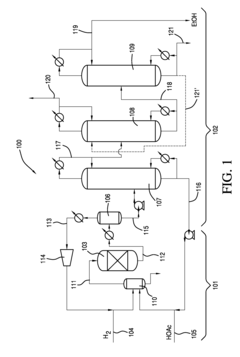
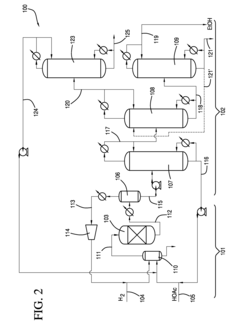
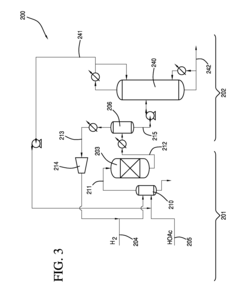
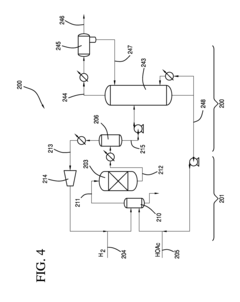
How to Improve Ethyl Acetate Extraction Methods?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Extraction Background and Objectives
Ethyl acetate extraction has been a cornerstone technique in chemical and pharmaceutical industries for decades. This method, which leverages the unique properties of ethyl acetate as a solvent, has proven invaluable in separating and purifying various organic compounds. The evolution of this extraction technique can be traced back to the early 20th century, with significant advancements occurring in the latter half of the century as industrial demands grew.
The primary objective in improving ethyl acetate extraction methods is to enhance efficiency, reduce environmental impact, and increase the purity of extracted compounds. These goals are driven by the increasing demands of industries such as pharmaceuticals, food processing, and fine chemicals, where high-purity products and cost-effective processes are paramount.
Recent technological trends in ethyl acetate extraction focus on optimizing solvent usage, minimizing waste generation, and improving extraction yields. Innovations in this field are closely tied to advancements in green chemistry principles, aiming to develop more sustainable and environmentally friendly extraction processes. This includes exploring novel extraction techniques such as microwave-assisted extraction and ultrasound-assisted extraction, which can potentially reduce solvent consumption and extraction time.
Another significant trend is the integration of automation and process control systems in ethyl acetate extraction. This shift towards smart extraction processes allows for real-time monitoring and adjustment of extraction parameters, leading to more consistent and higher-quality outputs. The incorporation of machine learning algorithms for process optimization is an emerging area that holds promise for further improvements in extraction efficiency.
The global push towards sustainability has also spurred research into bio-based alternatives to traditional ethyl acetate, derived from renewable resources. This aligns with the broader objective of developing greener extraction methods that maintain or surpass the effectiveness of conventional techniques while reducing the carbon footprint of the extraction process.
As we look towards the future of ethyl acetate extraction, the technical objectives are multifaceted. They include developing more selective extraction methods to improve compound purity, designing scalable and energy-efficient extraction systems, and creating closed-loop processes that minimize solvent loss and environmental impact. Additionally, there is a growing interest in combining ethyl acetate extraction with other separation techniques to create hybrid systems that offer superior performance in complex separation challenges.
The primary objective in improving ethyl acetate extraction methods is to enhance efficiency, reduce environmental impact, and increase the purity of extracted compounds. These goals are driven by the increasing demands of industries such as pharmaceuticals, food processing, and fine chemicals, where high-purity products and cost-effective processes are paramount.
Recent technological trends in ethyl acetate extraction focus on optimizing solvent usage, minimizing waste generation, and improving extraction yields. Innovations in this field are closely tied to advancements in green chemistry principles, aiming to develop more sustainable and environmentally friendly extraction processes. This includes exploring novel extraction techniques such as microwave-assisted extraction and ultrasound-assisted extraction, which can potentially reduce solvent consumption and extraction time.
Another significant trend is the integration of automation and process control systems in ethyl acetate extraction. This shift towards smart extraction processes allows for real-time monitoring and adjustment of extraction parameters, leading to more consistent and higher-quality outputs. The incorporation of machine learning algorithms for process optimization is an emerging area that holds promise for further improvements in extraction efficiency.
The global push towards sustainability has also spurred research into bio-based alternatives to traditional ethyl acetate, derived from renewable resources. This aligns with the broader objective of developing greener extraction methods that maintain or surpass the effectiveness of conventional techniques while reducing the carbon footprint of the extraction process.
As we look towards the future of ethyl acetate extraction, the technical objectives are multifaceted. They include developing more selective extraction methods to improve compound purity, designing scalable and energy-efficient extraction systems, and creating closed-loop processes that minimize solvent loss and environmental impact. Additionally, there is a growing interest in combining ethyl acetate extraction with other separation techniques to create hybrid systems that offer superior performance in complex separation challenges.
Market Analysis for Ethyl Acetate Extraction Applications
The global market for ethyl acetate extraction applications has been experiencing steady growth, driven by increasing demand across various industries. The pharmaceutical sector remains a key consumer, utilizing ethyl acetate extraction in the production of active pharmaceutical ingredients and drug formulations. This industry's expansion, particularly in emerging markets, is expected to fuel further demand for improved extraction methods.
In the food and beverage industry, ethyl acetate extraction is widely used for flavor and fragrance extraction, as well as in the decaffeination of coffee and tea. With consumers increasingly seeking natural and clean-label products, there is a growing need for more efficient and environmentally friendly extraction processes. This trend is likely to drive innovation in ethyl acetate extraction methods, focusing on reducing solvent consumption and improving product purity.
The cosmetics and personal care industry also represents a significant market for ethyl acetate extraction, particularly in the production of natural extracts and essential oils. As the global cosmetics market continues to expand, driven by rising disposable incomes and changing consumer preferences, the demand for advanced extraction techniques is expected to grow in parallel.
Environmental concerns and stringent regulations regarding solvent use and waste management are shaping market dynamics. This has led to increased interest in green extraction technologies and solvent recycling systems, creating opportunities for companies that can develop more sustainable ethyl acetate extraction methods.
The Asia-Pacific region is emerging as a key growth market for ethyl acetate extraction applications, driven by rapid industrialization, increasing manufacturing activities, and growing consumer markets. China and India, in particular, are expected to be major contributors to market growth, with their expanding pharmaceutical and chemical industries.
In terms of market challenges, fluctuations in raw material prices, particularly ethanol and acetic acid used in ethyl acetate production, can impact the overall cost-effectiveness of extraction processes. Additionally, the availability of alternative solvents and extraction methods poses a competitive threat to traditional ethyl acetate extraction techniques.
The COVID-19 pandemic has had a mixed impact on the market. While it initially disrupted supply chains and manufacturing activities, it has also accelerated demand in certain sectors, such as pharmaceuticals and sanitizers, which rely on ethyl acetate extraction processes.
Looking ahead, the market for ethyl acetate extraction applications is poised for growth, with a focus on developing more efficient, sustainable, and cost-effective extraction methods. Companies that can innovate in these areas are likely to gain a competitive edge in this evolving market landscape.
In the food and beverage industry, ethyl acetate extraction is widely used for flavor and fragrance extraction, as well as in the decaffeination of coffee and tea. With consumers increasingly seeking natural and clean-label products, there is a growing need for more efficient and environmentally friendly extraction processes. This trend is likely to drive innovation in ethyl acetate extraction methods, focusing on reducing solvent consumption and improving product purity.
The cosmetics and personal care industry also represents a significant market for ethyl acetate extraction, particularly in the production of natural extracts and essential oils. As the global cosmetics market continues to expand, driven by rising disposable incomes and changing consumer preferences, the demand for advanced extraction techniques is expected to grow in parallel.
Environmental concerns and stringent regulations regarding solvent use and waste management are shaping market dynamics. This has led to increased interest in green extraction technologies and solvent recycling systems, creating opportunities for companies that can develop more sustainable ethyl acetate extraction methods.
The Asia-Pacific region is emerging as a key growth market for ethyl acetate extraction applications, driven by rapid industrialization, increasing manufacturing activities, and growing consumer markets. China and India, in particular, are expected to be major contributors to market growth, with their expanding pharmaceutical and chemical industries.
In terms of market challenges, fluctuations in raw material prices, particularly ethanol and acetic acid used in ethyl acetate production, can impact the overall cost-effectiveness of extraction processes. Additionally, the availability of alternative solvents and extraction methods poses a competitive threat to traditional ethyl acetate extraction techniques.
The COVID-19 pandemic has had a mixed impact on the market. While it initially disrupted supply chains and manufacturing activities, it has also accelerated demand in certain sectors, such as pharmaceuticals and sanitizers, which rely on ethyl acetate extraction processes.
Looking ahead, the market for ethyl acetate extraction applications is poised for growth, with a focus on developing more efficient, sustainable, and cost-effective extraction methods. Companies that can innovate in these areas are likely to gain a competitive edge in this evolving market landscape.
Current Challenges in Ethyl Acetate Extraction Techniques
Ethyl acetate extraction is a widely used technique in various industries, including pharmaceuticals, food processing, and chemical manufacturing. However, despite its prevalence, several challenges persist in current extraction methods, hindering efficiency and overall performance.
One of the primary challenges is the relatively low extraction efficiency of ethyl acetate for certain compounds. This limitation often necessitates multiple extraction cycles or larger solvent volumes, leading to increased processing time and higher solvent consumption. The issue is particularly pronounced when dealing with complex matrices or compounds with low partition coefficients in ethyl acetate.
Another significant challenge is the formation of emulsions during the extraction process. Emulsions can severely impede phase separation, resulting in longer settling times and potential loss of target compounds. This problem is especially prevalent when extracting from aqueous solutions containing surfactants or high concentrations of proteins.
The volatility of ethyl acetate poses additional challenges in extraction processes. Its low boiling point (77.1°C) can lead to significant solvent loss during extraction, particularly in open systems or at elevated temperatures. This not only increases operational costs but also raises environmental and safety concerns due to increased volatile organic compound (VOC) emissions.
Selectivity issues present another hurdle in ethyl acetate extraction. While ethyl acetate is an excellent solvent for many organic compounds, it may also co-extract unwanted substances, necessitating additional purification steps. This lack of selectivity can be particularly problematic in applications requiring high-purity extracts, such as in the pharmaceutical industry.
The potential for hydrolysis of ethyl acetate in aqueous environments, especially under acidic or basic conditions, can lead to the formation of acetic acid and ethanol. This degradation not only reduces extraction efficiency but can also introduce impurities into the final product, complicating downstream processing and potentially affecting product quality.
Scale-up challenges are also significant in industrial applications of ethyl acetate extraction. Maintaining consistent extraction efficiency and phase separation dynamics when transitioning from laboratory to industrial scale can be difficult, often requiring substantial process optimization and equipment redesign.
Lastly, the environmental impact of ethyl acetate extraction methods is an increasing concern. While ethyl acetate is considered less toxic compared to many other organic solvents, its production and disposal still contribute to environmental pollution. The need for more sustainable and environmentally friendly extraction processes is driving research into alternative solvents and extraction techniques.
One of the primary challenges is the relatively low extraction efficiency of ethyl acetate for certain compounds. This limitation often necessitates multiple extraction cycles or larger solvent volumes, leading to increased processing time and higher solvent consumption. The issue is particularly pronounced when dealing with complex matrices or compounds with low partition coefficients in ethyl acetate.
Another significant challenge is the formation of emulsions during the extraction process. Emulsions can severely impede phase separation, resulting in longer settling times and potential loss of target compounds. This problem is especially prevalent when extracting from aqueous solutions containing surfactants or high concentrations of proteins.
The volatility of ethyl acetate poses additional challenges in extraction processes. Its low boiling point (77.1°C) can lead to significant solvent loss during extraction, particularly in open systems or at elevated temperatures. This not only increases operational costs but also raises environmental and safety concerns due to increased volatile organic compound (VOC) emissions.
Selectivity issues present another hurdle in ethyl acetate extraction. While ethyl acetate is an excellent solvent for many organic compounds, it may also co-extract unwanted substances, necessitating additional purification steps. This lack of selectivity can be particularly problematic in applications requiring high-purity extracts, such as in the pharmaceutical industry.
The potential for hydrolysis of ethyl acetate in aqueous environments, especially under acidic or basic conditions, can lead to the formation of acetic acid and ethanol. This degradation not only reduces extraction efficiency but can also introduce impurities into the final product, complicating downstream processing and potentially affecting product quality.
Scale-up challenges are also significant in industrial applications of ethyl acetate extraction. Maintaining consistent extraction efficiency and phase separation dynamics when transitioning from laboratory to industrial scale can be difficult, often requiring substantial process optimization and equipment redesign.
Lastly, the environmental impact of ethyl acetate extraction methods is an increasing concern. While ethyl acetate is considered less toxic compared to many other organic solvents, its production and disposal still contribute to environmental pollution. The need for more sustainable and environmentally friendly extraction processes is driving research into alternative solvents and extraction techniques.
Existing Ethyl Acetate Extraction Methodologies
01 Optimization of extraction conditions
Improving ethyl acetate extraction efficiency involves optimizing various parameters such as temperature, pressure, solvent-to-feed ratio, and extraction time. These factors significantly influence the yield and quality of the extracted compounds. Careful adjustment of these conditions can lead to enhanced extraction efficiency and improved product quality.- Optimization of extraction conditions: Improving ethyl acetate extraction efficiency involves optimizing various parameters such as temperature, pressure, solvent-to-feed ratio, and extraction time. These factors significantly influence the yield and quality of the extracted compounds. Careful adjustment of these conditions can lead to enhanced extraction efficiency and reduced solvent consumption.
- Use of ultrasonic-assisted extraction: Ultrasonic-assisted extraction can significantly improve the efficiency of ethyl acetate extraction. The ultrasonic waves create cavitation bubbles that enhance mass transfer and cell wall disruption, leading to faster and more complete extraction of target compounds. This method can reduce extraction time and solvent consumption while increasing yield.
- Microwave-assisted extraction technique: Microwave-assisted extraction is an effective method to enhance ethyl acetate extraction efficiency. The microwave energy rapidly heats the solvent and sample, causing cell rupture and improving the release of target compounds. This technique can significantly reduce extraction time and increase yield compared to conventional methods.
- Continuous flow extraction systems: Implementing continuous flow extraction systems can improve the efficiency of ethyl acetate extraction. These systems allow for better control of extraction parameters, increased mass transfer, and reduced solvent consumption. Continuous flow extraction can lead to higher yields and improved product quality compared to batch extraction methods.
- Solvent recycling and purification: Incorporating solvent recycling and purification systems can enhance the overall efficiency of ethyl acetate extraction processes. These systems allow for the recovery and reuse of ethyl acetate, reducing solvent consumption and operational costs. Additionally, purification steps ensure the quality of the recycled solvent, maintaining extraction efficiency over multiple cycles.
02 Use of advanced extraction techniques
Employing advanced extraction techniques like ultrasound-assisted extraction, microwave-assisted extraction, or supercritical fluid extraction can significantly improve the efficiency of ethyl acetate extraction. These methods can reduce extraction time, increase yield, and potentially improve the quality of extracted compounds compared to traditional methods.Expand Specific Solutions03 Solvent recycling and purification
Implementing solvent recycling and purification systems can enhance the overall efficiency of the ethyl acetate extraction process. This approach not only reduces solvent consumption and waste but also ensures consistent extraction quality by maintaining solvent purity. Techniques such as distillation or membrane separation can be used for solvent recovery.Expand Specific Solutions04 Pre-treatment of raw materials
Pre-treating raw materials before ethyl acetate extraction can significantly improve extraction efficiency. Methods such as grinding, drying, or enzymatic treatment can increase the surface area of the material, break down cell walls, or modify the matrix, facilitating better solvent penetration and enhancing the release of target compounds.Expand Specific Solutions05 Continuous extraction systems
Implementing continuous extraction systems, as opposed to batch processes, can improve the overall efficiency of ethyl acetate extraction. Continuous systems allow for better control of extraction parameters, reduced processing time, and potentially higher yields. This approach is particularly beneficial for large-scale industrial applications.Expand Specific Solutions
Key Players in Ethyl Acetate Extraction Industry
The ethyl acetate extraction methods market is in a mature stage, with a global market size estimated to be in the billions of dollars. The technology is well-established, with ongoing research focused on improving efficiency and sustainability. Key players like Celanese International Corp., Rhodia Operations SASU, and Johnson Matthey Davy Technologies Ltd. are driving innovation in this field. Emerging companies such as Viridis Chemical LLC are introducing bio-based alternatives, indicating a shift towards more environmentally friendly processes. Academic institutions like National Taiwan University and South China University of Technology are contributing to research and development, further advancing the technology. The competitive landscape is characterized by a mix of established chemical companies and innovative startups, with a growing emphasis on sustainable extraction methods.
Celanese International Corp.
Technical Solution: Celanese has developed an advanced ethyl acetate extraction method using a proprietary solvent system. This system employs a combination of ethyl acetate and a co-solvent to enhance extraction efficiency. The process utilizes a multi-stage counter-current extraction setup, which maximizes the contact between the solvent and the target compounds. Celanese has also implemented a novel membrane-based separation technique to purify the extracted product, reducing energy consumption in the distillation step. The company has integrated process intensification strategies, such as microreactor technology, to improve mass transfer and reaction kinetics[1][3]. Additionally, Celanese has developed a green chemistry approach, using bio-based feedstocks for ethyl acetate production, which aligns with sustainability goals[2].
Strengths: High extraction efficiency, reduced energy consumption, improved product purity, and sustainable production. Weaknesses: Potentially higher initial capital costs and the need for specialized equipment.
Johnson Matthey Davy Technologies Ltd.
Technical Solution: Johnson Matthey Davy Technologies has innovated in ethyl acetate extraction by developing a catalytic distillation process. This method combines reaction and separation in a single unit operation, significantly improving process efficiency. The company's approach uses a heterogeneous catalyst fixed in the distillation column, allowing for continuous production and separation of ethyl acetate. The process operates at lower temperatures compared to conventional methods, reducing energy requirements[4]. Johnson Matthey has also implemented advanced process control systems, utilizing real-time analytics and machine learning algorithms to optimize extraction parameters dynamically. Their technology incorporates a heat integration network, recovering and reusing thermal energy throughout the process[5].
Strengths: Improved process efficiency, reduced energy consumption, continuous operation, and advanced process control. Weaknesses: Complexity in catalyst management and potential limitations in handling certain feedstocks.
Innovative Approaches in Ethyl Acetate Extraction
Process for producing an ethyl acetate solvent and co-production of ethanol
PatentInactiveUS20110190531A1
Innovation
- A process involving the hydrogenation of acetic acid in the presence of a catalyst, followed by a series of distillation columns to separate and recover ethanol and ethyl acetate solvent, with specific catalyst compositions and conditions to optimize ethanol and ethyl acetate production, including the use of platinum-based catalysts and modified silica supports.
System and method for rapid extraction and purification of ethyl acetate after pressurized esterification synthesis
PatentActiveKR1020210050511A
Innovation
- A system comprising a reaction rectifying column, purification column, deoxidation column, and recovery column, utilizing demineralized water for extraction and purification, along with controlled pressures and temperatures, to enhance the extraction and purification of ethyl acetate.
Environmental Impact of Ethyl Acetate Extraction Processes
The environmental impact of ethyl acetate extraction processes is a critical consideration in the quest to improve extraction methods. Ethyl acetate, while widely used in various industries, poses several environmental challenges that need to be addressed.
One of the primary concerns is the volatile organic compound (VOC) emissions associated with ethyl acetate use. These emissions contribute to air pollution and can lead to the formation of ground-level ozone, which has detrimental effects on human health and ecosystems. Industrial facilities using ethyl acetate extraction methods must implement robust emission control systems to mitigate these impacts.
Water pollution is another significant issue. Ethyl acetate, if not properly managed, can contaminate water sources through spills or improper disposal. This contamination can harm aquatic life and potentially affect drinking water supplies. Implementing closed-loop systems and efficient wastewater treatment processes is crucial to minimize water pollution risks.
The production and disposal of ethyl acetate also have environmental implications. The manufacturing process requires energy and resources, contributing to carbon emissions and resource depletion. End-of-life considerations for ethyl acetate and its byproducts necessitate proper disposal methods to prevent environmental contamination.
Biodegradability is a key factor in assessing the long-term environmental impact of ethyl acetate. While it is considered more biodegradable than some other solvents, its persistence in the environment can still be problematic. Research into enhancing the biodegradability of ethyl acetate or developing more environmentally friendly alternatives is ongoing.
The potential for accidental releases and their environmental consequences must also be considered. Proper storage, handling, and transportation protocols are essential to prevent spills that could lead to soil and groundwater contamination.
To improve ethyl acetate extraction methods from an environmental perspective, several strategies can be employed. These include developing more efficient extraction processes that reduce solvent consumption, implementing advanced recycling and recovery systems to minimize waste, and exploring greener alternatives that maintain extraction efficacy while reducing environmental impact.
Regulatory compliance and adherence to environmental standards play a crucial role in mitigating the environmental impact of ethyl acetate extraction processes. Industries must stay abreast of evolving regulations and proactively implement best practices to ensure sustainable operations.
One of the primary concerns is the volatile organic compound (VOC) emissions associated with ethyl acetate use. These emissions contribute to air pollution and can lead to the formation of ground-level ozone, which has detrimental effects on human health and ecosystems. Industrial facilities using ethyl acetate extraction methods must implement robust emission control systems to mitigate these impacts.
Water pollution is another significant issue. Ethyl acetate, if not properly managed, can contaminate water sources through spills or improper disposal. This contamination can harm aquatic life and potentially affect drinking water supplies. Implementing closed-loop systems and efficient wastewater treatment processes is crucial to minimize water pollution risks.
The production and disposal of ethyl acetate also have environmental implications. The manufacturing process requires energy and resources, contributing to carbon emissions and resource depletion. End-of-life considerations for ethyl acetate and its byproducts necessitate proper disposal methods to prevent environmental contamination.
Biodegradability is a key factor in assessing the long-term environmental impact of ethyl acetate. While it is considered more biodegradable than some other solvents, its persistence in the environment can still be problematic. Research into enhancing the biodegradability of ethyl acetate or developing more environmentally friendly alternatives is ongoing.
The potential for accidental releases and their environmental consequences must also be considered. Proper storage, handling, and transportation protocols are essential to prevent spills that could lead to soil and groundwater contamination.
To improve ethyl acetate extraction methods from an environmental perspective, several strategies can be employed. These include developing more efficient extraction processes that reduce solvent consumption, implementing advanced recycling and recovery systems to minimize waste, and exploring greener alternatives that maintain extraction efficacy while reducing environmental impact.
Regulatory compliance and adherence to environmental standards play a crucial role in mitigating the environmental impact of ethyl acetate extraction processes. Industries must stay abreast of evolving regulations and proactively implement best practices to ensure sustainable operations.
Safety Considerations in Ethyl Acetate Extraction
Safety considerations are paramount in ethyl acetate extraction processes due to the chemical's flammable and potentially hazardous nature. Proper handling and storage of ethyl acetate are crucial to prevent accidents and ensure worker safety. The compound has a low flash point of -4°C (25°F), making it highly flammable and prone to ignition from static electricity or open flames. Therefore, extraction processes must be conducted in well-ventilated areas with appropriate fire suppression systems in place.
Personal protective equipment (PPE) is essential for workers involved in ethyl acetate extraction. This includes chemical-resistant gloves, safety goggles, and respiratory protection when necessary. Proper training on the safe handling of ethyl acetate and emergency procedures should be provided to all personnel involved in the extraction process.
The storage of ethyl acetate requires special attention. It should be kept in tightly sealed containers in a cool, dry place away from sources of heat or ignition. Proper grounding and bonding procedures must be followed during transfer operations to prevent the buildup of static electricity, which could lead to fires or explosions.
Exposure to ethyl acetate vapors can cause irritation to the eyes, nose, and throat, as well as headaches and dizziness. Therefore, adequate ventilation is crucial in extraction areas to maintain airborne concentrations below the permissible exposure limits set by regulatory agencies such as OSHA. Regular air monitoring should be conducted to ensure compliance with these limits.
Spill response procedures should be established and regularly practiced. In the event of a spill, the area should be immediately evacuated, and only trained personnel with appropriate PPE should handle the cleanup. Absorbent materials specifically designed for organic solvents should be used, and contaminated materials must be disposed of as hazardous waste.
Environmental considerations are also important in ethyl acetate extraction. The compound can be harmful to aquatic life and should not be released into the environment. Proper waste management procedures, including the use of sealed containers and approved disposal methods, must be implemented to prevent environmental contamination.
Regular maintenance and inspection of extraction equipment are essential to prevent leaks and ensure the integrity of the system. This includes checking seals, valves, and connections for signs of wear or damage. Any issues should be promptly addressed to maintain safe operating conditions.
By implementing these safety measures and continuously educating workers on the potential hazards associated with ethyl acetate extraction, the risks can be significantly mitigated, leading to a safer and more efficient extraction process.
Personal protective equipment (PPE) is essential for workers involved in ethyl acetate extraction. This includes chemical-resistant gloves, safety goggles, and respiratory protection when necessary. Proper training on the safe handling of ethyl acetate and emergency procedures should be provided to all personnel involved in the extraction process.
The storage of ethyl acetate requires special attention. It should be kept in tightly sealed containers in a cool, dry place away from sources of heat or ignition. Proper grounding and bonding procedures must be followed during transfer operations to prevent the buildup of static electricity, which could lead to fires or explosions.
Exposure to ethyl acetate vapors can cause irritation to the eyes, nose, and throat, as well as headaches and dizziness. Therefore, adequate ventilation is crucial in extraction areas to maintain airborne concentrations below the permissible exposure limits set by regulatory agencies such as OSHA. Regular air monitoring should be conducted to ensure compliance with these limits.
Spill response procedures should be established and regularly practiced. In the event of a spill, the area should be immediately evacuated, and only trained personnel with appropriate PPE should handle the cleanup. Absorbent materials specifically designed for organic solvents should be used, and contaminated materials must be disposed of as hazardous waste.
Environmental considerations are also important in ethyl acetate extraction. The compound can be harmful to aquatic life and should not be released into the environment. Proper waste management procedures, including the use of sealed containers and approved disposal methods, must be implemented to prevent environmental contamination.
Regular maintenance and inspection of extraction equipment are essential to prevent leaks and ensure the integrity of the system. This includes checking seals, valves, and connections for signs of wear or damage. Any issues should be promptly addressed to maintain safe operating conditions.
By implementing these safety measures and continuously educating workers on the potential hazards associated with ethyl acetate extraction, the risks can be significantly mitigated, leading to a safer and more efficient extraction process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!