Ethyl Acetate’s Role in Streamlining Industrial Processes
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, plays a crucial role in streamlining various industrial processes. This colorless liquid, characterized by its fruity odor, is widely recognized for its exceptional solvent properties and reactivity, making it an indispensable component in numerous applications across diverse industries.
In the chemical industry, ethyl acetate serves as a key intermediate in the production of numerous compounds, facilitating the synthesis of pharmaceuticals, plastics, and other specialty chemicals. Its ability to dissolve a wide range of organic substances makes it an ideal solvent for many reactions, enhancing efficiency and yield in chemical processes.
The paint and coatings industry heavily relies on ethyl acetate as a low-boiling solvent. Its rapid evaporation rate and excellent solvency for various resins and pigments contribute to the formulation of high-quality paints, lacquers, and varnishes. This property enables faster drying times and improved finish quality, significantly streamlining production processes in this sector.
In the pharmaceutical industry, ethyl acetate plays a vital role in the extraction and purification of active pharmaceutical ingredients (APIs). Its selective solubility and ease of removal make it an excellent choice for liquid-liquid extraction processes, helping to isolate and purify valuable compounds efficiently.
The food and beverage industry utilizes ethyl acetate as a flavoring agent and in the production of artificial fruit essences. Its natural occurrence in many fruits and its low toxicity make it a preferred choice for enhancing flavors in various food products. Additionally, it serves as an extraction solvent in the decaffeination of coffee and tea, offering a more environmentally friendly alternative to traditional methods.
Ethyl acetate's role extends to the electronics industry, where it is employed in the production of printed circuit boards and in cleaning processes. Its ability to dissolve flux residues without damaging sensitive components makes it an invaluable tool in maintaining the quality and reliability of electronic devices.
In the realm of adhesives and sealants, ethyl acetate acts as a crucial solvent and diluent. It enhances the performance of adhesives by improving their spreading properties and accelerating drying times, thereby increasing production efficiency in industries ranging from packaging to construction.
The textile industry benefits from ethyl acetate's use in dyeing processes and as a cleaning agent for machinery. Its effectiveness in dissolving various dyes and its low residue properties contribute to improved color fastness and overall product quality.
As industries continue to seek more efficient and environmentally friendly processes, ethyl acetate's role in streamlining industrial operations is likely to expand further. Its relatively low toxicity, biodegradability, and versatile properties position it as a sustainable choice for many applications, aligning with the growing emphasis on green chemistry and sustainable industrial practices.
In the chemical industry, ethyl acetate serves as a key intermediate in the production of numerous compounds, facilitating the synthesis of pharmaceuticals, plastics, and other specialty chemicals. Its ability to dissolve a wide range of organic substances makes it an ideal solvent for many reactions, enhancing efficiency and yield in chemical processes.
The paint and coatings industry heavily relies on ethyl acetate as a low-boiling solvent. Its rapid evaporation rate and excellent solvency for various resins and pigments contribute to the formulation of high-quality paints, lacquers, and varnishes. This property enables faster drying times and improved finish quality, significantly streamlining production processes in this sector.
In the pharmaceutical industry, ethyl acetate plays a vital role in the extraction and purification of active pharmaceutical ingredients (APIs). Its selective solubility and ease of removal make it an excellent choice for liquid-liquid extraction processes, helping to isolate and purify valuable compounds efficiently.
The food and beverage industry utilizes ethyl acetate as a flavoring agent and in the production of artificial fruit essences. Its natural occurrence in many fruits and its low toxicity make it a preferred choice for enhancing flavors in various food products. Additionally, it serves as an extraction solvent in the decaffeination of coffee and tea, offering a more environmentally friendly alternative to traditional methods.
Ethyl acetate's role extends to the electronics industry, where it is employed in the production of printed circuit boards and in cleaning processes. Its ability to dissolve flux residues without damaging sensitive components makes it an invaluable tool in maintaining the quality and reliability of electronic devices.
In the realm of adhesives and sealants, ethyl acetate acts as a crucial solvent and diluent. It enhances the performance of adhesives by improving their spreading properties and accelerating drying times, thereby increasing production efficiency in industries ranging from packaging to construction.
The textile industry benefits from ethyl acetate's use in dyeing processes and as a cleaning agent for machinery. Its effectiveness in dissolving various dyes and its low residue properties contribute to improved color fastness and overall product quality.
As industries continue to seek more efficient and environmentally friendly processes, ethyl acetate's role in streamlining industrial operations is likely to expand further. Its relatively low toxicity, biodegradability, and versatile properties position it as a sustainable choice for many applications, aligning with the growing emphasis on green chemistry and sustainable industrial practices.
Industrial Applications
Ethyl acetate plays a crucial role in streamlining industrial processes across various sectors. In the chemical industry, it serves as a versatile solvent for a wide range of applications, including the production of paints, coatings, and adhesives. Its low toxicity and high solvency power make it an ideal choice for these processes, enhancing efficiency and reducing environmental impact.
In the pharmaceutical industry, ethyl acetate is extensively used in the extraction and purification of active pharmaceutical ingredients (APIs). Its ability to selectively dissolve certain compounds while leaving others undissolved makes it invaluable in the separation and isolation of drug molecules. This property significantly streamlines the manufacturing process of many medications, reducing production time and costs.
The food and beverage industry also benefits from ethyl acetate's unique properties. It is commonly used as an extraction solvent in the production of decaffeinated coffee and tea. The process involves using ethyl acetate to selectively remove caffeine from coffee beans or tea leaves without affecting the flavor compounds, resulting in a more efficient and cost-effective decaffeination process compared to traditional methods.
In the electronics industry, ethyl acetate plays a crucial role in the production of printed circuit boards (PCBs). It is used as a cleaning agent to remove flux residues after soldering, ensuring the reliability and longevity of electronic components. Its fast evaporation rate and low residue properties make it an excellent choice for this application, improving production speed and product quality.
The fragrance and flavor industry relies heavily on ethyl acetate for the extraction and concentration of natural essences. Its ability to dissolve a wide range of organic compounds makes it an ideal solvent for extracting aromatic compounds from plant materials. This application streamlines the production of perfumes, essential oils, and food flavorings, allowing for more efficient and cost-effective manufacturing processes.
In the textile industry, ethyl acetate is used in the production of synthetic fibers, particularly in the spinning of cellulose acetate fibers. Its role as a solvent in this process helps to dissolve and reshape cellulose, enabling the creation of various textile products with specific properties. This application has significantly improved the efficiency and versatility of textile manufacturing.
The automotive industry also benefits from ethyl acetate's properties in the production of car parts and finishes. It is used in the formulation of automotive paints and coatings, providing excellent solvency for resins and pigments. This results in improved application properties, faster drying times, and enhanced durability of automotive finishes, streamlining the production and assembly processes in car manufacturing.
In the pharmaceutical industry, ethyl acetate is extensively used in the extraction and purification of active pharmaceutical ingredients (APIs). Its ability to selectively dissolve certain compounds while leaving others undissolved makes it invaluable in the separation and isolation of drug molecules. This property significantly streamlines the manufacturing process of many medications, reducing production time and costs.
The food and beverage industry also benefits from ethyl acetate's unique properties. It is commonly used as an extraction solvent in the production of decaffeinated coffee and tea. The process involves using ethyl acetate to selectively remove caffeine from coffee beans or tea leaves without affecting the flavor compounds, resulting in a more efficient and cost-effective decaffeination process compared to traditional methods.
In the electronics industry, ethyl acetate plays a crucial role in the production of printed circuit boards (PCBs). It is used as a cleaning agent to remove flux residues after soldering, ensuring the reliability and longevity of electronic components. Its fast evaporation rate and low residue properties make it an excellent choice for this application, improving production speed and product quality.
The fragrance and flavor industry relies heavily on ethyl acetate for the extraction and concentration of natural essences. Its ability to dissolve a wide range of organic compounds makes it an ideal solvent for extracting aromatic compounds from plant materials. This application streamlines the production of perfumes, essential oils, and food flavorings, allowing for more efficient and cost-effective manufacturing processes.
In the textile industry, ethyl acetate is used in the production of synthetic fibers, particularly in the spinning of cellulose acetate fibers. Its role as a solvent in this process helps to dissolve and reshape cellulose, enabling the creation of various textile products with specific properties. This application has significantly improved the efficiency and versatility of textile manufacturing.
The automotive industry also benefits from ethyl acetate's properties in the production of car parts and finishes. It is used in the formulation of automotive paints and coatings, providing excellent solvency for resins and pigments. This results in improved application properties, faster drying times, and enhanced durability of automotive finishes, streamlining the production and assembly processes in car manufacturing.
Production Challenges
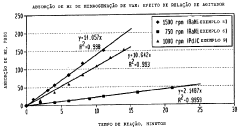
The production of ethyl acetate faces several challenges that impact its efficiency and cost-effectiveness in industrial processes. One of the primary issues is the equilibrium limitation in the esterification reaction between ethanol and acetic acid. This equilibrium constraint results in incomplete conversion, necessitating additional separation and purification steps, which increase production costs and energy consumption.
Another significant challenge is the corrosive nature of the reactants and catalysts used in ethyl acetate production. Acetic acid, in particular, can cause severe corrosion to equipment and pipelines, leading to increased maintenance costs and potential safety hazards. This corrosion issue also limits the choice of materials for reactor construction and storage tanks, often requiring expensive corrosion-resistant alloys.
The presence of water as a byproduct in the esterification reaction poses additional complications. Water forms azeotropes with both ethanol and ethyl acetate, making the separation process more complex and energy-intensive. Conventional distillation techniques are often insufficient to achieve high-purity ethyl acetate, necessitating the use of advanced separation methods such as azeotropic distillation or pervaporation.
Temperature control during the production process is another critical challenge. The esterification reaction is exothermic, and maintaining optimal temperature conditions is crucial for maximizing yield and product quality. Inadequate temperature control can lead to side reactions, reduced conversion rates, and potential safety risks due to runaway reactions.
Catalyst efficiency and longevity also present ongoing challenges in ethyl acetate production. Homogeneous catalysts, such as sulfuric acid, are effective but difficult to separate from the product stream and can cause equipment corrosion. Heterogeneous catalysts offer easier separation but may suffer from deactivation over time, requiring frequent regeneration or replacement.
Environmental concerns and regulatory compliance add another layer of complexity to ethyl acetate production. The volatile organic compound (VOC) emissions associated with the process necessitate the implementation of stringent emission control measures. Additionally, the handling and disposal of waste streams, particularly those containing residual acids or catalysts, require careful management to meet environmental regulations.
Scaling up production while maintaining product quality and process efficiency remains a persistent challenge. As demand for ethyl acetate grows, manufacturers must optimize reactor designs, heat transfer systems, and separation processes to achieve higher throughput without compromising on product purity or increasing operational costs disproportionately.
Another significant challenge is the corrosive nature of the reactants and catalysts used in ethyl acetate production. Acetic acid, in particular, can cause severe corrosion to equipment and pipelines, leading to increased maintenance costs and potential safety hazards. This corrosion issue also limits the choice of materials for reactor construction and storage tanks, often requiring expensive corrosion-resistant alloys.
The presence of water as a byproduct in the esterification reaction poses additional complications. Water forms azeotropes with both ethanol and ethyl acetate, making the separation process more complex and energy-intensive. Conventional distillation techniques are often insufficient to achieve high-purity ethyl acetate, necessitating the use of advanced separation methods such as azeotropic distillation or pervaporation.
Temperature control during the production process is another critical challenge. The esterification reaction is exothermic, and maintaining optimal temperature conditions is crucial for maximizing yield and product quality. Inadequate temperature control can lead to side reactions, reduced conversion rates, and potential safety risks due to runaway reactions.
Catalyst efficiency and longevity also present ongoing challenges in ethyl acetate production. Homogeneous catalysts, such as sulfuric acid, are effective but difficult to separate from the product stream and can cause equipment corrosion. Heterogeneous catalysts offer easier separation but may suffer from deactivation over time, requiring frequent regeneration or replacement.
Environmental concerns and regulatory compliance add another layer of complexity to ethyl acetate production. The volatile organic compound (VOC) emissions associated with the process necessitate the implementation of stringent emission control measures. Additionally, the handling and disposal of waste streams, particularly those containing residual acids or catalysts, require careful management to meet environmental regulations.
Scaling up production while maintaining product quality and process efficiency remains a persistent challenge. As demand for ethyl acetate grows, manufacturers must optimize reactor designs, heat transfer systems, and separation processes to achieve higher throughput without compromising on product purity or increasing operational costs disproportionately.
Current Production Methods
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also utilized in extraction processes, as a reaction medium, and in the production of other chemicals.
- Ethyl acetate in polymer and material science: Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of various polymers, as a solvent for resins, and in the development of composite materials. The compound's properties make it suitable for use in coating formulations and adhesive systems.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on addressing environmental and safety concerns related to ethyl acetate. This includes developing eco-friendly production methods, improving handling and storage practices, and exploring alternatives to reduce environmental impact. Safety measures for transportation and use of ethyl acetate are also considered.
- Novel synthesis routes and catalysts for ethyl acetate: Innovative approaches to synthesizing ethyl acetate are explored, including the development of new catalysts and reaction pathways. These methods aim to improve efficiency, reduce energy consumption, and enhance selectivity in the production of ethyl acetate. Novel catalysts and process conditions are investigated to optimize the synthesis process.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes, such as solvent extraction, as a reaction medium, and in the production of other chemicals. Its versatile properties make it valuable in industries like pharmaceuticals, coatings, and adhesives.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications, including the production of synthetic fibers, plastics, and composite materials. It is used in processes such as dissolution, precipitation, and as a component in formulations.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices.Expand Specific Solutions05 Novel applications and formulations containing ethyl acetate
Innovative uses and formulations incorporating ethyl acetate are being explored, such as in cleaning products, personal care items, and specialized industrial applications. These developments aim to leverage the unique properties of ethyl acetate in new ways.Expand Specific Solutions
Key Manufacturers
The ethyl acetate market is in a mature growth stage, with a global market size expected to reach $4.3 billion by 2026. The technology for its production is well-established, with major players like Celanese International Corp., Eastman Chemical Co., and BP Chemicals Ltd. leading the industry. These companies have developed advanced processes for ethyl acetate synthesis, focusing on improving efficiency and sustainability. The market is characterized by intense competition and ongoing research to optimize production methods. Emerging players such as Nantong Baichuan New Material Co., Ltd. and Jiangsu Baichuan High-Tech New Materials Co., Ltd. are also making strides in the industry, particularly in the Asian market. The technology's maturity is evident in its widespread industrial applications, ranging from coatings to pharmaceuticals.
Celanese International Corp.
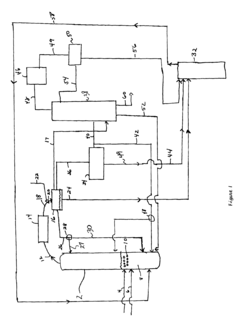
Technical Solution: Celanese has developed an advanced process for ethyl acetate production using reactive distillation technology. This innovative approach combines reaction and separation in a single unit operation, significantly improving process efficiency[1]. The company's method utilizes ethanol and acetic acid as feedstocks, employing a strong acid catalyst to facilitate the esterification reaction. The reactive distillation column is designed with optimized internal structures to enhance mass transfer and reaction kinetics, resulting in higher conversion rates and product purity[3]. Celanese's process also incorporates energy integration techniques, such as heat recovery systems, to minimize energy consumption and reduce operational costs[5].
Strengths: High conversion efficiency, reduced equipment footprint, lower energy consumption. Weaknesses: Potential catalyst deactivation issues, complexity in process control and optimization.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has pioneered a novel ethyl acetate production method using a two-step process involving methanol carbonylation and transesterification. In the first step, methanol is carbonylated to produce methyl acetate using a rhodium-based catalyst system[2]. The second step involves the transesterification of methyl acetate with ethanol to form ethyl acetate. This process offers advantages in terms of raw material flexibility, as it can utilize syngas (CO and H2) derived from various sources, including natural gas or coal[4]. Eastman's technology also incorporates advanced separation techniques, such as pressure-swing distillation, to achieve high-purity ethyl acetate while minimizing energy consumption[6].
Strengths: Versatile feedstock options, high product purity, potential for integration with other chemical processes. Weaknesses: Multi-step process complexity, reliance on precious metal catalysts.
Innovative Synthesis Routes
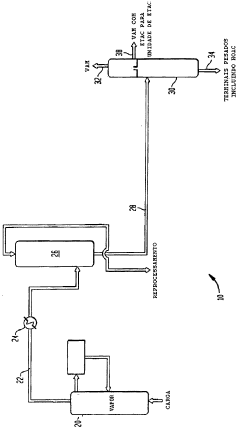
Co-production of vinyl acetate and ethyl acetate
PatentInactiveBRPI0518929A2
Innovation
- A co-production process that involves reacting ethylene, acetic acid, and oxygen to form vinyl acetate and ethyl acetate, followed by distillation to separate and hydrogenate the vinyl acetate in a sidestream to produce ethyl acetate, reducing capital costs and energy consumption by integrating the production processes.
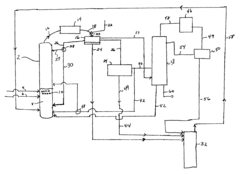
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Environmental Impact
The use of ethyl acetate in industrial processes has significant environmental implications that warrant careful consideration. As a volatile organic compound (VOC), ethyl acetate can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. However, its relatively low toxicity and high biodegradability make it a more environmentally friendly alternative to many other solvents used in industrial applications.
In terms of air quality, ethyl acetate emissions from industrial processes can be effectively controlled through the implementation of proper ventilation systems and the use of activated carbon adsorption technologies. These measures can significantly reduce the release of ethyl acetate vapors into the environment, minimizing its impact on air quality and worker health. Additionally, the development of closed-loop systems and solvent recovery processes has further reduced the environmental footprint of ethyl acetate usage in many industries.
Water pollution is another environmental concern associated with ethyl acetate. While the compound is highly soluble in water, its rapid biodegradation in aquatic environments mitigates long-term ecological impacts. Nevertheless, proper wastewater treatment is essential to prevent the release of high concentrations of ethyl acetate into water bodies. Advanced oxidation processes and biological treatment systems have proven effective in removing ethyl acetate from industrial effluents.
From a lifecycle perspective, the production of ethyl acetate through sustainable methods, such as the use of bio-based feedstocks, can significantly reduce its overall environmental impact. The shift towards green chemistry principles in ethyl acetate synthesis aligns with global efforts to reduce dependence on fossil fuel-derived chemicals and minimize carbon footprints.
In the context of waste management, the recyclability of ethyl acetate presents opportunities for circular economy practices. Distillation and purification techniques allow for the recovery and reuse of ethyl acetate in industrial processes, reducing waste generation and the need for virgin material production. This not only conserves resources but also decreases the environmental burden associated with disposal.
As industries continue to streamline their processes using ethyl acetate, ongoing research and development efforts focus on enhancing its environmental profile. This includes exploring novel production methods with lower energy requirements, developing more efficient recovery systems, and investigating potential substitutes for applications where ethyl acetate's use may pose environmental risks. The continuous improvement of environmental management strategies and technologies associated with ethyl acetate usage is crucial for maintaining its role as a valuable industrial solvent while minimizing ecological impacts.
In terms of air quality, ethyl acetate emissions from industrial processes can be effectively controlled through the implementation of proper ventilation systems and the use of activated carbon adsorption technologies. These measures can significantly reduce the release of ethyl acetate vapors into the environment, minimizing its impact on air quality and worker health. Additionally, the development of closed-loop systems and solvent recovery processes has further reduced the environmental footprint of ethyl acetate usage in many industries.
Water pollution is another environmental concern associated with ethyl acetate. While the compound is highly soluble in water, its rapid biodegradation in aquatic environments mitigates long-term ecological impacts. Nevertheless, proper wastewater treatment is essential to prevent the release of high concentrations of ethyl acetate into water bodies. Advanced oxidation processes and biological treatment systems have proven effective in removing ethyl acetate from industrial effluents.
From a lifecycle perspective, the production of ethyl acetate through sustainable methods, such as the use of bio-based feedstocks, can significantly reduce its overall environmental impact. The shift towards green chemistry principles in ethyl acetate synthesis aligns with global efforts to reduce dependence on fossil fuel-derived chemicals and minimize carbon footprints.
In the context of waste management, the recyclability of ethyl acetate presents opportunities for circular economy practices. Distillation and purification techniques allow for the recovery and reuse of ethyl acetate in industrial processes, reducing waste generation and the need for virgin material production. This not only conserves resources but also decreases the environmental burden associated with disposal.
As industries continue to streamline their processes using ethyl acetate, ongoing research and development efforts focus on enhancing its environmental profile. This includes exploring novel production methods with lower energy requirements, developing more efficient recovery systems, and investigating potential substitutes for applications where ethyl acetate's use may pose environmental risks. The continuous improvement of environmental management strategies and technologies associated with ethyl acetate usage is crucial for maintaining its role as a valuable industrial solvent while minimizing ecological impacts.
Regulatory Compliance
Regulatory compliance plays a crucial role in the industrial use of ethyl acetate, as it ensures the safe and responsible handling of this versatile chemical compound. The production, storage, transportation, and application of ethyl acetate are subject to various regulations and standards set by governmental agencies and industry organizations worldwide.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers handling ethyl acetate in industrial settings. These regulations aim to protect human health and the environment from potential risks associated with the compound's use.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register ethyl acetate and provide safety information. Additionally, the Classification, Labeling, and Packaging (CLP) regulation ensures proper hazard communication for ethyl acetate and other chemicals used in industrial processes.
Many countries have adopted the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which provides a standardized approach to communicating chemical hazards. This system includes specific labeling requirements and safety data sheets for ethyl acetate, facilitating its safe use across international borders.
In the context of streamlining industrial processes, regulatory compliance for ethyl acetate usage often involves implementing robust safety management systems. These systems typically include regular risk assessments, employee training programs, and the development of standard operating procedures for handling and storage. Companies must also maintain detailed records of ethyl acetate usage, disposal, and any incidents or near-misses related to its handling.
Environmental regulations often require industries to monitor and control emissions of volatile organic compounds (VOCs), including ethyl acetate. This has led to the development and implementation of various emission control technologies, such as thermal oxidizers and carbon adsorption systems, which help industries comply with air quality standards while using ethyl acetate in their processes.
As sustainability becomes an increasingly important focus for industries worldwide, regulatory frameworks are evolving to encourage the use of more environmentally friendly alternatives or processes. This trend may impact the future use of ethyl acetate in certain applications, potentially driving innovation in green chemistry and sustainable industrial practices.
Compliance with these regulations not only ensures legal operation but also contributes to the overall efficiency and safety of industrial processes involving ethyl acetate. By adhering to these standards, companies can minimize risks, protect workers and the environment, and maintain their social license to operate in an increasingly environmentally conscious global market.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers handling ethyl acetate in industrial settings. These regulations aim to protect human health and the environment from potential risks associated with the compound's use.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register ethyl acetate and provide safety information. Additionally, the Classification, Labeling, and Packaging (CLP) regulation ensures proper hazard communication for ethyl acetate and other chemicals used in industrial processes.
Many countries have adopted the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which provides a standardized approach to communicating chemical hazards. This system includes specific labeling requirements and safety data sheets for ethyl acetate, facilitating its safe use across international borders.
In the context of streamlining industrial processes, regulatory compliance for ethyl acetate usage often involves implementing robust safety management systems. These systems typically include regular risk assessments, employee training programs, and the development of standard operating procedures for handling and storage. Companies must also maintain detailed records of ethyl acetate usage, disposal, and any incidents or near-misses related to its handling.
Environmental regulations often require industries to monitor and control emissions of volatile organic compounds (VOCs), including ethyl acetate. This has led to the development and implementation of various emission control technologies, such as thermal oxidizers and carbon adsorption systems, which help industries comply with air quality standards while using ethyl acetate in their processes.
As sustainability becomes an increasingly important focus for industries worldwide, regulatory frameworks are evolving to encourage the use of more environmentally friendly alternatives or processes. This trend may impact the future use of ethyl acetate in certain applications, potentially driving innovation in green chemistry and sustainable industrial practices.
Compliance with these regulations not only ensures legal operation but also contributes to the overall efficiency and safety of industrial processes involving ethyl acetate. By adhering to these standards, companies can minimize risks, protect workers and the environment, and maintain their social license to operate in an increasingly environmentally conscious global market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!