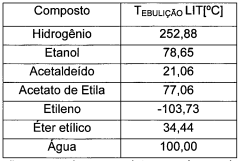
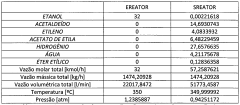
How to Drive Sustainable Tech with Ethyl Acetate Solutions?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Tech Evolution
The evolution of ethyl acetate technology has been marked by significant advancements in production methods, applications, and sustainability efforts. Initially, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid, a process that dates back to the early 20th century. This traditional method, while effective, was energy-intensive and often relied on petrochemical feedstocks.
As environmental concerns grew in the latter half of the 20th century, researchers began exploring more sustainable production routes. The 1980s saw the development of bio-based ethyl acetate production, utilizing fermentation processes to derive ethanol from renewable resources such as corn and sugarcane. This shift marked a crucial step towards reducing the carbon footprint of ethyl acetate manufacturing.
The 1990s brought about further refinements in catalytic processes, improving reaction efficiency and reducing energy consumption. Heterogeneous catalysts, such as ion-exchange resins, gained prominence, offering easier separation and recycling compared to homogeneous catalysts. This period also saw increased focus on process intensification, with the introduction of reactive distillation techniques that combined reaction and separation steps, significantly enhancing production efficiency.
The turn of the millennium heralded a new era of green chemistry principles applied to ethyl acetate production. Researchers began exploring the use of supercritical carbon dioxide as a solvent and reactant, offering a more environmentally friendly alternative to traditional organic solvents. Additionally, the development of enzymatic processes for ethyl acetate synthesis gained traction, promising milder reaction conditions and higher selectivity.
In recent years, the focus has shifted towards circular economy concepts in ethyl acetate production. Waste valorization strategies have emerged, with researchers investigating the use of agricultural and industrial waste streams as feedstocks. For instance, the conversion of lignocellulosic biomass to ethyl acetate has shown promising results, offering a dual benefit of waste reduction and sustainable chemical production.
The latest frontier in ethyl acetate technology involves the integration of artificial intelligence and machine learning in process optimization. These tools are being employed to fine-tune reaction conditions, predict catalyst performance, and enhance overall production efficiency. Furthermore, the development of novel reactor designs, such as microreactors and flow chemistry systems, is pushing the boundaries of process intensification, enabling more precise control over reaction parameters and reducing waste generation.
As we look to the future, the evolution of ethyl acetate technology is likely to continue its trajectory towards greater sustainability and efficiency. Emerging areas of research include the development of bio-based catalysts, the exploration of electrochemical synthesis routes, and the integration of renewable energy sources in production processes. These advancements promise to further reduce the environmental impact of ethyl acetate production while meeting the growing global demand for this versatile solvent.
As environmental concerns grew in the latter half of the 20th century, researchers began exploring more sustainable production routes. The 1980s saw the development of bio-based ethyl acetate production, utilizing fermentation processes to derive ethanol from renewable resources such as corn and sugarcane. This shift marked a crucial step towards reducing the carbon footprint of ethyl acetate manufacturing.
The 1990s brought about further refinements in catalytic processes, improving reaction efficiency and reducing energy consumption. Heterogeneous catalysts, such as ion-exchange resins, gained prominence, offering easier separation and recycling compared to homogeneous catalysts. This period also saw increased focus on process intensification, with the introduction of reactive distillation techniques that combined reaction and separation steps, significantly enhancing production efficiency.
The turn of the millennium heralded a new era of green chemistry principles applied to ethyl acetate production. Researchers began exploring the use of supercritical carbon dioxide as a solvent and reactant, offering a more environmentally friendly alternative to traditional organic solvents. Additionally, the development of enzymatic processes for ethyl acetate synthesis gained traction, promising milder reaction conditions and higher selectivity.
In recent years, the focus has shifted towards circular economy concepts in ethyl acetate production. Waste valorization strategies have emerged, with researchers investigating the use of agricultural and industrial waste streams as feedstocks. For instance, the conversion of lignocellulosic biomass to ethyl acetate has shown promising results, offering a dual benefit of waste reduction and sustainable chemical production.
The latest frontier in ethyl acetate technology involves the integration of artificial intelligence and machine learning in process optimization. These tools are being employed to fine-tune reaction conditions, predict catalyst performance, and enhance overall production efficiency. Furthermore, the development of novel reactor designs, such as microreactors and flow chemistry systems, is pushing the boundaries of process intensification, enabling more precise control over reaction parameters and reducing waste generation.
As we look to the future, the evolution of ethyl acetate technology is likely to continue its trajectory towards greater sustainability and efficiency. Emerging areas of research include the development of bio-based catalysts, the exploration of electrochemical synthesis routes, and the integration of renewable energy sources in production processes. These advancements promise to further reduce the environmental impact of ethyl acetate production while meeting the growing global demand for this versatile solvent.
Sustainable Solvent Market Analysis
The sustainable solvent market has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations. Ethyl acetate, a versatile and eco-friendly solvent, has emerged as a key player in this market. The global ethyl acetate market size was valued at USD 3.3 billion in 2020 and is projected to reach USD 4.9 billion by 2028, growing at a CAGR of 5.2% during the forecast period.
The demand for sustainable solvents, particularly ethyl acetate, is being fueled by various industries, including paints and coatings, pharmaceuticals, adhesives, and food and beverages. The paints and coatings industry remains the largest consumer of ethyl acetate, accounting for approximately 35% of the market share. This is primarily due to the solvent's low toxicity, fast evaporation rate, and excellent solvency for a wide range of resins and polymers.
In the pharmaceutical sector, ethyl acetate is increasingly being used as a more environmentally friendly alternative to traditional solvents in drug manufacturing processes. The food and beverage industry is also adopting ethyl acetate for various applications, such as decaffeination of coffee and tea, and as a flavoring agent, due to its low toxicity and biodegradability.
Geographically, Asia-Pacific dominates the ethyl acetate market, accounting for over 40% of the global market share. This is attributed to the rapid industrialization, growing population, and increasing demand from end-use industries in countries like China and India. North America and Europe follow, with a combined market share of approximately 35%, driven by stringent environmental regulations and a shift towards sustainable manufacturing practices.
The market is characterized by the presence of several key players, including Celanese Corporation, Eastman Chemical Company, and INEOS. These companies are focusing on expanding their production capacities and developing bio-based ethyl acetate to meet the growing demand for sustainable solvents.
Despite the positive outlook, the ethyl acetate market faces challenges such as volatile raw material prices and competition from other eco-friendly solvents. However, ongoing research and development efforts are aimed at improving production processes and exploring new applications, which are expected to create lucrative opportunities for market growth.
In conclusion, the sustainable solvent market, particularly for ethyl acetate, shows promising growth potential. The increasing adoption of environmentally friendly practices across industries, coupled with supportive government regulations, is expected to drive the demand for ethyl acetate as a sustainable solvent solution in the coming years.
The demand for sustainable solvents, particularly ethyl acetate, is being fueled by various industries, including paints and coatings, pharmaceuticals, adhesives, and food and beverages. The paints and coatings industry remains the largest consumer of ethyl acetate, accounting for approximately 35% of the market share. This is primarily due to the solvent's low toxicity, fast evaporation rate, and excellent solvency for a wide range of resins and polymers.
In the pharmaceutical sector, ethyl acetate is increasingly being used as a more environmentally friendly alternative to traditional solvents in drug manufacturing processes. The food and beverage industry is also adopting ethyl acetate for various applications, such as decaffeination of coffee and tea, and as a flavoring agent, due to its low toxicity and biodegradability.
Geographically, Asia-Pacific dominates the ethyl acetate market, accounting for over 40% of the global market share. This is attributed to the rapid industrialization, growing population, and increasing demand from end-use industries in countries like China and India. North America and Europe follow, with a combined market share of approximately 35%, driven by stringent environmental regulations and a shift towards sustainable manufacturing practices.
The market is characterized by the presence of several key players, including Celanese Corporation, Eastman Chemical Company, and INEOS. These companies are focusing on expanding their production capacities and developing bio-based ethyl acetate to meet the growing demand for sustainable solvents.
Despite the positive outlook, the ethyl acetate market faces challenges such as volatile raw material prices and competition from other eco-friendly solvents. However, ongoing research and development efforts are aimed at improving production processes and exploring new applications, which are expected to create lucrative opportunities for market growth.
In conclusion, the sustainable solvent market, particularly for ethyl acetate, shows promising growth potential. The increasing adoption of environmentally friendly practices across industries, coupled with supportive government regulations, is expected to drive the demand for ethyl acetate as a sustainable solvent solution in the coming years.
Ethyl Acetate Challenges
The use of ethyl acetate as a sustainable solution in technology faces several significant challenges. One of the primary obstacles is its volatile organic compound (VOC) status, which raises environmental and health concerns. Despite being less harmful than many other solvents, ethyl acetate still contributes to air pollution and can pose risks in high concentrations, necessitating careful handling and emission control measures.
Another challenge lies in the production process of ethyl acetate. While it can be derived from renewable resources, such as biomass, the current industrial production methods often rely on petrochemical feedstocks. This dependency on non-renewable resources contradicts the goal of sustainability, creating a need for more eco-friendly production techniques that can match the scale and efficiency of traditional methods.
The cost-effectiveness of ethyl acetate solutions presents another hurdle. Although ethyl acetate is generally less expensive than some alternative solvents, implementing sustainable production methods and meeting stringent environmental regulations can increase overall costs. This economic factor can deter widespread adoption, particularly in industries where profit margins are slim.
Regulatory compliance poses an additional challenge. As environmental regulations become more stringent globally, the use of ethyl acetate, despite its relative safety, may face increasing scrutiny. Companies must navigate a complex landscape of local, national, and international regulations, which can vary significantly across different regions.
The performance characteristics of ethyl acetate in certain applications can also be limiting. While it excels in many areas, such as its use as a solvent in coatings and adhesives, it may not be suitable for all technological applications. Its relatively low boiling point and high volatility can be disadvantageous in some processes, requiring the development of specialized formulations or application techniques.
Lastly, the challenge of perception and market acceptance cannot be overlooked. Despite its potential as a more sustainable option, ethyl acetate still faces competition from both traditional solvents and newer, marketed as "green" alternatives. Overcoming entrenched practices and convincing stakeholders to switch to ethyl acetate-based solutions requires substantial education and demonstration of its benefits.
Addressing these challenges requires a multifaceted approach, combining technological innovation, policy support, and market education. Developing more sustainable production methods, improving application techniques, and demonstrating the long-term environmental and economic benefits of ethyl acetate solutions are crucial steps in overcoming these obstacles and driving sustainable technology forward.
Another challenge lies in the production process of ethyl acetate. While it can be derived from renewable resources, such as biomass, the current industrial production methods often rely on petrochemical feedstocks. This dependency on non-renewable resources contradicts the goal of sustainability, creating a need for more eco-friendly production techniques that can match the scale and efficiency of traditional methods.
The cost-effectiveness of ethyl acetate solutions presents another hurdle. Although ethyl acetate is generally less expensive than some alternative solvents, implementing sustainable production methods and meeting stringent environmental regulations can increase overall costs. This economic factor can deter widespread adoption, particularly in industries where profit margins are slim.
Regulatory compliance poses an additional challenge. As environmental regulations become more stringent globally, the use of ethyl acetate, despite its relative safety, may face increasing scrutiny. Companies must navigate a complex landscape of local, national, and international regulations, which can vary significantly across different regions.
The performance characteristics of ethyl acetate in certain applications can also be limiting. While it excels in many areas, such as its use as a solvent in coatings and adhesives, it may not be suitable for all technological applications. Its relatively low boiling point and high volatility can be disadvantageous in some processes, requiring the development of specialized formulations or application techniques.
Lastly, the challenge of perception and market acceptance cannot be overlooked. Despite its potential as a more sustainable option, ethyl acetate still faces competition from both traditional solvents and newer, marketed as "green" alternatives. Overcoming entrenched practices and convincing stakeholders to switch to ethyl acetate-based solutions requires substantial education and demonstration of its benefits.
Addressing these challenges requires a multifaceted approach, combining technological innovation, policy support, and market education. Developing more sustainable production methods, improving application techniques, and demonstrating the long-term environmental and economic benefits of ethyl acetate solutions are crucial steps in overcoming these obstacles and driving sustainable technology forward.
Current Ethyl Acetate Applications
01 Purification and separation processes
Ethyl acetate solutions are commonly used in purification and separation processes. These processes may involve distillation, extraction, or other techniques to isolate specific compounds or remove impurities. The high volatility and solvent properties of ethyl acetate make it suitable for these applications in various industries.- Purification and separation processes: Ethyl acetate solutions are commonly used in purification and separation processes. These processes may involve distillation, extraction, or other techniques to isolate specific compounds or remove impurities. The use of ethyl acetate as a solvent in these processes can be advantageous due to its relatively low boiling point and good solvency properties.
- Synthesis and chemical reactions: Ethyl acetate solutions play a role in various synthesis and chemical reaction processes. They can serve as reaction media, solvents for reactants, or be involved in the formation of specific compounds. The use of ethyl acetate in these applications can influence reaction kinetics, yield, and product purity.
- Extraction of natural products: Ethyl acetate solutions are utilized in the extraction of natural products from plant materials or other sources. This process can involve the isolation of essential oils, flavors, fragrances, or other valuable compounds. The solvent properties of ethyl acetate make it suitable for extracting a wide range of organic compounds.
- Formulation of coatings and adhesives: Ethyl acetate solutions are used in the formulation of various coatings and adhesives. The solvent properties of ethyl acetate allow for the dissolution of resins, polymers, and other components used in these products. Its relatively fast evaporation rate can contribute to the drying and curing processes of coatings and adhesives.
- Cleaning and degreasing applications: Ethyl acetate solutions find use in cleaning and degreasing applications across various industries. The solvent's ability to dissolve a wide range of organic compounds makes it effective for removing oils, greases, and other contaminants from surfaces. It can be used in both industrial and consumer cleaning products.
02 Synthesis and chemical reactions
Ethyl acetate solutions play a role in various chemical synthesis processes and reactions. They can serve as a solvent medium for organic reactions, esterification processes, or as a reactant itself. The use of ethyl acetate in these applications can influence reaction rates, yields, and product purity.Expand Specific Solutions03 Pharmaceutical and cosmetic applications
Ethyl acetate solutions find applications in the pharmaceutical and cosmetic industries. They can be used in the formulation of drugs, extraction of active ingredients from natural sources, or as a component in cosmetic products. The solvent properties of ethyl acetate make it useful for dissolving various organic compounds used in these industries.Expand Specific Solutions04 Industrial cleaning and degreasing
Ethyl acetate solutions are employed in industrial cleaning and degreasing processes. Their ability to dissolve oils, greases, and other organic contaminants makes them effective for cleaning metal surfaces, electronic components, and other industrial equipment. These solutions can be used in various concentrations depending on the specific cleaning requirements.Expand Specific Solutions05 Environmental and safety considerations
The use of ethyl acetate solutions requires consideration of environmental and safety aspects. This includes proper handling, storage, and disposal methods to minimize environmental impact and ensure worker safety. Additionally, research into alternative solvents or processes to reduce the use of volatile organic compounds (VOCs) like ethyl acetate is ongoing in various industries.Expand Specific Solutions
Key Ethyl Acetate Producers
The sustainable technology solutions using ethyl acetate are in a growth phase, with increasing market size driven by environmental concerns and regulatory pressures. The global market for green solvents, including ethyl acetate, is projected to expand significantly in the coming years. Technologically, the field is advancing rapidly, with major players like Celanese International Corp., China Petroleum & Chemical Corp., and Eastman Chemical Co. leading innovation. These companies are developing more efficient and sustainable production methods for ethyl acetate, focusing on bio-based feedstocks and improved catalytic processes. Academic institutions such as the University of Campinas and Nanjing Tech University are contributing to research and development, particularly in areas of green chemistry and process optimization.
Celanese International Corp.
Technical Solution: Celanese has developed a sustainable ethyl acetate production process using bioethanol as a feedstock. Their technology involves a two-step reaction: first, the dehydrogenation of ethanol to acetaldehyde, followed by the Tishchenko reaction to form ethyl acetate. This process reduces carbon footprint by up to 50% compared to traditional fossil-based methods[1]. The company has also implemented advanced catalysts and process optimizations to improve yield and energy efficiency, achieving over 95% selectivity for ethyl acetate[3].
Strengths: Significantly reduced carbon footprint, high selectivity, and use of renewable feedstock. Weaknesses: Potential higher production costs compared to conventional methods and dependence on bioethanol availability.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to ethyl acetate production using a coupled process of methanol carbonylation and hydrogenation. This method utilizes syngas (CO + H2) derived from coal or natural gas as the primary feedstock. The process involves first producing acetic acid through methanol carbonylation, followed by its hydrogenation to ethanol, which is then esterified with the remaining acetic acid to form ethyl acetate[2]. Sinopec has optimized this process to achieve high conversion rates and selectivity, with reported yields of over 90%[4].
Strengths: Utilizes abundant coal or natural gas resources, high yield, and integration with existing petrochemical infrastructure. Weaknesses: Still relies on fossil fuels, potentially higher carbon footprint compared to bio-based methods.
Innovative Ethyl Acetate Research
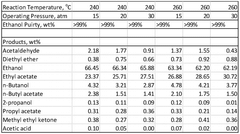
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
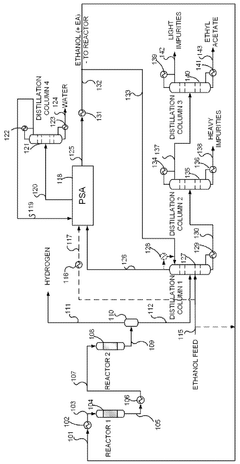
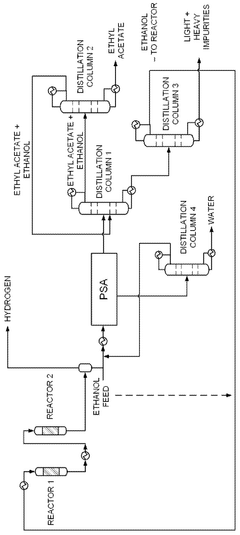
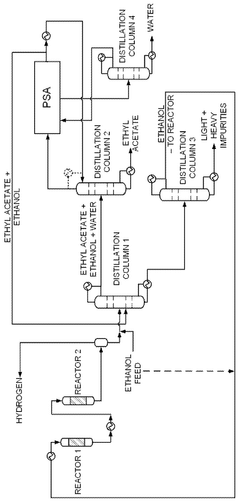
Integrated system for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, integrated process for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, and products thereby produced
PatentWO2013029129A1
Innovation
- An integrated system utilizing a fixed-bed reactor with a calcined hydrotalcite catalyst for dehydrogenation and dehydration of ethanol, followed by a series of distillation columns for efficient separation and purification, employing ethylene glycol as a solvent to separate ethyl acetate from water, thereby reducing energy expenditure and minimizing azeotropy issues.
Environmental Impact Assessment
The environmental impact assessment of ethyl acetate solutions in driving sustainable technology is a critical aspect that requires thorough examination. Ethyl acetate, a widely used solvent in various industries, presents both opportunities and challenges in terms of environmental sustainability.
One of the primary environmental benefits of ethyl acetate is its biodegradability. Unlike many other solvents, ethyl acetate breaks down relatively quickly in the environment, reducing long-term pollution risks. This characteristic makes it an attractive option for industries seeking to minimize their ecological footprint. Additionally, ethyl acetate is not considered a volatile organic compound (VOC) in many jurisdictions, which can contribute to improved air quality in manufacturing settings.
However, the production and use of ethyl acetate are not without environmental concerns. The manufacturing process typically involves the esterification of ethanol and acetic acid, both of which are derived from petrochemical sources. This reliance on fossil fuels raises questions about the overall sustainability of ethyl acetate production. To address this issue, research into bio-based ethyl acetate production methods, using renewable feedstocks, is gaining traction.
Water pollution is another potential environmental risk associated with ethyl acetate. Although it is less toxic than many alternative solvents, improper disposal or accidental spills can still impact aquatic ecosystems. Implementing robust waste management and spill prevention protocols is essential for mitigating these risks.
Energy consumption during the production and purification of ethyl acetate also contributes to its environmental impact. The distillation process required to achieve high-purity ethyl acetate is energy-intensive, leading to increased carbon emissions. Innovations in energy-efficient production methods and the use of renewable energy sources in manufacturing facilities can help reduce this carbon footprint.
When considering the lifecycle assessment of ethyl acetate solutions, it's important to evaluate their performance in comparison to alternative technologies. In many applications, ethyl acetate-based solutions can offer improved efficiency and reduced waste generation, potentially offsetting some of the environmental impacts associated with their production.
The recyclability of ethyl acetate is another factor that enhances its environmental profile. With proper recovery and purification systems in place, a significant portion of the solvent can be reclaimed and reused, reducing the need for continuous production of new material. This closed-loop approach aligns well with circular economy principles and can substantially decrease the overall environmental impact of ethyl acetate use in sustainable technologies.
One of the primary environmental benefits of ethyl acetate is its biodegradability. Unlike many other solvents, ethyl acetate breaks down relatively quickly in the environment, reducing long-term pollution risks. This characteristic makes it an attractive option for industries seeking to minimize their ecological footprint. Additionally, ethyl acetate is not considered a volatile organic compound (VOC) in many jurisdictions, which can contribute to improved air quality in manufacturing settings.
However, the production and use of ethyl acetate are not without environmental concerns. The manufacturing process typically involves the esterification of ethanol and acetic acid, both of which are derived from petrochemical sources. This reliance on fossil fuels raises questions about the overall sustainability of ethyl acetate production. To address this issue, research into bio-based ethyl acetate production methods, using renewable feedstocks, is gaining traction.
Water pollution is another potential environmental risk associated with ethyl acetate. Although it is less toxic than many alternative solvents, improper disposal or accidental spills can still impact aquatic ecosystems. Implementing robust waste management and spill prevention protocols is essential for mitigating these risks.
Energy consumption during the production and purification of ethyl acetate also contributes to its environmental impact. The distillation process required to achieve high-purity ethyl acetate is energy-intensive, leading to increased carbon emissions. Innovations in energy-efficient production methods and the use of renewable energy sources in manufacturing facilities can help reduce this carbon footprint.
When considering the lifecycle assessment of ethyl acetate solutions, it's important to evaluate their performance in comparison to alternative technologies. In many applications, ethyl acetate-based solutions can offer improved efficiency and reduced waste generation, potentially offsetting some of the environmental impacts associated with their production.
The recyclability of ethyl acetate is another factor that enhances its environmental profile. With proper recovery and purification systems in place, a significant portion of the solvent can be reclaimed and reused, reducing the need for continuous production of new material. This closed-loop approach aligns well with circular economy principles and can substantially decrease the overall environmental impact of ethyl acetate use in sustainable technologies.
Circular Economy Integration
Integrating ethyl acetate solutions into the circular economy framework presents a significant opportunity for driving sustainable technology. This approach aligns with the principles of resource efficiency, waste reduction, and environmental preservation. By implementing circular economy strategies, the ethyl acetate industry can minimize its ecological footprint while maximizing economic value.
One key aspect of circular economy integration for ethyl acetate solutions is the development of closed-loop production systems. These systems aim to recapture and reuse ethyl acetate throughout the manufacturing process, reducing the need for virgin materials and minimizing waste generation. Advanced distillation and purification techniques can be employed to recover ethyl acetate from waste streams, enabling its reintroduction into the production cycle.
Another crucial element is the design of products and processes that facilitate easy disassembly and material recovery at the end of their lifecycle. This approach, known as "design for circularity," ensures that ethyl acetate-based products can be efficiently recycled or repurposed, extending their useful life and reducing the demand for new production. Collaborations between manufacturers, designers, and recycling facilities are essential to optimize these circular design strategies.
The adoption of bio-based feedstocks for ethyl acetate production represents a significant step towards circular economy integration. By utilizing renewable resources such as agricultural waste or sustainably sourced biomass, the industry can reduce its reliance on fossil-based raw materials. This shift not only decreases the carbon footprint of ethyl acetate production but also creates new value streams for agricultural and forestry sectors.
Implementing digital technologies and data analytics can enhance the efficiency of circular economy practices in the ethyl acetate industry. Advanced monitoring systems and predictive maintenance algorithms can optimize resource use, minimize waste, and extend equipment lifespan. Additionally, blockchain technology can improve supply chain transparency, enabling better tracking of materials and facilitating the creation of circular economy networks.
Collaboration and partnerships across the value chain are crucial for successful circular economy integration. By fostering relationships between suppliers, manufacturers, consumers, and waste management entities, the ethyl acetate industry can create robust circular ecosystems. These partnerships can lead to innovative solutions for material recovery, shared infrastructure for recycling, and the development of new business models that prioritize resource efficiency and sustainability.
Policy support and regulatory frameworks play a vital role in accelerating the transition towards a circular economy for ethyl acetate solutions. Governments can incentivize circular practices through tax benefits, grants, or preferential procurement policies. Additionally, standardization of circular economy metrics and reporting can help businesses quantify and communicate their progress, driving further adoption of sustainable practices across the industry.
One key aspect of circular economy integration for ethyl acetate solutions is the development of closed-loop production systems. These systems aim to recapture and reuse ethyl acetate throughout the manufacturing process, reducing the need for virgin materials and minimizing waste generation. Advanced distillation and purification techniques can be employed to recover ethyl acetate from waste streams, enabling its reintroduction into the production cycle.
Another crucial element is the design of products and processes that facilitate easy disassembly and material recovery at the end of their lifecycle. This approach, known as "design for circularity," ensures that ethyl acetate-based products can be efficiently recycled or repurposed, extending their useful life and reducing the demand for new production. Collaborations between manufacturers, designers, and recycling facilities are essential to optimize these circular design strategies.
The adoption of bio-based feedstocks for ethyl acetate production represents a significant step towards circular economy integration. By utilizing renewable resources such as agricultural waste or sustainably sourced biomass, the industry can reduce its reliance on fossil-based raw materials. This shift not only decreases the carbon footprint of ethyl acetate production but also creates new value streams for agricultural and forestry sectors.
Implementing digital technologies and data analytics can enhance the efficiency of circular economy practices in the ethyl acetate industry. Advanced monitoring systems and predictive maintenance algorithms can optimize resource use, minimize waste, and extend equipment lifespan. Additionally, blockchain technology can improve supply chain transparency, enabling better tracking of materials and facilitating the creation of circular economy networks.
Collaboration and partnerships across the value chain are crucial for successful circular economy integration. By fostering relationships between suppliers, manufacturers, consumers, and waste management entities, the ethyl acetate industry can create robust circular ecosystems. These partnerships can lead to innovative solutions for material recovery, shared infrastructure for recycling, and the development of new business models that prioritize resource efficiency and sustainability.
Policy support and regulatory frameworks play a vital role in accelerating the transition towards a circular economy for ethyl acetate solutions. Governments can incentivize circular practices through tax benefits, grants, or preferential procurement policies. Additionally, standardization of circular economy metrics and reporting can help businesses quantify and communicate their progress, driving further adoption of sustainable practices across the industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!