Emerging Advances in Ethyl Acetate Utilization
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the molecular formula CH3COOC2H5, has been a staple in various industries for decades. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its widespread use stems from its unique properties, including low toxicity, high solvency, and rapid evaporation rate.
In the chemical industry, ethyl acetate serves as a crucial solvent for a wide range of applications. It is extensively used in the production of paints, coatings, and adhesives, where its ability to dissolve various resins and polymers is highly valued. The pharmaceutical sector relies on ethyl acetate for the extraction and purification of drugs, while the food industry employs it as a flavoring agent and in the decaffeination of coffee and tea.
Recent years have witnessed a surge in the demand for ethyl acetate, driven by the growth of end-use industries and the increasing preference for eco-friendly solvents. The global ethyl acetate market has been experiencing steady growth, with projections indicating continued expansion in the coming years. This growth is particularly pronounced in emerging economies, where rapid industrialization and urbanization are fueling demand across various sectors.
The production of ethyl acetate has evolved significantly over time. Traditional methods, such as the Fischer esterification process, have been complemented by more efficient and sustainable approaches. Modern production techniques focus on improving yield, reducing energy consumption, and minimizing environmental impact. Catalytic processes and continuous flow reactors are among the innovations that have enhanced the efficiency of ethyl acetate synthesis.
As environmental concerns gain prominence, the ethyl acetate industry is adapting to meet sustainability goals. Efforts are underway to develop bio-based production methods, utilizing renewable feedstocks instead of petrochemical sources. This shift aligns with the growing trend towards green chemistry and circular economy principles, positioning ethyl acetate as a more environmentally friendly alternative to some traditional solvents.
The versatility of ethyl acetate continues to drive research into new applications and improved production methods. Emerging areas of interest include its use in advanced materials, such as in the fabrication of nanostructures and in the development of high-performance coatings. Additionally, the potential of ethyl acetate in energy storage applications and as a platform chemical for the synthesis of value-added products is being actively explored.
In the chemical industry, ethyl acetate serves as a crucial solvent for a wide range of applications. It is extensively used in the production of paints, coatings, and adhesives, where its ability to dissolve various resins and polymers is highly valued. The pharmaceutical sector relies on ethyl acetate for the extraction and purification of drugs, while the food industry employs it as a flavoring agent and in the decaffeination of coffee and tea.
Recent years have witnessed a surge in the demand for ethyl acetate, driven by the growth of end-use industries and the increasing preference for eco-friendly solvents. The global ethyl acetate market has been experiencing steady growth, with projections indicating continued expansion in the coming years. This growth is particularly pronounced in emerging economies, where rapid industrialization and urbanization are fueling demand across various sectors.
The production of ethyl acetate has evolved significantly over time. Traditional methods, such as the Fischer esterification process, have been complemented by more efficient and sustainable approaches. Modern production techniques focus on improving yield, reducing energy consumption, and minimizing environmental impact. Catalytic processes and continuous flow reactors are among the innovations that have enhanced the efficiency of ethyl acetate synthesis.
As environmental concerns gain prominence, the ethyl acetate industry is adapting to meet sustainability goals. Efforts are underway to develop bio-based production methods, utilizing renewable feedstocks instead of petrochemical sources. This shift aligns with the growing trend towards green chemistry and circular economy principles, positioning ethyl acetate as a more environmentally friendly alternative to some traditional solvents.
The versatility of ethyl acetate continues to drive research into new applications and improved production methods. Emerging areas of interest include its use in advanced materials, such as in the fabrication of nanostructures and in the development of high-performance coatings. Additionally, the potential of ethyl acetate in energy storage applications and as a platform chemical for the synthesis of value-added products is being actively explored.
Market Demand Analysis
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. The demand for ethyl acetate is primarily fueled by its widespread use as a solvent in paints, coatings, adhesives, and the pharmaceutical industry. The increasing focus on eco-friendly and low-VOC (Volatile Organic Compound) products has further boosted the adoption of ethyl acetate as a preferred solvent in many applications.
In the paints and coatings industry, ethyl acetate is witnessing a surge in demand due to its excellent solvency properties and quick evaporation rate. This sector is expected to remain a significant driver for ethyl acetate consumption, particularly in emerging economies where construction and infrastructure development activities are on the rise.
The adhesives industry is another key consumer of ethyl acetate, with growing applications in packaging, woodworking, and automotive sectors. The shift towards water-based adhesives has somewhat impacted the demand, but ethyl acetate continues to hold a strong position in solvent-based adhesive formulations.
In the pharmaceutical sector, ethyl acetate is increasingly being utilized as a reaction medium and in the production of various drugs. The growing pharmaceutical industry, especially in developing countries, is expected to contribute significantly to the market growth of ethyl acetate.
The food and beverage industry presents a promising avenue for ethyl acetate utilization. Its application as a flavoring agent and in the decaffeination of tea and coffee is gaining traction. The rising consumer preference for natural and organic products is likely to drive further innovation in this area.
Emerging applications of ethyl acetate in the electronics industry, particularly in the production of flexible displays and electronic components, are opening new market opportunities. This trend is expected to contribute to the diversification of ethyl acetate's application portfolio.
Geographically, Asia-Pacific remains the largest consumer and producer of ethyl acetate, with China leading the market. The region's robust industrial growth, particularly in paints, coatings, and adhesives sectors, continues to drive demand. North America and Europe follow, with steady demand from established industries and increasing focus on sustainable chemical solutions.
The market is also witnessing a shift towards bio-based ethyl acetate, driven by sustainability concerns and regulatory pressures. This trend is expected to shape the future market dynamics, potentially creating new opportunities for producers who can offer environmentally friendly alternatives.
In the paints and coatings industry, ethyl acetate is witnessing a surge in demand due to its excellent solvency properties and quick evaporation rate. This sector is expected to remain a significant driver for ethyl acetate consumption, particularly in emerging economies where construction and infrastructure development activities are on the rise.
The adhesives industry is another key consumer of ethyl acetate, with growing applications in packaging, woodworking, and automotive sectors. The shift towards water-based adhesives has somewhat impacted the demand, but ethyl acetate continues to hold a strong position in solvent-based adhesive formulations.
In the pharmaceutical sector, ethyl acetate is increasingly being utilized as a reaction medium and in the production of various drugs. The growing pharmaceutical industry, especially in developing countries, is expected to contribute significantly to the market growth of ethyl acetate.
The food and beverage industry presents a promising avenue for ethyl acetate utilization. Its application as a flavoring agent and in the decaffeination of tea and coffee is gaining traction. The rising consumer preference for natural and organic products is likely to drive further innovation in this area.
Emerging applications of ethyl acetate in the electronics industry, particularly in the production of flexible displays and electronic components, are opening new market opportunities. This trend is expected to contribute to the diversification of ethyl acetate's application portfolio.
Geographically, Asia-Pacific remains the largest consumer and producer of ethyl acetate, with China leading the market. The region's robust industrial growth, particularly in paints, coatings, and adhesives sectors, continues to drive demand. North America and Europe follow, with steady demand from established industries and increasing focus on sustainable chemical solutions.
The market is also witnessing a shift towards bio-based ethyl acetate, driven by sustainability concerns and regulatory pressures. This trend is expected to shape the future market dynamics, potentially creating new opportunities for producers who can offer environmentally friendly alternatives.
Technical Challenges
The utilization of ethyl acetate faces several technical challenges that hinder its widespread adoption and efficiency in various applications. One of the primary obstacles is the optimization of production processes to enhance yield and purity while reducing energy consumption and environmental impact. Current manufacturing methods often involve energy-intensive steps and generate significant waste, necessitating the development of more sustainable and cost-effective production techniques.
Another critical challenge lies in the purification and separation of ethyl acetate from reaction mixtures. Conventional distillation methods are energy-intensive and may not always achieve the desired level of purity for high-end applications. Advanced separation technologies, such as membrane-based processes or reactive distillation, are being explored but require further refinement to become economically viable at industrial scales.
The stability of ethyl acetate under various conditions poses another technical hurdle. Its tendency to hydrolyze in the presence of water or undergo decomposition at elevated temperatures limits its applicability in certain processes. Developing stabilization techniques or modified formulations that enhance the compound's resistance to degradation without compromising its desirable properties is an ongoing area of research.
In the realm of materials science, incorporating ethyl acetate into polymer matrices or composite materials presents challenges related to compatibility and long-term stability. Researchers are working on improving the interfacial interactions between ethyl acetate and various substrates to enhance its performance in coatings, adhesives, and other advanced materials.
The recovery and recycling of ethyl acetate from waste streams and end-of-life products represent another significant technical challenge. Developing efficient and economical methods for capturing and purifying used ethyl acetate is crucial for improving the overall sustainability of its lifecycle. This includes addressing issues such as the removal of contaminants and the restoration of the solvent's original properties.
From an application perspective, expanding the use of ethyl acetate in emerging fields such as 3D printing, advanced electronics, and biomedical applications requires overcoming specific technical barriers. These may include improving its compatibility with novel materials, enhancing its performance under extreme conditions, or modifying its properties to meet specialized requirements in these cutting-edge domains.
Lastly, the development of novel catalysts and reaction systems for the synthesis and transformation of ethyl acetate remains an active area of research. Improving catalytic efficiency, selectivity, and durability could lead to breakthroughs in both production and utilization processes, potentially opening up new avenues for ethyl acetate in chemical synthesis and industrial applications.
Another critical challenge lies in the purification and separation of ethyl acetate from reaction mixtures. Conventional distillation methods are energy-intensive and may not always achieve the desired level of purity for high-end applications. Advanced separation technologies, such as membrane-based processes or reactive distillation, are being explored but require further refinement to become economically viable at industrial scales.
The stability of ethyl acetate under various conditions poses another technical hurdle. Its tendency to hydrolyze in the presence of water or undergo decomposition at elevated temperatures limits its applicability in certain processes. Developing stabilization techniques or modified formulations that enhance the compound's resistance to degradation without compromising its desirable properties is an ongoing area of research.
In the realm of materials science, incorporating ethyl acetate into polymer matrices or composite materials presents challenges related to compatibility and long-term stability. Researchers are working on improving the interfacial interactions between ethyl acetate and various substrates to enhance its performance in coatings, adhesives, and other advanced materials.
The recovery and recycling of ethyl acetate from waste streams and end-of-life products represent another significant technical challenge. Developing efficient and economical methods for capturing and purifying used ethyl acetate is crucial for improving the overall sustainability of its lifecycle. This includes addressing issues such as the removal of contaminants and the restoration of the solvent's original properties.
From an application perspective, expanding the use of ethyl acetate in emerging fields such as 3D printing, advanced electronics, and biomedical applications requires overcoming specific technical barriers. These may include improving its compatibility with novel materials, enhancing its performance under extreme conditions, or modifying its properties to meet specialized requirements in these cutting-edge domains.
Lastly, the development of novel catalysts and reaction systems for the synthesis and transformation of ethyl acetate remains an active area of research. Improving catalytic efficiency, selectivity, and durability could lead to breakthroughs in both production and utilization processes, potentially opening up new avenues for ethyl acetate in chemical synthesis and industrial applications.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and separation methods. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse manufacturing applications.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques in chemical and pharmaceutical industries.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and volatility.
- Novel applications and formulations of ethyl acetate: Innovative uses and formulations of ethyl acetate are being explored in various fields. These include its application in advanced materials, coatings, adhesives, and specialized chemical processes, expanding its utility beyond traditional solvent applications.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in diverse chemical processes, such as solvent extraction, as a reaction medium, and in the production of other chemicals. Its properties make it suitable for various industrial applications, including pharmaceuticals and polymer synthesis.Expand Specific Solutions03 Ethyl acetate in coating and adhesive formulations
Ethyl acetate is employed in the formulation of coatings, adhesives, and related products. Its use as a solvent and component in these applications contributes to improved performance and characteristics of the final products.Expand Specific Solutions04 Recovery and recycling of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes are described. These techniques aim to improve efficiency, reduce waste, and minimize environmental impact in ethyl acetate-based operations.Expand Specific Solutions05 Ethyl acetate in extraction and separation processes
Ethyl acetate is used in various extraction and separation processes, including the isolation of natural products, purification of chemicals, and as a component in chromatographic techniques. Its properties make it an effective solvent for these applications.Expand Specific Solutions
Key Industry Players
The emerging advances in ethyl acetate utilization are shaping a competitive landscape characterized by a maturing industry with significant growth potential. The market is experiencing expansion due to increasing applications across various sectors, including pharmaceuticals, food, and electronics. Technologically, the field is progressing rapidly, with companies like Celanese International Corp., BASF Corp., and DuPont de Nemours, Inc. leading innovation. These industry giants, along with emerging players such as Viridis Chemical LLC, are driving advancements in bio-based ethyl acetate production and sustainable manufacturing processes. The involvement of academic institutions like Nanjing Tech University and South China University of Technology further accelerates research and development, indicating a collaborative ecosystem fostering technological maturity and market growth.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using advanced catalysts and reactive distillation technology. This method combines esterification and distillation in a single unit operation, significantly improving efficiency[1]. The process utilizes ethanol and acetic acid as feedstocks, employing a heterogeneous acid catalyst. The reactive distillation column is designed with optimized internal packing and liquid distribution systems, enhancing mass transfer and reaction rates. This results in higher conversion rates, reduced energy consumption, and improved product purity compared to conventional batch processes[3].
Strengths: Higher efficiency, reduced energy consumption, improved product purity. Weaknesses: Potentially higher initial capital investment, complexity in process control.
BASF Corp.
Technical Solution: BASF has pioneered a green chemistry approach to ethyl acetate production, focusing on sustainability and circular economy principles. Their process utilizes bio-based feedstocks, specifically ethanol derived from renewable sources such as agricultural waste. The company has developed a proprietary catalyst system that enables direct oxidative esterification of ethanol to ethyl acetate, bypassing the need for acetic acid as an intermediate[2]. This one-step process operates at lower temperatures and pressures compared to traditional methods, reducing energy requirements. Additionally, BASF has implemented advanced process control systems and heat integration techniques to further optimize energy efficiency and minimize waste[4].
Strengths: Sustainable feedstock, reduced process steps, lower energy consumption. Weaknesses: Potential limitations in scalability, dependency on bio-ethanol availability.
Innovative Technologies
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
Process for production of ethyl acetate
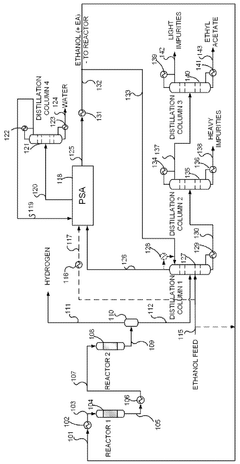
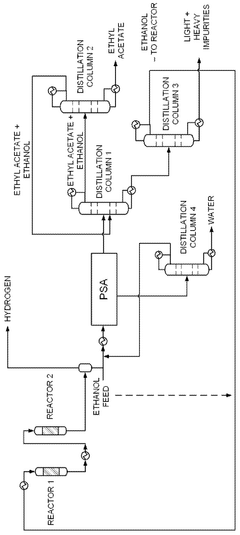
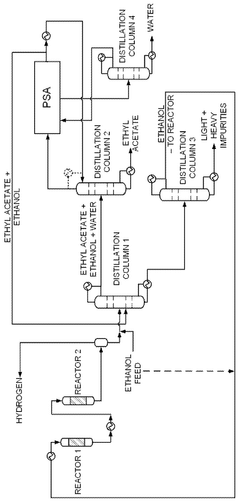
PatentWO2025072073A1
Innovation
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
Environmental Impact
The environmental impact of ethyl acetate utilization is a critical consideration in the emerging advances of this versatile organic compound. As industries seek more sustainable practices, the environmental footprint of ethyl acetate production and use has come under scrutiny. Traditional manufacturing processes for ethyl acetate often rely on petrochemical feedstocks, contributing to carbon emissions and resource depletion. However, recent advancements have focused on developing bio-based production methods, utilizing renewable resources such as agricultural waste and biomass.
These green synthesis routes significantly reduce the carbon footprint associated with ethyl acetate production. Fermentation processes using microorganisms to convert sugars into ethyl acetate have shown promising results, offering a more environmentally friendly alternative to conventional methods. Additionally, the use of waste streams from other industries as raw materials for ethyl acetate production represents a step towards circular economy principles, minimizing overall environmental impact.
In terms of usage, ethyl acetate's role as a solvent in various industries has both positive and negative environmental implications. On one hand, it serves as a less toxic alternative to many other organic solvents, reducing the potential for harmful emissions and worker exposure. Its relatively low boiling point also allows for easier recovery and recycling in industrial processes, minimizing waste generation.
However, the volatile nature of ethyl acetate raises concerns about its contribution to air pollution and the formation of ground-level ozone when released into the atmosphere. To address this, advancements in emission control technologies and improved handling practices have been developed to minimize fugitive emissions during production and use.
The end-of-life management of ethyl acetate-containing products is another area of environmental focus. Innovations in recycling and recovery techniques have improved the ability to reclaim and reuse ethyl acetate from waste streams, reducing the need for virgin production and associated environmental impacts. Furthermore, research into biodegradable alternatives and bio-based derivatives of ethyl acetate is ongoing, aiming to create more environmentally benign options for applications where recovery is not feasible.
As regulations on volatile organic compounds (VOCs) become more stringent globally, the pressure to develop low-emission formulations and application methods for ethyl acetate-based products has intensified. This has led to advancements in encapsulation technologies, controlled release systems, and alternative application techniques that minimize evaporative losses and environmental dispersion.
In conclusion, the environmental impact of ethyl acetate utilization is a complex issue that encompasses its entire lifecycle. While challenges remain, emerging advances in production methods, usage practices, and end-of-life management are steadily improving the environmental profile of this important industrial chemical. The ongoing shift towards bio-based sources and circular economy principles promises to further reduce the environmental footprint of ethyl acetate in the coming years.
These green synthesis routes significantly reduce the carbon footprint associated with ethyl acetate production. Fermentation processes using microorganisms to convert sugars into ethyl acetate have shown promising results, offering a more environmentally friendly alternative to conventional methods. Additionally, the use of waste streams from other industries as raw materials for ethyl acetate production represents a step towards circular economy principles, minimizing overall environmental impact.
In terms of usage, ethyl acetate's role as a solvent in various industries has both positive and negative environmental implications. On one hand, it serves as a less toxic alternative to many other organic solvents, reducing the potential for harmful emissions and worker exposure. Its relatively low boiling point also allows for easier recovery and recycling in industrial processes, minimizing waste generation.
However, the volatile nature of ethyl acetate raises concerns about its contribution to air pollution and the formation of ground-level ozone when released into the atmosphere. To address this, advancements in emission control technologies and improved handling practices have been developed to minimize fugitive emissions during production and use.
The end-of-life management of ethyl acetate-containing products is another area of environmental focus. Innovations in recycling and recovery techniques have improved the ability to reclaim and reuse ethyl acetate from waste streams, reducing the need for virgin production and associated environmental impacts. Furthermore, research into biodegradable alternatives and bio-based derivatives of ethyl acetate is ongoing, aiming to create more environmentally benign options for applications where recovery is not feasible.
As regulations on volatile organic compounds (VOCs) become more stringent globally, the pressure to develop low-emission formulations and application methods for ethyl acetate-based products has intensified. This has led to advancements in encapsulation technologies, controlled release systems, and alternative application techniques that minimize evaporative losses and environmental dispersion.
In conclusion, the environmental impact of ethyl acetate utilization is a complex issue that encompasses its entire lifecycle. While challenges remain, emerging advances in production methods, usage practices, and end-of-life management are steadily improving the environmental profile of this important industrial chemical. The ongoing shift towards bio-based sources and circular economy principles promises to further reduce the environmental footprint of ethyl acetate in the coming years.
Regulatory Framework
The regulatory framework surrounding ethyl acetate utilization is evolving to address emerging advances in its applications. Governments worldwide are implementing stricter regulations to ensure the safe and sustainable use of this versatile compound. Environmental protection agencies are focusing on emissions control and waste management practices related to ethyl acetate production and usage.
In the United States, the Environmental Protection Agency (EPA) has established guidelines for ethyl acetate handling under the Toxic Substances Control Act (TSCA). These regulations cover aspects such as storage, transportation, and disposal of ethyl acetate. The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for workers in industries utilizing ethyl acetate, emphasizing the importance of proper ventilation and personal protective equipment.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to register ethyl acetate and provide safety data. This framework aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances.
In Asia, countries like China and Japan have also strengthened their regulatory oversight. China's Ministry of Ecology and Environment has included ethyl acetate in its list of priority pollutants, mandating stricter emission controls and monitoring requirements. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of ethyl acetate, focusing on risk assessment and management.
As new applications for ethyl acetate emerge, particularly in green chemistry and sustainable manufacturing, regulatory bodies are adapting their frameworks to accommodate these innovations. There is a growing emphasis on promoting the use of bio-based ethyl acetate, derived from renewable resources, as an environmentally friendly alternative to petrochemical-based production.
Regulatory agencies are also addressing the potential health impacts of long-term exposure to ethyl acetate. This includes updating occupational exposure limits and implementing more comprehensive health monitoring programs for workers in industries that heavily utilize this compound.
International cooperation is increasing to harmonize regulations across borders, facilitating global trade while maintaining high safety and environmental standards. Organizations such as the OECD are working to develop consistent guidelines for the assessment and management of chemicals, including ethyl acetate.
As the regulatory landscape continues to evolve, companies involved in ethyl acetate production and utilization must stay informed and adapt their practices to ensure compliance. This dynamic regulatory environment is driving innovation in safer handling techniques, more efficient production methods, and the development of alternative, more sustainable sources of ethyl acetate.
In the United States, the Environmental Protection Agency (EPA) has established guidelines for ethyl acetate handling under the Toxic Substances Control Act (TSCA). These regulations cover aspects such as storage, transportation, and disposal of ethyl acetate. The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for workers in industries utilizing ethyl acetate, emphasizing the importance of proper ventilation and personal protective equipment.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to register ethyl acetate and provide safety data. This framework aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances.
In Asia, countries like China and Japan have also strengthened their regulatory oversight. China's Ministry of Ecology and Environment has included ethyl acetate in its list of priority pollutants, mandating stricter emission controls and monitoring requirements. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of ethyl acetate, focusing on risk assessment and management.
As new applications for ethyl acetate emerge, particularly in green chemistry and sustainable manufacturing, regulatory bodies are adapting their frameworks to accommodate these innovations. There is a growing emphasis on promoting the use of bio-based ethyl acetate, derived from renewable resources, as an environmentally friendly alternative to petrochemical-based production.
Regulatory agencies are also addressing the potential health impacts of long-term exposure to ethyl acetate. This includes updating occupational exposure limits and implementing more comprehensive health monitoring programs for workers in industries that heavily utilize this compound.
International cooperation is increasing to harmonize regulations across borders, facilitating global trade while maintaining high safety and environmental standards. Organizations such as the OECD are working to develop consistent guidelines for the assessment and management of chemicals, including ethyl acetate.
As the regulatory landscape continues to evolve, companies involved in ethyl acetate production and utilization must stay informed and adapt their practices to ensure compliance. This dynamic regulatory environment is driving innovation in safer handling techniques, more efficient production methods, and the development of alternative, more sustainable sources of ethyl acetate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!