate in sustainable industries
Exploration of new and existing applications of ethyl acetate in industries focused on sustainability, such as green chemistry, eco-friendly coatings, or biodegradable plastics. This includes research into how ethyl acetate can replace less sustainable solvents in various processes.Expand Specific Solutions03 Recycling and recovery of ethyl acetate
Development of efficient recycling and recovery systems for ethyl acetate to minimize waste and promote circular economy principles. This may involve advanced separation techniques, purification methods, or innovative process designs to reuse ethyl acetate in industrial applications.Expand Specific Solutions04 Ethyl acetate in sustainable packaging solutions
Utilization of ethyl acetate in the development of sustainable packaging materials, potentially as a solvent in the production of bio-based or biodegradable packaging. This includes research into improving the properties of such materials to make them competitive with traditional packaging options.Expand Specific Solutions05 Life cycle assessment and sustainability metrics for ethyl acetate
Implementation of comprehensive life cycle assessments and development of sustainability metrics specific to ethyl acetate production and use. This involves analyzing the environmental impact of ethyl acetate throughout its lifecycle and establishing benchmarks for sustainable production and application.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is in a mature growth stage, with a global market size expected to reach $4.3 billion by 2027. The technology for producing ethyl acetate is well-established, with major players like Celanese, BASF, and Eastman Chemical dominating the traditional petrochemical-based production. However, there is increasing focus on sustainable bio-based ethyl acetate, with companies like LanzaTech and Viridis Chemical developing innovative fermentation and bioconversion processes. This shift towards green chemistry is driving new research collaborations between industry leaders and academic institutions like MIT and Tianjin University to improve production efficiency and explore novel applications in sectors such as coatings, pharmaceuticals, and flexible packaging.
Celanese International Corp.
Technical Solution: Celanese has focused on developing a highly efficient, integrated production process for ethyl acetate. Their approach combines advanced process intensification techniques with innovative reactor designs to maximize yield and minimize energy consumption. The company has implemented a proprietary catalytic system that allows for direct ethanol dehydrogenation to ethyl acetate, bypassing the traditional acetic acid intermediate step[5]. This streamlined process significantly reduces the number of unit operations and associated energy requirements. Celanese has also invested in AI-driven process optimization tools to continuously fine-tune reaction conditions, further improving efficiency and product quality[6]. The company has successfully scaled up this technology and implemented it in several of their global production facilities.
Strengths: Simplified production process, reduced energy consumption, and potential for lower production costs. Weaknesses: Potential challenges in catalyst longevity and regeneration, and higher initial capital investment for new plant designs.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel approach to ethyl acetate production focusing on circular economy principles. Their technology involves the use of advanced molecular recycling to break down post-consumer plastic waste into basic chemical building blocks, which are then used to synthesize ethyl acetate[3]. This process not only addresses the issue of plastic waste but also reduces reliance on fossil-based raw materials. Eastman has also implemented a proprietary catalytic system that enhances reaction rates and selectivity, leading to improved product quality and reduced energy consumption[4]. The company has invested in large-scale facilities to commercialize this technology, aiming to produce circular ethyl acetate at industrial scale.
Strengths: Addresses plastic waste issue, reduces fossil fuel dependence, and aligns with circular economy goals. Weaknesses: Complexity of sorting and processing diverse plastic waste streams, and potential quality variations in the recycled feedstock.
Innovative Formulations
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
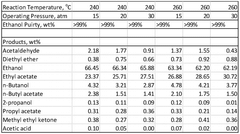
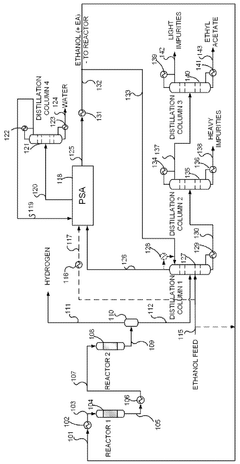
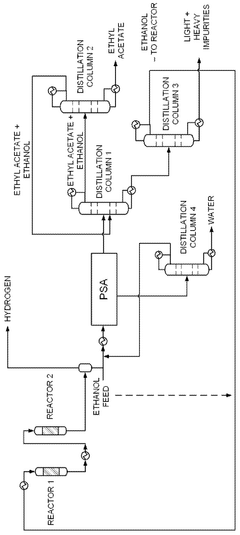
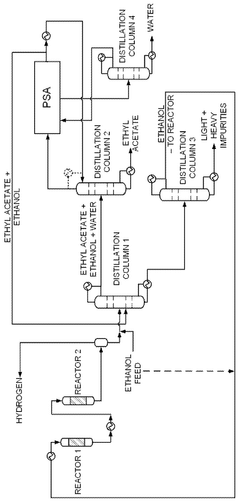
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Environmental Impact
Ethyl acetate, a widely used solvent in various industries, has gained attention for its potential role in sustainable practices. When considering its environmental impact, it is crucial to examine both the positive and negative aspects of its production and use.
The production of ethyl acetate traditionally involves the esterification of ethanol and acetic acid, which can be derived from renewable resources. This process has a lower carbon footprint compared to the production of many other solvents, particularly those derived from petroleum. Furthermore, ethyl acetate is biodegradable, breaking down into harmless components in the environment, which significantly reduces its long-term ecological impact.
However, the environmental benefits of ethyl acetate are not without challenges. The production process, while less harmful than some alternatives, still requires energy and resources. The use of catalysts and the potential for byproducts in the manufacturing process can lead to waste that needs proper management. Additionally, the volatility of ethyl acetate means it can contribute to air pollution if not properly handled during use and storage.
In terms of water pollution, ethyl acetate has a relatively low toxicity to aquatic life compared to many other organic solvents. However, large spills or improper disposal can still have localized impacts on water ecosystems. It is essential to implement proper handling and disposal protocols to minimize these risks.
One of the most significant environmental advantages of ethyl acetate is its potential for recycling and reuse. Many industries have implemented solvent recovery systems that can recapture and purify used ethyl acetate, reducing the need for new production and minimizing waste. This circular approach aligns well with sustainable manufacturing principles and can significantly reduce the overall environmental footprint of processes using this solvent.
The use of ethyl acetate as a replacement for more harmful solvents in various applications also contributes to its positive environmental impact. For instance, in the production of low-VOC (Volatile Organic Compound) paints and coatings, ethyl acetate can replace solvents that have higher ozone depletion potential or greater toxicity.
Looking towards the future, research into green chemistry techniques for ethyl acetate production, such as using enzymatic processes or renewable feedstocks, holds promise for further reducing its environmental impact. These advancements could potentially make ethyl acetate an even more sustainable choice in various industrial applications.
The production of ethyl acetate traditionally involves the esterification of ethanol and acetic acid, which can be derived from renewable resources. This process has a lower carbon footprint compared to the production of many other solvents, particularly those derived from petroleum. Furthermore, ethyl acetate is biodegradable, breaking down into harmless components in the environment, which significantly reduces its long-term ecological impact.
However, the environmental benefits of ethyl acetate are not without challenges. The production process, while less harmful than some alternatives, still requires energy and resources. The use of catalysts and the potential for byproducts in the manufacturing process can lead to waste that needs proper management. Additionally, the volatility of ethyl acetate means it can contribute to air pollution if not properly handled during use and storage.
In terms of water pollution, ethyl acetate has a relatively low toxicity to aquatic life compared to many other organic solvents. However, large spills or improper disposal can still have localized impacts on water ecosystems. It is essential to implement proper handling and disposal protocols to minimize these risks.
One of the most significant environmental advantages of ethyl acetate is its potential for recycling and reuse. Many industries have implemented solvent recovery systems that can recapture and purify used ethyl acetate, reducing the need for new production and minimizing waste. This circular approach aligns well with sustainable manufacturing principles and can significantly reduce the overall environmental footprint of processes using this solvent.
The use of ethyl acetate as a replacement for more harmful solvents in various applications also contributes to its positive environmental impact. For instance, in the production of low-VOC (Volatile Organic Compound) paints and coatings, ethyl acetate can replace solvents that have higher ozone depletion potential or greater toxicity.
Looking towards the future, research into green chemistry techniques for ethyl acetate production, such as using enzymatic processes or renewable feedstocks, holds promise for further reducing its environmental impact. These advancements could potentially make ethyl acetate an even more sustainable choice in various industrial applications.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate plays a crucial role in shaping its sustainable success. As a widely used solvent in various industries, ethyl acetate is subject to a complex web of regulations that govern its production, use, and disposal. These regulations aim to ensure environmental protection, worker safety, and consumer health.
At the international level, organizations such as the United Nations and the World Health Organization provide guidelines for the safe handling and use of ethyl acetate. These guidelines often serve as a foundation for national and regional regulations. The European Union, for instance, has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to assess and manage the risks associated with ethyl acetate and other chemicals.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and importers, as well as guidelines for the safe handling and disposal of ethyl acetate. Additionally, the Occupational Safety and Health Administration (OSHA) sets workplace exposure limits and safety standards for ethyl acetate use in industrial settings.
Many countries have implemented their own regulatory frameworks for ethyl acetate, often aligning with international standards while addressing specific national concerns. These regulations typically cover aspects such as production processes, storage, transportation, labeling, and waste management. Compliance with these regulations is essential for companies operating in the ethyl acetate market, as non-compliance can result in significant penalties and reputational damage.
The regulatory landscape also extends to the end-use applications of ethyl acetate. For example, in the food and beverage industry, the use of ethyl acetate as a flavoring agent or extraction solvent is subject to strict regulations by food safety authorities. Similarly, in the pharmaceutical industry, the use of ethyl acetate in drug manufacturing must comply with Good Manufacturing Practice (GMP) guidelines and other pharmaceutical regulations.
As sustainability becomes an increasingly important focus for both regulators and businesses, new regulations are emerging that specifically address the environmental impact of ethyl acetate production and use. These include requirements for reducing emissions, implementing cleaner production technologies, and exploring bio-based alternatives. Companies that proactively adapt to these evolving regulations are better positioned to achieve long-term sustainable success in the ethyl acetate market.
At the international level, organizations such as the United Nations and the World Health Organization provide guidelines for the safe handling and use of ethyl acetate. These guidelines often serve as a foundation for national and regional regulations. The European Union, for instance, has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to assess and manage the risks associated with ethyl acetate and other chemicals.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and importers, as well as guidelines for the safe handling and disposal of ethyl acetate. Additionally, the Occupational Safety and Health Administration (OSHA) sets workplace exposure limits and safety standards for ethyl acetate use in industrial settings.
Many countries have implemented their own regulatory frameworks for ethyl acetate, often aligning with international standards while addressing specific national concerns. These regulations typically cover aspects such as production processes, storage, transportation, labeling, and waste management. Compliance with these regulations is essential for companies operating in the ethyl acetate market, as non-compliance can result in significant penalties and reputational damage.
The regulatory landscape also extends to the end-use applications of ethyl acetate. For example, in the food and beverage industry, the use of ethyl acetate as a flavoring agent or extraction solvent is subject to strict regulations by food safety authorities. Similarly, in the pharmaceutical industry, the use of ethyl acetate in drug manufacturing must comply with Good Manufacturing Practice (GMP) guidelines and other pharmaceutical regulations.
As sustainability becomes an increasingly important focus for both regulators and businesses, new regulations are emerging that specifically address the environmental impact of ethyl acetate production and use. These include requirements for reducing emissions, implementing cleaner production technologies, and exploring bio-based alternatives. Companies that proactively adapt to these evolving regulations are better positioned to achieve long-term sustainable success in the ethyl acetate market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!