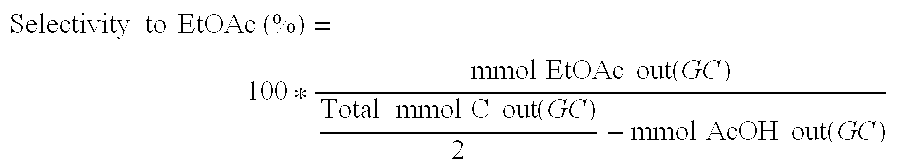The Strategic Evolution of Ethyl Acetate in Technology
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Tech Background and Objectives
Ethyl acetate, a versatile organic compound, has played a significant role in technological advancements across various industries. Its journey from a simple chemical to a key component in cutting-edge applications exemplifies the strategic evolution of materials in technology. The development of ethyl acetate has been driven by the increasing demand for environmentally friendly solvents and the need for more efficient production processes.
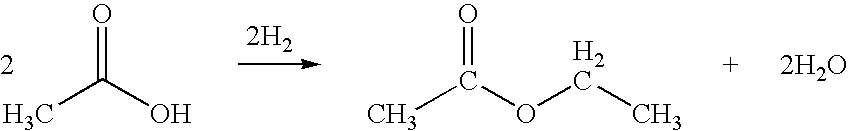
The historical background of ethyl acetate dates back to its first synthesis in the 19th century. Initially used primarily as a solvent in the production of nitrocellulose, its applications have expanded dramatically over time. The evolution of ethyl acetate technology has been marked by continuous improvements in production methods, from traditional esterification processes to more advanced catalytic approaches.
In recent years, the focus has shifted towards developing greener and more sustainable production methods for ethyl acetate. This shift aligns with global efforts to reduce environmental impact and promote circular economy principles. Researchers and industry players are exploring bio-based feedstocks and innovative catalytic systems to enhance the sustainability profile of ethyl acetate production.
The technological objectives for ethyl acetate are multifaceted. One primary goal is to optimize production efficiency, reducing energy consumption and minimizing waste generation. Another critical objective is to expand its application scope, particularly in emerging fields such as nanotechnology and advanced materials science. Additionally, there is a growing emphasis on developing ethyl acetate-based formulations with enhanced performance characteristics for specific industrial applications.
The strategic importance of ethyl acetate in technology is underscored by its role in enabling advancements in diverse sectors. In the electronics industry, high-purity ethyl acetate is crucial for manufacturing processes, including the production of flexible displays and advanced semiconductors. In the pharmaceutical sector, it serves as a key solvent in drug formulation and synthesis, contributing to the development of novel therapeutic agents.
Looking ahead, the strategic evolution of ethyl acetate technology is expected to focus on several key areas. These include the development of bio-based production routes, the exploration of new catalytic systems for improved selectivity and yield, and the integration of ethyl acetate into advanced material composites. Furthermore, there is growing interest in leveraging ethyl acetate's properties for innovative applications in energy storage, smart coatings, and sustainable packaging solutions.
As technology continues to advance, the strategic importance of ethyl acetate is likely to grow. Its versatility, coupled with ongoing research and development efforts, positions it as a critical component in the pursuit of more sustainable and efficient technological solutions across multiple industries.
The historical background of ethyl acetate dates back to its first synthesis in the 19th century. Initially used primarily as a solvent in the production of nitrocellulose, its applications have expanded dramatically over time. The evolution of ethyl acetate technology has been marked by continuous improvements in production methods, from traditional esterification processes to more advanced catalytic approaches.
In recent years, the focus has shifted towards developing greener and more sustainable production methods for ethyl acetate. This shift aligns with global efforts to reduce environmental impact and promote circular economy principles. Researchers and industry players are exploring bio-based feedstocks and innovative catalytic systems to enhance the sustainability profile of ethyl acetate production.
The technological objectives for ethyl acetate are multifaceted. One primary goal is to optimize production efficiency, reducing energy consumption and minimizing waste generation. Another critical objective is to expand its application scope, particularly in emerging fields such as nanotechnology and advanced materials science. Additionally, there is a growing emphasis on developing ethyl acetate-based formulations with enhanced performance characteristics for specific industrial applications.
The strategic importance of ethyl acetate in technology is underscored by its role in enabling advancements in diverse sectors. In the electronics industry, high-purity ethyl acetate is crucial for manufacturing processes, including the production of flexible displays and advanced semiconductors. In the pharmaceutical sector, it serves as a key solvent in drug formulation and synthesis, contributing to the development of novel therapeutic agents.
Looking ahead, the strategic evolution of ethyl acetate technology is expected to focus on several key areas. These include the development of bio-based production routes, the exploration of new catalytic systems for improved selectivity and yield, and the integration of ethyl acetate into advanced material composites. Furthermore, there is growing interest in leveraging ethyl acetate's properties for innovative applications in energy storage, smart coatings, and sustainable packaging solutions.
As technology continues to advance, the strategic importance of ethyl acetate is likely to grow. Its versatility, coupled with ongoing research and development efforts, positions it as a critical component in the pursuit of more sustainable and efficient technological solutions across multiple industries.
Market Demand Analysis for Ethyl Acetate
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. The demand for ethyl acetate is primarily fueled by its extensive use as a solvent in paints, coatings, and adhesives, which collectively account for a significant portion of its consumption. The packaging industry, particularly flexible packaging, has emerged as a key growth driver for ethyl acetate, with increasing demand for eco-friendly and high-performance packaging solutions.
In the pharmaceutical sector, ethyl acetate plays a crucial role as a solvent in drug formulation and extraction processes. The growing pharmaceutical industry, especially in emerging economies, is expected to contribute substantially to the market's expansion. Additionally, the food and beverage industry utilizes ethyl acetate as a flavoring agent and in the production of artificial fruit essences, further bolstering its market demand.
The automotive industry's recovery and the increasing adoption of water-based coatings have created new opportunities for ethyl acetate in automotive paints and coatings. Moreover, the shift towards sustainable and bio-based chemicals has led to the development of bio-ethyl acetate, derived from renewable resources, which is gaining traction among environmentally conscious consumers and industries.
Geographically, Asia-Pacific dominates the ethyl acetate market, with China and India being the major consumers and producers. The region's rapid industrialization, growing population, and increasing disposable income are driving the demand for products that utilize ethyl acetate. North America and Europe follow, with steady demand from established industries and a focus on sustainable alternatives.
The market is also influenced by fluctuations in raw material prices, particularly ethanol and acetic acid. These price variations can impact the overall production costs and, consequently, the market dynamics of ethyl acetate. Despite this challenge, the market is expected to maintain its growth trajectory, supported by technological advancements in production processes and the development of novel applications.
As industries continue to seek cost-effective and environmentally friendly solvents, ethyl acetate is well-positioned to meet these requirements. Its low toxicity, high solvency, and favorable environmental profile make it an attractive option across various applications. The market is projected to witness further expansion, driven by innovations in product formulations and the exploration of new end-use industries.
In the pharmaceutical sector, ethyl acetate plays a crucial role as a solvent in drug formulation and extraction processes. The growing pharmaceutical industry, especially in emerging economies, is expected to contribute substantially to the market's expansion. Additionally, the food and beverage industry utilizes ethyl acetate as a flavoring agent and in the production of artificial fruit essences, further bolstering its market demand.
The automotive industry's recovery and the increasing adoption of water-based coatings have created new opportunities for ethyl acetate in automotive paints and coatings. Moreover, the shift towards sustainable and bio-based chemicals has led to the development of bio-ethyl acetate, derived from renewable resources, which is gaining traction among environmentally conscious consumers and industries.
Geographically, Asia-Pacific dominates the ethyl acetate market, with China and India being the major consumers and producers. The region's rapid industrialization, growing population, and increasing disposable income are driving the demand for products that utilize ethyl acetate. North America and Europe follow, with steady demand from established industries and a focus on sustainable alternatives.
The market is also influenced by fluctuations in raw material prices, particularly ethanol and acetic acid. These price variations can impact the overall production costs and, consequently, the market dynamics of ethyl acetate. Despite this challenge, the market is expected to maintain its growth trajectory, supported by technological advancements in production processes and the development of novel applications.
As industries continue to seek cost-effective and environmentally friendly solvents, ethyl acetate is well-positioned to meet these requirements. Its low toxicity, high solvency, and favorable environmental profile make it an attractive option across various applications. The market is projected to witness further expansion, driven by innovations in product formulations and the exploration of new end-use industries.
Current Status and Challenges in Ethyl Acetate Production
The current status of ethyl acetate production is characterized by a mature industrial process, primarily based on the esterification of ethanol with acetic acid. This method, while well-established, faces challenges in terms of sustainability and efficiency. Global production capacity has seen steady growth, with Asia-Pacific emerging as a dominant region due to increasing demand in various industries.
One of the main challenges in ethyl acetate production is the reliance on petrochemical feedstocks. The traditional process uses ethanol derived from fossil fuels, raising concerns about long-term sustainability and environmental impact. This has led to a growing interest in developing bio-based production methods, utilizing renewable resources such as biomass-derived ethanol.
Energy efficiency remains a significant challenge in the current production processes. The esterification reaction requires high temperatures and pressures, resulting in substantial energy consumption. Improving energy efficiency without compromising product quality or yield is a key focus area for manufacturers and researchers alike.
Another pressing issue is the volatility of raw material prices, particularly ethanol and acetic acid. These fluctuations can significantly impact production costs and profit margins, making it difficult for manufacturers to maintain consistent pricing and supply. This challenge is further compounded by the global nature of the ethyl acetate market, where regional price disparities can affect competitiveness.
Water management in ethyl acetate production presents both environmental and economic challenges. The process generates wastewater that requires treatment before disposal, adding to production costs and environmental concerns. Developing more efficient water recycling systems and reducing overall water consumption are important goals for the industry.
The purity of ethyl acetate produced is another critical factor. Many high-tech applications, such as in the electronics industry, require ultra-pure ethyl acetate. Meeting these stringent purity requirements while maintaining cost-effectiveness is an ongoing challenge for producers.
Regulatory compliance is becoming increasingly complex, with stricter environmental and safety regulations being implemented globally. Manufacturers must adapt their processes to meet these evolving standards, which often requires significant investment in new technologies and equipment.
Lastly, the industry faces challenges in scaling up new, more sustainable production methods. While promising alternatives like enzymatic processes and bio-based feedstocks have been developed in laboratory settings, translating these innovations into large-scale, economically viable production remains a significant hurdle. Overcoming these scaling challenges is crucial for the future evolution of ethyl acetate production technology.
One of the main challenges in ethyl acetate production is the reliance on petrochemical feedstocks. The traditional process uses ethanol derived from fossil fuels, raising concerns about long-term sustainability and environmental impact. This has led to a growing interest in developing bio-based production methods, utilizing renewable resources such as biomass-derived ethanol.
Energy efficiency remains a significant challenge in the current production processes. The esterification reaction requires high temperatures and pressures, resulting in substantial energy consumption. Improving energy efficiency without compromising product quality or yield is a key focus area for manufacturers and researchers alike.
Another pressing issue is the volatility of raw material prices, particularly ethanol and acetic acid. These fluctuations can significantly impact production costs and profit margins, making it difficult for manufacturers to maintain consistent pricing and supply. This challenge is further compounded by the global nature of the ethyl acetate market, where regional price disparities can affect competitiveness.
Water management in ethyl acetate production presents both environmental and economic challenges. The process generates wastewater that requires treatment before disposal, adding to production costs and environmental concerns. Developing more efficient water recycling systems and reducing overall water consumption are important goals for the industry.
The purity of ethyl acetate produced is another critical factor. Many high-tech applications, such as in the electronics industry, require ultra-pure ethyl acetate. Meeting these stringent purity requirements while maintaining cost-effectiveness is an ongoing challenge for producers.
Regulatory compliance is becoming increasingly complex, with stricter environmental and safety regulations being implemented globally. Manufacturers must adapt their processes to meet these evolving standards, which often requires significant investment in new technologies and equipment.
Lastly, the industry faces challenges in scaling up new, more sustainable production methods. While promising alternatives like enzymatic processes and bio-based feedstocks have been developed in laboratory settings, translating these innovations into large-scale, economically viable production remains a significant hurdle. Overcoming these scaling challenges is crucial for the future evolution of ethyl acetate production technology.
Current Technical Solutions for Ethyl Acetate Synthesis
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, chromatography, and other separation techniques. These processes are used in the purification of natural products, pharmaceuticals, and other chemicals.
- Environmental and safety considerations for ethyl acetate: The use and handling of ethyl acetate involve environmental and safety considerations. This includes methods for reducing emissions, improving workplace safety, and developing more sustainable production processes. Efforts are made to minimize the environmental impact of ethyl acetate production and use.
- Novel derivatives and modifications of ethyl acetate: Research into novel derivatives and modifications of ethyl acetate aims to enhance its properties or create new compounds with improved characteristics. This includes the development of new reaction pathways, functionalization of ethyl acetate, and the creation of ethyl acetate-based materials with specific properties.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. Its versatility makes it a valuable component in many industrial processes.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes due to its solvent properties. It is used to extract various compounds from mixtures and can be utilized in liquid-liquid extraction techniques. These applications are relevant in industries such as food processing and pharmaceutical manufacturing.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, enhancing recycling processes, and implementing safety measures for handling and storage of ethyl acetate in industrial settings.Expand Specific Solutions05 Novel applications and formulations of ethyl acetate
Innovative applications and formulations of ethyl acetate are being explored in various fields. These include its use in advanced materials, specialty coatings, and as a component in novel chemical processes. Research is ongoing to expand the utility of ethyl acetate beyond its traditional applications.Expand Specific Solutions
Key Players in Ethyl Acetate Industry
The strategic evolution of ethyl acetate in technology is characterized by a mature market with steady growth, driven by diverse industrial applications. The global market size is estimated to be in the billions of dollars, with a compound annual growth rate of around 5-6%. The technology has reached a high level of maturity, with established production processes and widespread adoption across industries. Key players like Celanese, China Petroleum & Chemical Corp., and Eastman Chemical Co. dominate the market, leveraging their extensive R&D capabilities and global production networks. Emerging trends include the development of bio-based ethyl acetate, with companies like Viridis Chemical LLC and LanzaTech NZ, Inc. focusing on sustainable production methods. Academic institutions such as Nanjing Tech University and the University of Florida are contributing to advancements in catalysis and process optimization, further enhancing the efficiency and sustainability of ethyl acetate production.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using advanced catalysts and reactive distillation technology. This method allows for the direct esterification of ethanol and acetic acid in a single, integrated unit operation. The process achieves high conversion rates and selectivity, resulting in a more energy-efficient and cost-effective production route compared to traditional methods[1][3]. Additionally, Celanese has implemented a proprietary purification system that reduces the number of distillation columns required, further optimizing the production process[2].
Strengths: High efficiency, reduced energy consumption, and lower production costs. Weaknesses: Potential dependency on specific catalyst formulations and higher initial capital investment for specialized equipment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to ethyl acetate production using a coupled process of methanol carbonylation and hydrogenation. This method utilizes syngas (CO + H2) as a raw material, which is first converted to acetic acid through methanol carbonylation. The acetic acid is then hydrogenated to ethanol, which reacts with the remaining acetic acid to form ethyl acetate[4]. Sinopec has also implemented advanced process control systems and heat integration techniques to optimize energy efficiency and product yield[5]. The company has successfully scaled up this technology in several of its petrochemical complexes across China[6].
Strengths: Utilization of readily available syngas, integration with existing petrochemical processes, and potential for cost reduction. Weaknesses: Complex process control requirements and potential sensitivity to syngas composition fluctuations.
Key Innovations in Ethyl Acetate Production
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentInactiveUS20100029980A1
Innovation
- A process utilizing a hydrogenating catalyst composed of metals like nickel, platinum, or palladium in combination with molybdenum, rhenium, zirconium, copper, or cobalt supported on catalysts such as silica or zeolites, which selectively converts acetic acid to ethyl acetate with high yield and selectivity.
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
Environmental Impact of Ethyl Acetate Production
The production and use of ethyl acetate have significant environmental implications that warrant careful consideration. The manufacturing process of ethyl acetate traditionally involves the esterification of ethanol with acetic acid, which can generate substantial amounts of waste and consume significant energy resources. This conventional method often relies on fossil fuel-based feedstocks, contributing to greenhouse gas emissions and climate change concerns.
However, recent technological advancements have led to more sustainable production methods. Bio-based ethyl acetate, derived from renewable resources such as corn or sugarcane, has emerged as a promising alternative. This approach reduces reliance on petrochemical feedstocks and potentially lowers the overall carbon footprint of ethyl acetate production. Additionally, innovative catalytic processes have been developed to improve reaction efficiency and reduce energy consumption during manufacturing.
The use of ethyl acetate in various industries also presents environmental challenges. As a volatile organic compound (VOC), ethyl acetate can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. This has led to increased regulatory scrutiny and the implementation of emission control measures in industrial settings. Many companies have adopted solvent recovery systems and closed-loop processes to minimize ethyl acetate emissions and reduce environmental impact.
In the realm of waste management, the disposal of ethyl acetate-containing products and byproducts requires careful handling. Improper disposal can lead to soil and water contamination, posing risks to ecosystems and human health. To address this, advanced treatment technologies such as activated carbon adsorption and biological degradation have been developed to effectively remove ethyl acetate from waste streams.
The lifecycle assessment of ethyl acetate has become an essential tool for evaluating its overall environmental impact. This comprehensive approach considers factors such as raw material extraction, production processes, transportation, use, and end-of-life disposal. Such assessments have driven efforts to optimize the entire value chain, leading to improvements in resource efficiency and the development of more environmentally friendly alternatives.
As industries strive for sustainability, the focus on green chemistry principles has intensified. Research into bio-catalytic processes and the use of supercritical carbon dioxide as a reaction medium for ethyl acetate synthesis represent promising directions for reducing environmental impact. These innovative approaches aim to minimize waste generation, improve atom economy, and enhance overall process sustainability.
However, recent technological advancements have led to more sustainable production methods. Bio-based ethyl acetate, derived from renewable resources such as corn or sugarcane, has emerged as a promising alternative. This approach reduces reliance on petrochemical feedstocks and potentially lowers the overall carbon footprint of ethyl acetate production. Additionally, innovative catalytic processes have been developed to improve reaction efficiency and reduce energy consumption during manufacturing.
The use of ethyl acetate in various industries also presents environmental challenges. As a volatile organic compound (VOC), ethyl acetate can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. This has led to increased regulatory scrutiny and the implementation of emission control measures in industrial settings. Many companies have adopted solvent recovery systems and closed-loop processes to minimize ethyl acetate emissions and reduce environmental impact.
In the realm of waste management, the disposal of ethyl acetate-containing products and byproducts requires careful handling. Improper disposal can lead to soil and water contamination, posing risks to ecosystems and human health. To address this, advanced treatment technologies such as activated carbon adsorption and biological degradation have been developed to effectively remove ethyl acetate from waste streams.
The lifecycle assessment of ethyl acetate has become an essential tool for evaluating its overall environmental impact. This comprehensive approach considers factors such as raw material extraction, production processes, transportation, use, and end-of-life disposal. Such assessments have driven efforts to optimize the entire value chain, leading to improvements in resource efficiency and the development of more environmentally friendly alternatives.
As industries strive for sustainability, the focus on green chemistry principles has intensified. Research into bio-catalytic processes and the use of supercritical carbon dioxide as a reaction medium for ethyl acetate synthesis represent promising directions for reducing environmental impact. These innovative approaches aim to minimize waste generation, improve atom economy, and enhance overall process sustainability.
Regulatory Framework for Ethyl Acetate Use
The regulatory framework for ethyl acetate use has evolved significantly over the years, reflecting growing awareness of its potential environmental and health impacts. In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The substance is classified as a volatile organic compound (VOC) due to its contribution to ground-level ozone formation.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in the workplace, setting the standard at 400 parts per million (ppm) for an 8-hour time-weighted average. This regulation aims to protect workers from potential health hazards associated with prolonged exposure to the chemical.
In the European Union, ethyl acetate is regulated under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework. Manufacturers and importers are required to register the substance and provide safety data to the European Chemicals Agency (ECHA). The EU has also implemented specific regulations for ethyl acetate use in food contact materials, setting migration limits to ensure consumer safety.
Japan's regulatory approach to ethyl acetate includes its listing under the Chemical Substances Control Law (CSCL) and the Industrial Safety and Health Law (ISHL). These regulations mandate proper labeling, handling, and storage of the substance, as well as regular workplace monitoring to ensure compliance with exposure limits.
Globally, the transportation of ethyl acetate is subject to strict regulations due to its flammability. The United Nations' Recommendations on the Transport of Dangerous Goods classifies ethyl acetate as a Class 3 Flammable Liquid, requiring specific packaging, labeling, and handling procedures during shipping.
Recent regulatory trends have focused on promoting the use of bio-based ethyl acetate as a more sustainable alternative to its petrochemical-derived counterpart. Several countries have introduced incentives and certification schemes to encourage the adoption of bio-based chemicals, including ethyl acetate, in various industrial applications.
As environmental concerns continue to shape policy, future regulatory developments may include stricter emission controls, enhanced recycling requirements, and increased emphasis on life cycle assessments for ethyl acetate production and use. These evolving regulations will likely drive innovation in production methods, application technologies, and end-of-life management strategies for ethyl acetate across various industries.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in the workplace, setting the standard at 400 parts per million (ppm) for an 8-hour time-weighted average. This regulation aims to protect workers from potential health hazards associated with prolonged exposure to the chemical.
In the European Union, ethyl acetate is regulated under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework. Manufacturers and importers are required to register the substance and provide safety data to the European Chemicals Agency (ECHA). The EU has also implemented specific regulations for ethyl acetate use in food contact materials, setting migration limits to ensure consumer safety.
Japan's regulatory approach to ethyl acetate includes its listing under the Chemical Substances Control Law (CSCL) and the Industrial Safety and Health Law (ISHL). These regulations mandate proper labeling, handling, and storage of the substance, as well as regular workplace monitoring to ensure compliance with exposure limits.
Globally, the transportation of ethyl acetate is subject to strict regulations due to its flammability. The United Nations' Recommendations on the Transport of Dangerous Goods classifies ethyl acetate as a Class 3 Flammable Liquid, requiring specific packaging, labeling, and handling procedures during shipping.
Recent regulatory trends have focused on promoting the use of bio-based ethyl acetate as a more sustainable alternative to its petrochemical-derived counterpart. Several countries have introduced incentives and certification schemes to encourage the adoption of bio-based chemicals, including ethyl acetate, in various industrial applications.
As environmental concerns continue to shape policy, future regulatory developments may include stricter emission controls, enhanced recycling requirements, and increased emphasis on life cycle assessments for ethyl acetate production and use. These evolving regulations will likely drive innovation in production methods, application technologies, and end-of-life management strategies for ethyl acetate across various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!