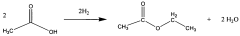
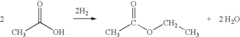
How Ethyl Acetate Mobilizes Industry for Environmental Change?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone a significant evolution since its discovery in the early 19th century. Initially synthesized as a laboratory curiosity, it quickly found applications in various industries due to its unique properties. The evolution of ethyl acetate can be traced through several key stages, each marked by technological advancements and expanding applications.
In the early stages, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This process, while effective, was limited in scale and efficiency. The mid-20th century saw a shift towards more industrial production methods, including the Tishchenko reaction, which allowed for larger-scale manufacturing.
The 1970s and 1980s marked a turning point in ethyl acetate production with the development of more environmentally friendly synthesis routes. This period saw the introduction of catalytic processes that significantly reduced energy consumption and waste generation. The use of heterogeneous catalysts, in particular, revolutionized the production process, allowing for continuous flow reactors and improved product purity.
The late 20th and early 21st centuries brought about a focus on sustainability in ethyl acetate production. This era saw the emergence of bio-based ethyl acetate, derived from renewable resources such as corn and sugarcane. The development of enzymatic processes for ethyl acetate synthesis further enhanced the green credentials of this versatile compound.
Recent years have witnessed a surge in research aimed at optimizing ethyl acetate production and utilization. Advanced separation techniques, such as reactive distillation and membrane technology, have been developed to improve process efficiency and reduce energy consumption. Additionally, the application of nanotechnology in catalyst design has opened new avenues for enhancing reaction selectivity and yield.
The evolution of ethyl acetate has not been limited to production methods alone. Its applications have expanded significantly over time, from traditional uses in solvents and coatings to more specialized roles in pharmaceuticals, electronics, and advanced materials. The compound's low toxicity and biodegradability have made it an increasingly attractive option in industries seeking to reduce their environmental footprint.
Looking ahead, the evolution of ethyl acetate is likely to continue along the path of sustainability and efficiency. Emerging technologies such as artificial intelligence and machine learning are being applied to optimize production processes and discover new applications. The integration of ethyl acetate into circular economy models, where it can be efficiently recycled and reused, represents a promising frontier in its ongoing evolution.
In the early stages, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This process, while effective, was limited in scale and efficiency. The mid-20th century saw a shift towards more industrial production methods, including the Tishchenko reaction, which allowed for larger-scale manufacturing.
The 1970s and 1980s marked a turning point in ethyl acetate production with the development of more environmentally friendly synthesis routes. This period saw the introduction of catalytic processes that significantly reduced energy consumption and waste generation. The use of heterogeneous catalysts, in particular, revolutionized the production process, allowing for continuous flow reactors and improved product purity.
The late 20th and early 21st centuries brought about a focus on sustainability in ethyl acetate production. This era saw the emergence of bio-based ethyl acetate, derived from renewable resources such as corn and sugarcane. The development of enzymatic processes for ethyl acetate synthesis further enhanced the green credentials of this versatile compound.
Recent years have witnessed a surge in research aimed at optimizing ethyl acetate production and utilization. Advanced separation techniques, such as reactive distillation and membrane technology, have been developed to improve process efficiency and reduce energy consumption. Additionally, the application of nanotechnology in catalyst design has opened new avenues for enhancing reaction selectivity and yield.
The evolution of ethyl acetate has not been limited to production methods alone. Its applications have expanded significantly over time, from traditional uses in solvents and coatings to more specialized roles in pharmaceuticals, electronics, and advanced materials. The compound's low toxicity and biodegradability have made it an increasingly attractive option in industries seeking to reduce their environmental footprint.
Looking ahead, the evolution of ethyl acetate is likely to continue along the path of sustainability and efficiency. Emerging technologies such as artificial intelligence and machine learning are being applied to optimize production processes and discover new applications. The integration of ethyl acetate into circular economy models, where it can be efficiently recycled and reused, represents a promising frontier in its ongoing evolution.
Market Demand Analysis
The market demand for ethyl acetate as an environmentally friendly solvent has been steadily increasing in recent years. This growth is primarily driven by the rising awareness of environmental issues and the stringent regulations imposed on volatile organic compounds (VOCs) emissions across various industries. Ethyl acetate, being a low-toxicity solvent with relatively low environmental impact, has emerged as a viable alternative to more harmful solvents in many applications.
In the coatings and paints industry, there is a significant shift towards water-based and low-VOC formulations. Ethyl acetate plays a crucial role in this transition, as it can be used as a coalescent in water-based paints and as a solvent in low-VOC coatings. The construction and automotive sectors, which are major consumers of paints and coatings, are driving this demand due to increasing environmental regulations and consumer preferences for eco-friendly products.
The pharmaceutical industry is another key market for ethyl acetate. As a Class 3 solvent according to ICH guidelines, it is considered less toxic and of lower risk to human health. This classification has led to its increased use in drug formulation and manufacturing processes, replacing more hazardous solvents. The growing pharmaceutical market, particularly in emerging economies, is expected to further boost the demand for ethyl acetate.
In the food and beverage industry, ethyl acetate is widely used as a flavoring agent and in the decaffeination of coffee and tea. The rising consumer demand for natural and clean label products has led to an increased preference for ethyl acetate over other synthetic solvents in food applications. This trend is particularly strong in the specialty coffee market, where ethyl acetate-based decaffeination is marketed as a more natural process.
The adhesives industry is also a significant consumer of ethyl acetate. With the growing emphasis on sustainable packaging and eco-friendly consumer goods, there is a rising demand for adhesives that use less harmful solvents. Ethyl acetate-based adhesives are gaining traction in flexible packaging, labeling, and woodworking applications, driven by their lower environmental impact and improved safety profile.
The global market for ethyl acetate is projected to grow at a compound annual growth rate (CAGR) of over 5% in the coming years. This growth is attributed to its increasing adoption in various end-use industries as a more environmentally friendly alternative. The Asia-Pacific region, particularly China and India, is expected to be the fastest-growing market due to rapid industrialization and the implementation of stricter environmental regulations.
In the coatings and paints industry, there is a significant shift towards water-based and low-VOC formulations. Ethyl acetate plays a crucial role in this transition, as it can be used as a coalescent in water-based paints and as a solvent in low-VOC coatings. The construction and automotive sectors, which are major consumers of paints and coatings, are driving this demand due to increasing environmental regulations and consumer preferences for eco-friendly products.
The pharmaceutical industry is another key market for ethyl acetate. As a Class 3 solvent according to ICH guidelines, it is considered less toxic and of lower risk to human health. This classification has led to its increased use in drug formulation and manufacturing processes, replacing more hazardous solvents. The growing pharmaceutical market, particularly in emerging economies, is expected to further boost the demand for ethyl acetate.
In the food and beverage industry, ethyl acetate is widely used as a flavoring agent and in the decaffeination of coffee and tea. The rising consumer demand for natural and clean label products has led to an increased preference for ethyl acetate over other synthetic solvents in food applications. This trend is particularly strong in the specialty coffee market, where ethyl acetate-based decaffeination is marketed as a more natural process.
The adhesives industry is also a significant consumer of ethyl acetate. With the growing emphasis on sustainable packaging and eco-friendly consumer goods, there is a rising demand for adhesives that use less harmful solvents. Ethyl acetate-based adhesives are gaining traction in flexible packaging, labeling, and woodworking applications, driven by their lower environmental impact and improved safety profile.
The global market for ethyl acetate is projected to grow at a compound annual growth rate (CAGR) of over 5% in the coming years. This growth is attributed to its increasing adoption in various end-use industries as a more environmentally friendly alternative. The Asia-Pacific region, particularly China and India, is expected to be the fastest-growing market due to rapid industrialization and the implementation of stricter environmental regulations.
Technical Challenges
The adoption of ethyl acetate as an environmentally friendly solvent faces several technical challenges that need to be addressed for widespread industrial implementation. One of the primary obstacles is the optimization of production processes to ensure cost-effectiveness and scalability. While ethyl acetate offers significant environmental benefits, its production costs are currently higher than those of traditional petroleum-based solvents, making it less economically viable for many industries.
Another challenge lies in the development of efficient recovery and recycling systems for ethyl acetate. As a volatile organic compound, it can be difficult to capture and reuse effectively, leading to potential emissions and loss of material. Improving recovery technologies is crucial for maximizing the environmental benefits and economic feasibility of ethyl acetate use in industrial applications.
The compatibility of ethyl acetate with existing industrial equipment and processes presents another hurdle. Many manufacturing facilities are designed for use with conventional solvents, and transitioning to ethyl acetate may require significant modifications to infrastructure and operating procedures. This includes addressing issues related to material compatibility, as ethyl acetate may interact differently with certain plastics, rubbers, and metals compared to traditional solvents.
Furthermore, the purity requirements for ethyl acetate in various applications pose a technical challenge. Achieving and maintaining high levels of purity during production and throughout the supply chain is essential for ensuring consistent performance across different industrial uses. This necessitates the development of advanced purification techniques and quality control measures.
The storage and transportation of ethyl acetate also present technical difficulties. Its relatively low flash point and high volatility require specialized handling and storage solutions to ensure safety and prevent loss through evaporation. This includes the need for appropriate containment systems, temperature control, and vapor recovery mechanisms during transport and storage.
Lastly, there is a need for comprehensive life cycle assessments to fully understand and quantify the environmental impact of ethyl acetate compared to alternative solvents. While it is generally considered more environmentally friendly, rigorous studies are required to account for all aspects of its production, use, and disposal across various industrial applications. This data is crucial for informing policy decisions and guiding industry adoption.
Addressing these technical challenges requires collaborative efforts between researchers, industry stakeholders, and policymakers. Innovations in green chemistry, process engineering, and materials science will be key to overcoming these obstacles and realizing the full potential of ethyl acetate as a catalyst for environmental change in industrial practices.
Another challenge lies in the development of efficient recovery and recycling systems for ethyl acetate. As a volatile organic compound, it can be difficult to capture and reuse effectively, leading to potential emissions and loss of material. Improving recovery technologies is crucial for maximizing the environmental benefits and economic feasibility of ethyl acetate use in industrial applications.
The compatibility of ethyl acetate with existing industrial equipment and processes presents another hurdle. Many manufacturing facilities are designed for use with conventional solvents, and transitioning to ethyl acetate may require significant modifications to infrastructure and operating procedures. This includes addressing issues related to material compatibility, as ethyl acetate may interact differently with certain plastics, rubbers, and metals compared to traditional solvents.
Furthermore, the purity requirements for ethyl acetate in various applications pose a technical challenge. Achieving and maintaining high levels of purity during production and throughout the supply chain is essential for ensuring consistent performance across different industrial uses. This necessitates the development of advanced purification techniques and quality control measures.
The storage and transportation of ethyl acetate also present technical difficulties. Its relatively low flash point and high volatility require specialized handling and storage solutions to ensure safety and prevent loss through evaporation. This includes the need for appropriate containment systems, temperature control, and vapor recovery mechanisms during transport and storage.
Lastly, there is a need for comprehensive life cycle assessments to fully understand and quantify the environmental impact of ethyl acetate compared to alternative solvents. While it is generally considered more environmentally friendly, rigorous studies are required to account for all aspects of its production, use, and disposal across various industrial applications. This data is crucial for informing policy decisions and guiding industry adoption.
Addressing these technical challenges requires collaborative efforts between researchers, industry stakeholders, and policymakers. Innovations in green chemistry, process engineering, and materials science will be key to overcoming these obstacles and realizing the full potential of ethyl acetate as a catalyst for environmental change in industrial practices.
Current Green Solutions
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These methods aim to improve the yield and purity of ethyl acetate, which is an important industrial solvent and chemical intermediate.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in diverse chemical processes, such as extraction, separation, and as a reaction medium. Its properties make it suitable for use in the production of various compounds, including pharmaceuticals, polymers, and other industrial chemicals.
- Ethyl acetate in coating and adhesive formulations: Ethyl acetate is commonly used as a solvent in coating and adhesive formulations. It provides desirable properties such as fast evaporation, low toxicity, and compatibility with various resins and polymers, making it suitable for use in paints, varnishes, and adhesives.
- Recovery and recycling of ethyl acetate: Methods for recovering and recycling ethyl acetate from industrial processes are described. These techniques aim to reduce waste, improve process efficiency, and minimize environmental impact by reusing the solvent in various applications.
- Ethyl acetate as a green solvent alternative: Ethyl acetate is explored as a more environmentally friendly solvent alternative in various applications. Its relatively low toxicity, biodegradability, and favorable properties make it suitable for replacing more harmful solvents in industrial processes and consumer products.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse manufacturing applications.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques used in chemical and pharmaceutical industries.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and volatility.Expand Specific Solutions05 Novel applications and formulations of ethyl acetate
Innovative uses and formulations of ethyl acetate are being explored in various fields. These include its incorporation in specialized coatings, adhesives, and composite materials, as well as its potential in emerging technologies and sustainable product development.Expand Specific Solutions
Key Industry Players
The ethyl acetate industry is in a transitional phase, driven by environmental concerns and sustainability initiatives. The market is experiencing moderate growth, with an estimated global size of $3.5 billion in 2021 and projected to reach $4.8 billion by 2026. Technologically, the industry is evolving towards greener production methods, with companies like LanzaTech NZ, Inc. and SABIC Global Technologies BV leading innovation in bio-based ethyl acetate production. Traditional players such as Celanese International Corp. and China Petroleum & Chemical Corp. are also adapting their processes to meet environmental standards. Research institutions like Tianjin University and Columbia University are contributing to advancements in catalytic processes and sustainable synthesis methods, indicating a collaborative effort to improve the industry's ecological footprint.
Celanese International Corp.
Technical Solution: Celanese has developed a cutting-edge ethyl acetate production process that focuses on sustainability and efficiency. Their VAntage® technology platform utilizes a proprietary catalyst system that enables the direct addition of ethylene to acetic acid, bypassing the need for ethanol as an intermediate[9]. This one-step process significantly reduces energy consumption and carbon footprint compared to traditional two-step methods. The technology achieves conversion rates of over 99.5% and selectivity exceeding 99%, resulting in high-purity ethyl acetate production[10]. Celanese has also implemented advanced process intensification techniques, such as dividing wall column distillation, which further reduces energy requirements and improves overall process economics. The company has successfully commercialized this technology, with multiple large-scale plants operational globally, demonstrating its robustness and scalability[11].
Strengths: Direct one-step process with high conversion and selectivity; Reduced energy consumption and carbon emissions; Proven commercial-scale technology. Weaknesses: Dependent on ethylene availability; May face challenges in regions with limited ethylene infrastructure.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a green ethyl acetate production process using bio-ethanol as a raw material. This innovative approach involves the dehydrogenation of ethanol to produce ethyl acetate, significantly reducing carbon emissions compared to traditional petrochemical routes[1]. The process utilizes a highly efficient copper-based catalyst system, achieving conversion rates of over 95% and selectivity exceeding 98%[2]. Sinopec has also implemented advanced distillation techniques to enhance product purity, meeting stringent industry standards. The company has successfully scaled up this technology, with several commercial plants now operational, demonstrating its commitment to sustainable chemical production[3].
Strengths: Utilizes renewable bio-ethanol, reducing carbon footprint; High conversion rates and selectivity; Commercially proven technology. Weaknesses: Dependent on bio-ethanol availability; May face competition from traditional petrochemical routes in terms of cost.
Innovative EA Processes
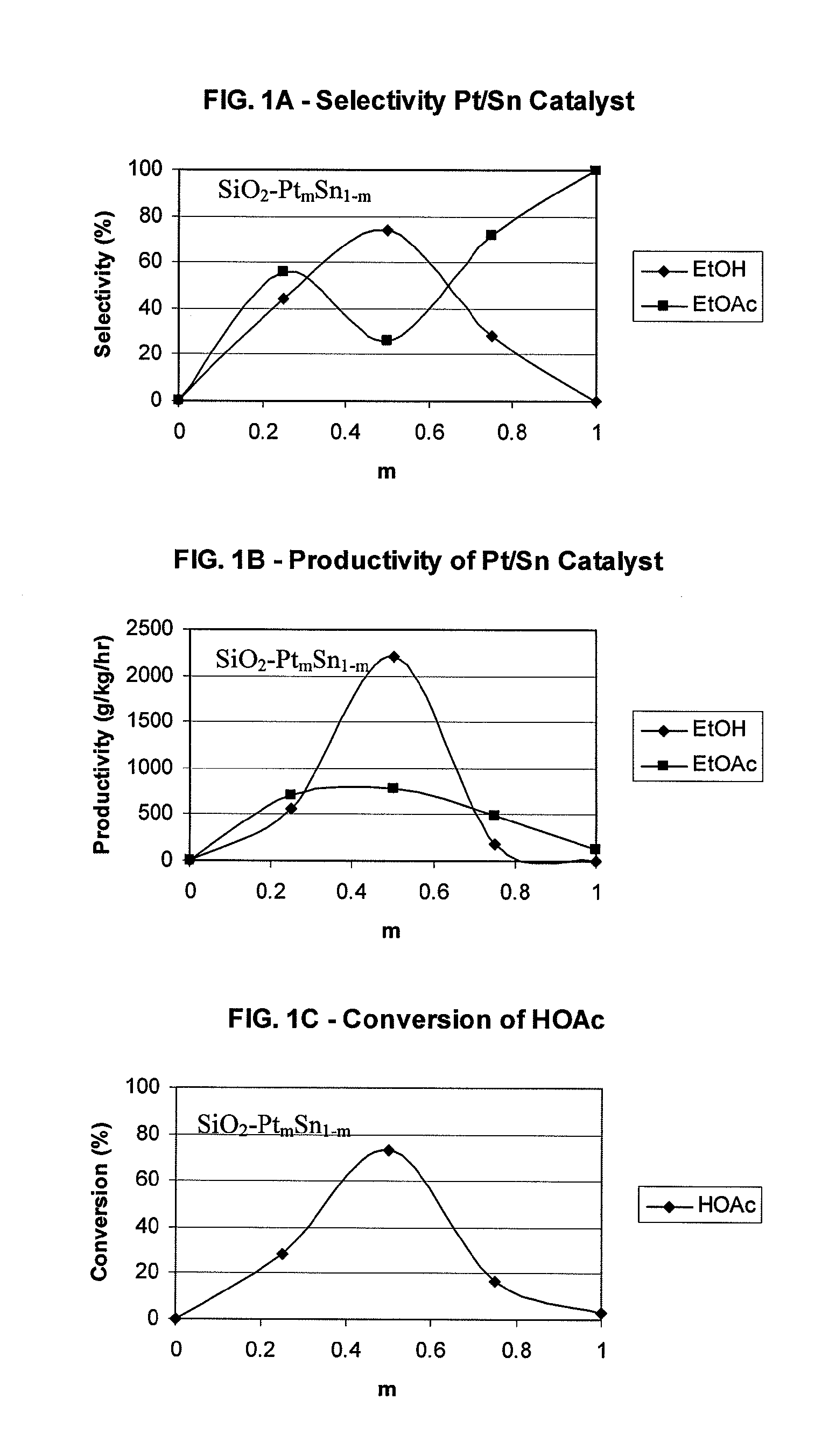
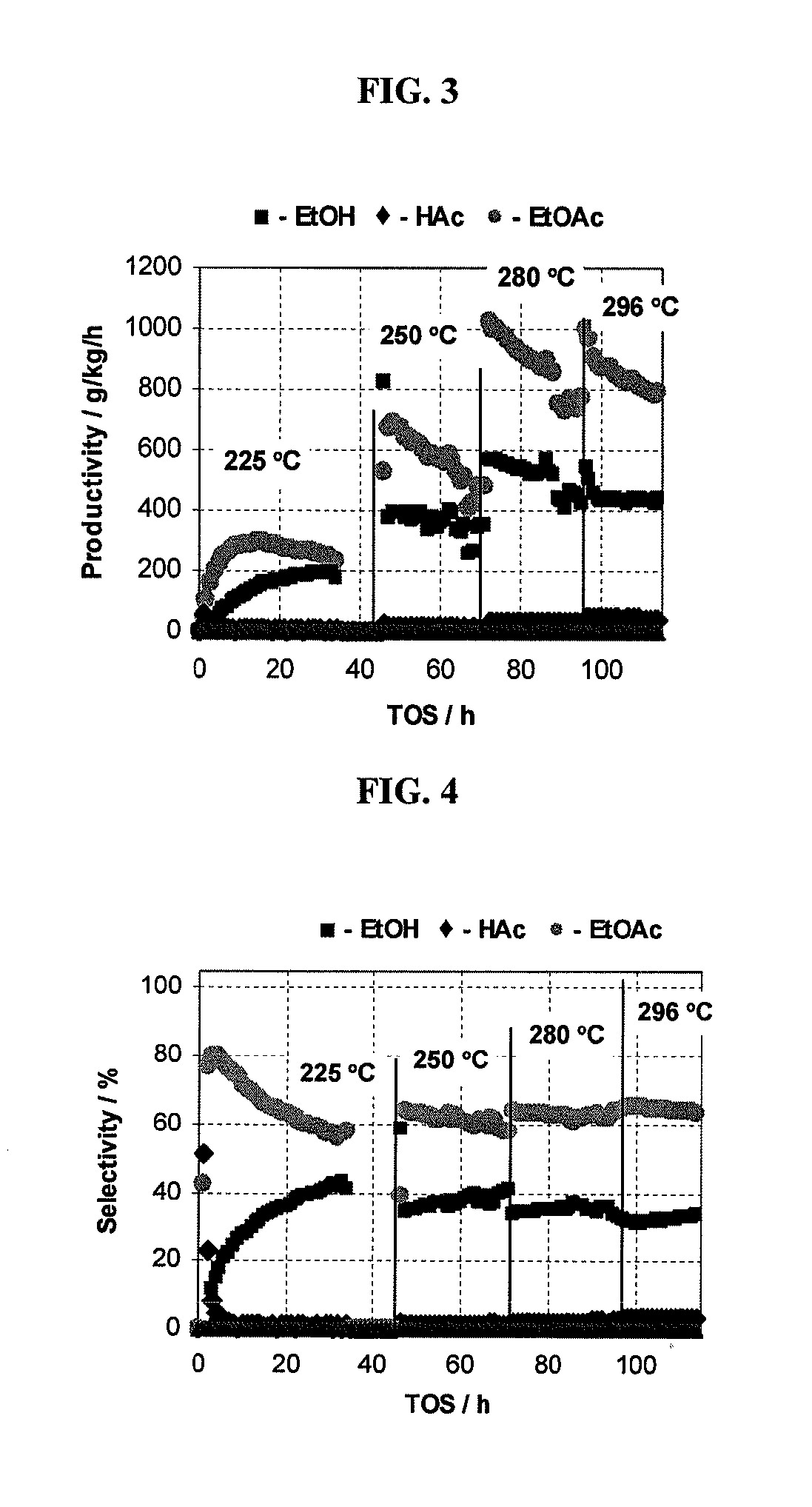
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentWO2010014145A2
Innovation
- A process utilizing a bimetallic catalyst supported on a suitable catalyst support, comprising metals like platinum, palladium, copper, and cobalt, which selectively hydrogenates acetic acid to ethyl acetate with high yield and selectivity, minimizing by-product formation.
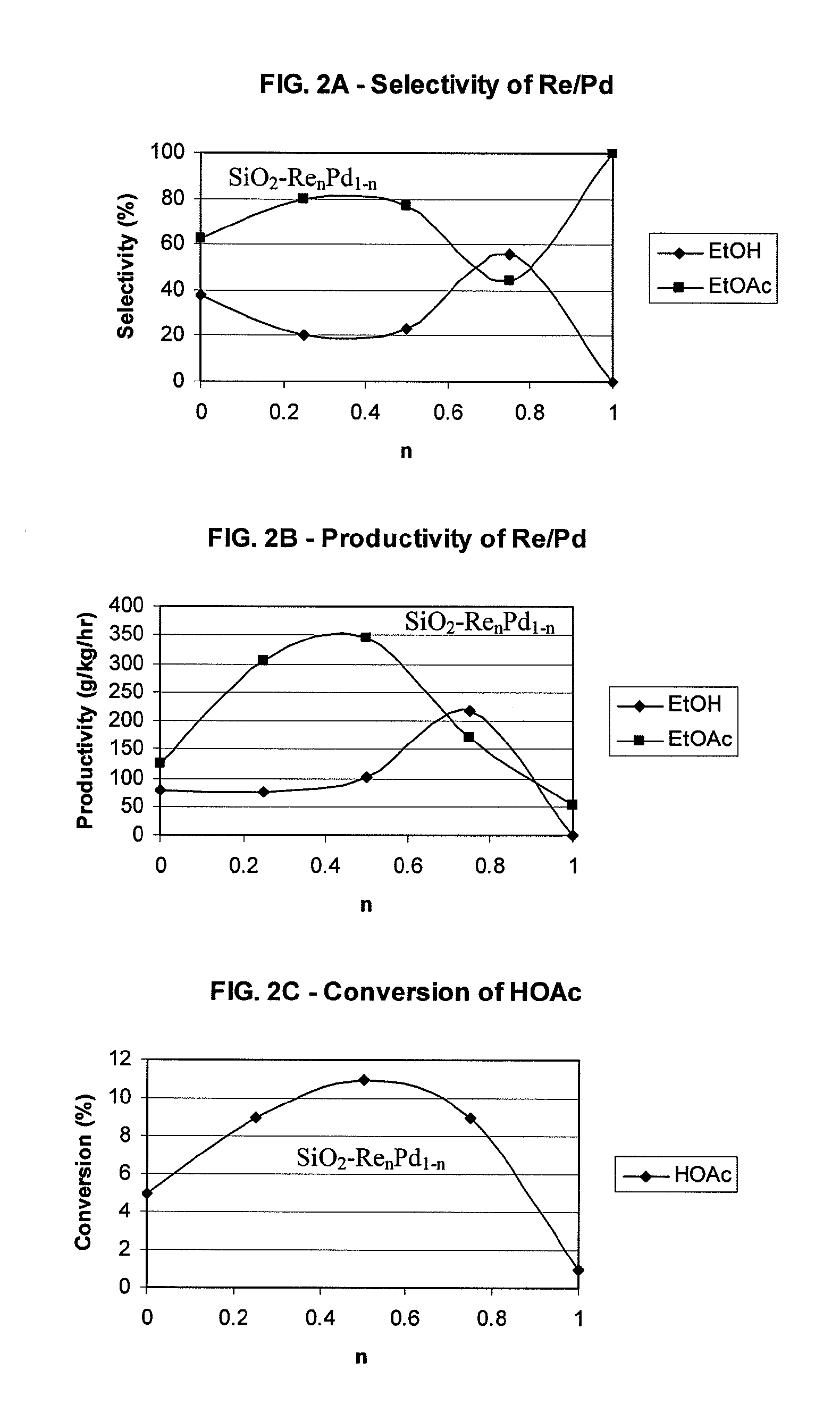
Processes for making ethyl acetate from acetic acid
PatentInactiveUS20100197959A1
Innovation
- A process utilizing catalysts comprising metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which are effective in hydrogenating acetic acid to produce ethyl acetate with high selectivity and minimizing by-product formation.
Environmental Regulations
Environmental regulations play a crucial role in shaping the adoption and use of ethyl acetate in various industries. As awareness of environmental issues grows, governments worldwide have implemented stricter regulations to control the emission of volatile organic compounds (VOCs) and reduce the environmental impact of industrial processes.
In the United States, the Environmental Protection Agency (EPA) has established guidelines for the use and disposal of ethyl acetate under the Clean Air Act and the Resource Conservation and Recovery Act. These regulations set limits on emissions and require proper handling and disposal methods for ethyl acetate and other solvents.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which affects the production, import, and use of ethyl acetate. This regulation aims to protect human health and the environment by requiring manufacturers and importers to assess and manage the risks associated with chemicals, including ethyl acetate.
Many countries have also introduced specific regulations targeting VOC emissions from industrial processes. For instance, China's Air Pollution Prevention and Control Law sets strict limits on VOC emissions from various industries, including those using ethyl acetate in their manufacturing processes.
These environmental regulations have driven industries to seek more sustainable alternatives and improve their production processes. As a result, many companies have invested in research and development to find ways to reduce ethyl acetate emissions or replace it with more environmentally friendly alternatives.
The paint and coatings industry, a major user of ethyl acetate, has been particularly affected by these regulations. Many manufacturers have reformulated their products to comply with VOC emission limits, leading to the development of low-VOC and zero-VOC coatings that use alternative solvents or water-based formulations.
In the pharmaceutical industry, where ethyl acetate is commonly used as a solvent in drug manufacturing, companies have implemented advanced recovery and recycling systems to minimize emissions and waste. This not only helps comply with regulations but also reduces production costs and improves overall efficiency.
The electronics industry has also been impacted by environmental regulations concerning ethyl acetate use. Manufacturers have developed new cleaning processes and formulations that reduce or eliminate the need for ethyl acetate, helping to minimize environmental impact and meet regulatory requirements.
As environmental regulations continue to evolve and become more stringent, industries are likely to face increasing pressure to find innovative solutions for reducing their reliance on ethyl acetate and other potentially harmful chemicals. This ongoing regulatory landscape serves as a significant driver for environmental change and technological advancement across various sectors.
In the United States, the Environmental Protection Agency (EPA) has established guidelines for the use and disposal of ethyl acetate under the Clean Air Act and the Resource Conservation and Recovery Act. These regulations set limits on emissions and require proper handling and disposal methods for ethyl acetate and other solvents.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which affects the production, import, and use of ethyl acetate. This regulation aims to protect human health and the environment by requiring manufacturers and importers to assess and manage the risks associated with chemicals, including ethyl acetate.
Many countries have also introduced specific regulations targeting VOC emissions from industrial processes. For instance, China's Air Pollution Prevention and Control Law sets strict limits on VOC emissions from various industries, including those using ethyl acetate in their manufacturing processes.
These environmental regulations have driven industries to seek more sustainable alternatives and improve their production processes. As a result, many companies have invested in research and development to find ways to reduce ethyl acetate emissions or replace it with more environmentally friendly alternatives.
The paint and coatings industry, a major user of ethyl acetate, has been particularly affected by these regulations. Many manufacturers have reformulated their products to comply with VOC emission limits, leading to the development of low-VOC and zero-VOC coatings that use alternative solvents or water-based formulations.
In the pharmaceutical industry, where ethyl acetate is commonly used as a solvent in drug manufacturing, companies have implemented advanced recovery and recycling systems to minimize emissions and waste. This not only helps comply with regulations but also reduces production costs and improves overall efficiency.
The electronics industry has also been impacted by environmental regulations concerning ethyl acetate use. Manufacturers have developed new cleaning processes and formulations that reduce or eliminate the need for ethyl acetate, helping to minimize environmental impact and meet regulatory requirements.
As environmental regulations continue to evolve and become more stringent, industries are likely to face increasing pressure to find innovative solutions for reducing their reliance on ethyl acetate and other potentially harmful chemicals. This ongoing regulatory landscape serves as a significant driver for environmental change and technological advancement across various sectors.
Economic Impact Analysis
The economic impact of ethyl acetate's role in mobilizing industry for environmental change is multifaceted and far-reaching. As a versatile and environmentally friendly solvent, ethyl acetate has the potential to significantly influence various sectors of the economy, particularly in industries seeking to reduce their environmental footprint.
In the manufacturing sector, the adoption of ethyl acetate as a replacement for more harmful solvents can lead to cost savings in waste management and regulatory compliance. Companies that switch to ethyl acetate may experience reduced expenses related to hazardous waste disposal and lower insurance premiums due to decreased workplace hazards. This shift can enhance operational efficiency and improve profit margins, potentially stimulating growth and job creation within the sector.
The agricultural industry stands to benefit economically from the increased demand for ethyl acetate. As a bio-based solvent derived from renewable resources such as corn and sugarcane, its production can create new revenue streams for farmers and rural communities. This diversification of agricultural output can contribute to the stability of rural economies and promote sustainable farming practices.
In the chemical industry, the transition towards ethyl acetate production can drive innovation and investment in green chemistry technologies. Companies that develop more efficient and sustainable production methods for ethyl acetate may gain a competitive edge in the global market. This can lead to increased exports, job creation in research and development, and the establishment of new specialized manufacturing facilities.
The packaging industry, particularly in food and beverage sectors, may experience significant economic impacts from the adoption of ethyl acetate-based adhesives and coatings. These environmentally friendly alternatives can help companies meet consumer demands for sustainable packaging, potentially increasing market share and brand value. The shift may also reduce costs associated with regulatory compliance and waste management in packaging production.
From a macroeconomic perspective, the widespread adoption of ethyl acetate can contribute to the growth of the green economy. This transition can attract investment in sustainable technologies, create new job opportunities in emerging green industries, and potentially lead to the development of specialized economic zones focused on eco-friendly production methods.
However, the economic impact is not without potential challenges. Industries heavily reliant on traditional solvents may face transition costs and potential job displacement. The shift to ethyl acetate production may require significant capital investment in new equipment and processes, which could pose financial challenges for smaller businesses.
In conclusion, the economic impact of ethyl acetate in driving environmental change is likely to be substantial, with potential benefits across multiple sectors. While there may be short-term adjustment costs, the long-term economic outlook suggests positive growth, innovation, and the creation of more sustainable industrial practices.
In the manufacturing sector, the adoption of ethyl acetate as a replacement for more harmful solvents can lead to cost savings in waste management and regulatory compliance. Companies that switch to ethyl acetate may experience reduced expenses related to hazardous waste disposal and lower insurance premiums due to decreased workplace hazards. This shift can enhance operational efficiency and improve profit margins, potentially stimulating growth and job creation within the sector.
The agricultural industry stands to benefit economically from the increased demand for ethyl acetate. As a bio-based solvent derived from renewable resources such as corn and sugarcane, its production can create new revenue streams for farmers and rural communities. This diversification of agricultural output can contribute to the stability of rural economies and promote sustainable farming practices.
In the chemical industry, the transition towards ethyl acetate production can drive innovation and investment in green chemistry technologies. Companies that develop more efficient and sustainable production methods for ethyl acetate may gain a competitive edge in the global market. This can lead to increased exports, job creation in research and development, and the establishment of new specialized manufacturing facilities.
The packaging industry, particularly in food and beverage sectors, may experience significant economic impacts from the adoption of ethyl acetate-based adhesives and coatings. These environmentally friendly alternatives can help companies meet consumer demands for sustainable packaging, potentially increasing market share and brand value. The shift may also reduce costs associated with regulatory compliance and waste management in packaging production.
From a macroeconomic perspective, the widespread adoption of ethyl acetate can contribute to the growth of the green economy. This transition can attract investment in sustainable technologies, create new job opportunities in emerging green industries, and potentially lead to the development of specialized economic zones focused on eco-friendly production methods.
However, the economic impact is not without potential challenges. Industries heavily reliant on traditional solvents may face transition costs and potential job displacement. The shift to ethyl acetate production may require significant capital investment in new equipment and processes, which could pose financial challenges for smaller businesses.
In conclusion, the economic impact of ethyl acetate in driving environmental change is likely to be substantial, with potential benefits across multiple sectors. While there may be short-term adjustment costs, the long-term economic outlook suggests positive growth, innovation, and the creation of more sustainable industrial practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!