How Plasma Surface Treatment Enhances Bio-compatibility
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasma Treatment Evolution and Biocompatibility Goals
Plasma surface treatment has evolved significantly since its inception in the mid-20th century. Initially developed for industrial applications in the 1960s, plasma technology was primarily used for cleaning and activating surfaces in manufacturing processes. The fundamental breakthrough came with the understanding that ionized gas could modify surface properties without altering bulk material characteristics, opening doors to numerous applications across industries.
The evolution of plasma treatment technology accelerated in the 1980s and 1990s with the development of various plasma generation methods, including radio frequency (RF), microwave, and atmospheric pressure plasma systems. These advancements allowed for more precise control over plasma parameters such as power density, gas composition, and treatment duration, enabling tailored surface modifications for specific applications.
In the biomedical field, plasma treatment emerged as a promising approach in the late 1990s when researchers began exploring its potential for enhancing the biocompatibility of medical devices and implants. The ability to modify surface chemistry, topography, and energy without using toxic chemicals aligned perfectly with the growing demand for safer biomedical materials and devices.
The primary goals of plasma treatment for biocompatibility enhancement have evolved to address several critical challenges in biomaterial science. First, improving cell adhesion and proliferation on synthetic surfaces by creating optimal surface energy and chemical functionality. Second, reducing bacterial adhesion and biofilm formation to minimize infection risks associated with implanted devices. Third, controlling protein adsorption patterns to guide subsequent biological responses in the host environment.
Recent technological advancements have focused on developing plasma systems that can operate at atmospheric pressure and ambient temperature, making the technology more accessible and applicable to temperature-sensitive biomaterials. Additionally, the integration of plasma treatment with other surface modification techniques has created synergistic approaches that offer unprecedented control over surface properties.
Looking forward, the field aims to achieve greater precision in surface modification, with goals including nanoscale patterning of bioactive molecules, gradient surface functionalization, and the development of smart, responsive surfaces that can adapt to biological environments. The ultimate objective is to create biomaterials that seamlessly integrate with biological systems, reducing rejection rates and improving long-term performance of medical implants and devices.
The convergence of plasma physics, materials science, and biomedical engineering continues to drive innovation in this field, with increasing focus on translating laboratory findings into clinically viable solutions that meet regulatory standards and can be manufactured at scale.
The evolution of plasma treatment technology accelerated in the 1980s and 1990s with the development of various plasma generation methods, including radio frequency (RF), microwave, and atmospheric pressure plasma systems. These advancements allowed for more precise control over plasma parameters such as power density, gas composition, and treatment duration, enabling tailored surface modifications for specific applications.
In the biomedical field, plasma treatment emerged as a promising approach in the late 1990s when researchers began exploring its potential for enhancing the biocompatibility of medical devices and implants. The ability to modify surface chemistry, topography, and energy without using toxic chemicals aligned perfectly with the growing demand for safer biomedical materials and devices.
The primary goals of plasma treatment for biocompatibility enhancement have evolved to address several critical challenges in biomaterial science. First, improving cell adhesion and proliferation on synthetic surfaces by creating optimal surface energy and chemical functionality. Second, reducing bacterial adhesion and biofilm formation to minimize infection risks associated with implanted devices. Third, controlling protein adsorption patterns to guide subsequent biological responses in the host environment.
Recent technological advancements have focused on developing plasma systems that can operate at atmospheric pressure and ambient temperature, making the technology more accessible and applicable to temperature-sensitive biomaterials. Additionally, the integration of plasma treatment with other surface modification techniques has created synergistic approaches that offer unprecedented control over surface properties.
Looking forward, the field aims to achieve greater precision in surface modification, with goals including nanoscale patterning of bioactive molecules, gradient surface functionalization, and the development of smart, responsive surfaces that can adapt to biological environments. The ultimate objective is to create biomaterials that seamlessly integrate with biological systems, reducing rejection rates and improving long-term performance of medical implants and devices.
The convergence of plasma physics, materials science, and biomedical engineering continues to drive innovation in this field, with increasing focus on translating laboratory findings into clinically viable solutions that meet regulatory standards and can be manufactured at scale.
Market Demand for Biocompatible Medical Devices
The global market for biocompatible medical devices has experienced substantial growth in recent years, driven by increasing healthcare expenditures, aging populations, and advancements in medical technology. According to market research, the biocompatible materials market reached $119.5 billion in 2022 and is projected to grow at a compound annual growth rate of 13.2% through 2030, highlighting the significant demand for devices with enhanced biocompatibility.
The healthcare sector's shift toward minimally invasive procedures has intensified the need for biocompatible implants and devices. Cardiovascular implants, orthopedic devices, dental implants, and drug delivery systems represent the largest segments within this market, collectively accounting for over 65% of the total market share. Particularly, the demand for cardiovascular stents with improved biocompatibility has surged due to the rising prevalence of cardiovascular diseases globally.
Regulatory requirements have become increasingly stringent regarding the biocompatibility of medical devices. The FDA in the United States and the European Medical Device Regulation (MDR) have established comprehensive frameworks for evaluating biocompatibility, compelling manufacturers to invest in advanced surface treatment technologies like plasma modification to meet these standards.
Patient expectations have evolved significantly, with consumers now demanding medical devices that not only perform their intended function but also integrate seamlessly with biological tissues, minimize adverse reactions, and provide longer service life. This consumer-driven demand has pushed manufacturers to prioritize biocompatibility in their product development pipelines.
Emerging economies, particularly in Asia-Pacific and Latin America, represent rapidly expanding markets for biocompatible medical devices. The growing middle class in these regions has increased access to advanced healthcare services, creating new opportunities for manufacturers of plasma-treated biocompatible devices. China and India alone are expected to contribute over 30% of the global market growth in the coming decade.
The COVID-19 pandemic has further accelerated market demand for biocompatible materials in critical care equipment, ventilators, and diagnostic devices. This unexpected surge has highlighted the importance of scalable surface treatment technologies like plasma modification that can be rapidly deployed to enhance biocompatibility across diverse medical applications.
Investment in research and development for biocompatible materials has seen a notable increase, with major medical device manufacturers allocating between 8-12% of their annual revenue to developing next-generation biocompatible surfaces. This investment trend underscores the strategic importance of biocompatibility in maintaining competitive advantage in the medical device industry.
The healthcare sector's shift toward minimally invasive procedures has intensified the need for biocompatible implants and devices. Cardiovascular implants, orthopedic devices, dental implants, and drug delivery systems represent the largest segments within this market, collectively accounting for over 65% of the total market share. Particularly, the demand for cardiovascular stents with improved biocompatibility has surged due to the rising prevalence of cardiovascular diseases globally.
Regulatory requirements have become increasingly stringent regarding the biocompatibility of medical devices. The FDA in the United States and the European Medical Device Regulation (MDR) have established comprehensive frameworks for evaluating biocompatibility, compelling manufacturers to invest in advanced surface treatment technologies like plasma modification to meet these standards.
Patient expectations have evolved significantly, with consumers now demanding medical devices that not only perform their intended function but also integrate seamlessly with biological tissues, minimize adverse reactions, and provide longer service life. This consumer-driven demand has pushed manufacturers to prioritize biocompatibility in their product development pipelines.
Emerging economies, particularly in Asia-Pacific and Latin America, represent rapidly expanding markets for biocompatible medical devices. The growing middle class in these regions has increased access to advanced healthcare services, creating new opportunities for manufacturers of plasma-treated biocompatible devices. China and India alone are expected to contribute over 30% of the global market growth in the coming decade.
The COVID-19 pandemic has further accelerated market demand for biocompatible materials in critical care equipment, ventilators, and diagnostic devices. This unexpected surge has highlighted the importance of scalable surface treatment technologies like plasma modification that can be rapidly deployed to enhance biocompatibility across diverse medical applications.
Investment in research and development for biocompatible materials has seen a notable increase, with major medical device manufacturers allocating between 8-12% of their annual revenue to developing next-generation biocompatible surfaces. This investment trend underscores the strategic importance of biocompatibility in maintaining competitive advantage in the medical device industry.
Current Plasma Surface Modification Challenges
Despite significant advancements in plasma surface treatment for biomedical applications, several critical challenges persist that limit the widespread implementation and effectiveness of these techniques. The primary challenge remains achieving consistent and uniform surface modification across complex three-dimensional implant geometries. Current plasma systems often struggle to deliver homogeneous treatment on intricate surfaces with recessed areas, leading to variable biocompatibility outcomes in different regions of the same device.
The stability and longevity of plasma-modified surfaces represent another significant hurdle. Many plasma treatments create modifications that degrade over time, particularly when exposed to biological environments. This aging effect can result in diminished biocompatibility performance during the implant's service life, raising concerns about long-term clinical outcomes and potentially necessitating additional treatments or replacements.
Process scalability presents substantial difficulties when transitioning from laboratory research to industrial production. Laboratory-scale plasma treatments that demonstrate excellent biocompatibility often face challenges in maintaining consistent results when scaled to commercial manufacturing volumes. This scale-up gap creates barriers for translating promising research into marketable medical devices.
Reproducibility issues further complicate plasma surface modification processes. Minor variations in treatment parameters, environmental conditions, or substrate properties can lead to significant differences in surface characteristics. This variability undermines quality control efforts and complicates regulatory approval pathways for plasma-treated biomedical devices.
The complex relationship between specific plasma parameters and resulting biocompatibility outcomes remains incompletely understood. Current approaches often rely on empirical optimization rather than predictive models, making it difficult to design targeted treatments for specific biological interactions. This knowledge gap slows innovation and increases development costs for new applications.
Energy consumption and environmental considerations pose additional challenges. Many plasma systems require significant power input and may utilize environmentally problematic gases or generate hazardous byproducts. As sustainability becomes increasingly important in manufacturing, developing more eco-friendly plasma treatment processes has become a pressing concern.
Regulatory hurdles specific to plasma-modified surfaces create further complications. The novelty of some plasma treatments means that standardized testing protocols and acceptance criteria may not adequately address their unique characteristics, creating uncertainty in the approval process for medical devices utilizing these technologies.
The stability and longevity of plasma-modified surfaces represent another significant hurdle. Many plasma treatments create modifications that degrade over time, particularly when exposed to biological environments. This aging effect can result in diminished biocompatibility performance during the implant's service life, raising concerns about long-term clinical outcomes and potentially necessitating additional treatments or replacements.
Process scalability presents substantial difficulties when transitioning from laboratory research to industrial production. Laboratory-scale plasma treatments that demonstrate excellent biocompatibility often face challenges in maintaining consistent results when scaled to commercial manufacturing volumes. This scale-up gap creates barriers for translating promising research into marketable medical devices.
Reproducibility issues further complicate plasma surface modification processes. Minor variations in treatment parameters, environmental conditions, or substrate properties can lead to significant differences in surface characteristics. This variability undermines quality control efforts and complicates regulatory approval pathways for plasma-treated biomedical devices.
The complex relationship between specific plasma parameters and resulting biocompatibility outcomes remains incompletely understood. Current approaches often rely on empirical optimization rather than predictive models, making it difficult to design targeted treatments for specific biological interactions. This knowledge gap slows innovation and increases development costs for new applications.
Energy consumption and environmental considerations pose additional challenges. Many plasma systems require significant power input and may utilize environmentally problematic gases or generate hazardous byproducts. As sustainability becomes increasingly important in manufacturing, developing more eco-friendly plasma treatment processes has become a pressing concern.
Regulatory hurdles specific to plasma-modified surfaces create further complications. The novelty of some plasma treatments means that standardized testing protocols and acceptance criteria may not adequately address their unique characteristics, creating uncertainty in the approval process for medical devices utilizing these technologies.
Established Plasma Modification Protocols
01 Plasma treatment for biomedical implants
Plasma surface treatment can be applied to biomedical implants to enhance their biocompatibility. This process modifies the surface properties of materials such as metals, polymers, and ceramics used in implants, improving cell adhesion, reducing bacterial colonization, and enhancing integration with surrounding tissues. The treatment creates functional groups on the surface that promote protein adsorption and subsequent cell attachment, leading to better acceptance by the body.- Plasma treatment for biocompatible medical implants: Plasma surface treatment can enhance the biocompatibility of medical implants by modifying surface properties without altering bulk characteristics. This technique improves cell adhesion, reduces rejection risk, and promotes tissue integration. The treatment creates functional groups on implant surfaces that facilitate protein adsorption and subsequent cell attachment, making it particularly valuable for orthopedic and dental implants where osseointegration is critical.
- Atmospheric plasma for biomaterial surface modification: Atmospheric plasma treatment offers advantages for biomaterial surface modification as it operates at ambient pressure without requiring vacuum systems. This technique can effectively alter surface wettability, introduce specific functional groups, and sterilize biomaterials simultaneously. The non-thermal nature of atmospheric plasma preserves the bulk properties of temperature-sensitive biomaterials while enhancing their biocompatibility through controlled surface oxidation and functionalization.
- Plasma-treated polymer surfaces for cell culture applications: Plasma treatment can modify polymer surfaces to improve their suitability for cell culture applications. By introducing specific functional groups and increasing surface energy, plasma-treated polymers show enhanced cell adhesion, proliferation, and differentiation. This approach enables the creation of biocompatible surfaces for tissue engineering scaffolds, cell culture dishes, and other biotechnology applications without using potentially cytotoxic chemical coatings.
- Plasma-assisted deposition of biocompatible coatings: Plasma technology can be used to deposit thin biocompatible coatings on various substrates. This process allows for the controlled deposition of materials such as hydroxyapatite, diamond-like carbon, or bioactive glass onto medical devices. The resulting coatings improve biocompatibility, reduce bacterial adhesion, and can incorporate therapeutic agents for controlled release. The plasma-assisted deposition ensures strong adhesion between the coating and substrate while maintaining the bioactive properties of the deposited material.
- Plasma sterilization and biocompatibility enhancement: Plasma treatment serves as an effective sterilization method that simultaneously enhances biocompatibility. Unlike conventional sterilization techniques that may leave toxic residues or damage sensitive materials, plasma sterilization eliminates microorganisms through reactive species while improving surface properties. The process can be optimized to remove organic contaminants, increase wettability, and create a more favorable interface for biological interactions, making it particularly valuable for biomedical devices that contact blood, tissue, or other biological fluids.
02 Plasma-modified polymer surfaces for tissue engineering
Plasma treatment can modify polymer surfaces for tissue engineering applications by altering surface chemistry, wettability, and topography. This modification enhances cell attachment, proliferation, and differentiation on scaffolds and other tissue engineering constructs. The treatment introduces specific functional groups like carboxyl, hydroxyl, or amine groups that improve protein adsorption and subsequent cellular interactions, making the materials more suitable for tissue regeneration applications.Expand Specific Solutions03 Low-temperature plasma sterilization for biocompatible materials
Low-temperature plasma treatment provides an effective sterilization method for biocompatible materials that cannot withstand conventional high-temperature sterilization processes. This technique eliminates microorganisms while preserving the structural integrity and biocompatibility of temperature-sensitive materials. The reactive species generated in the plasma effectively inactivate bacteria, viruses, and other pathogens without causing thermal damage to the treated surfaces.Expand Specific Solutions04 Plasma-assisted surface functionalization for improved biocompatibility
Plasma treatment can be used to functionalize material surfaces with specific chemical groups or biomolecules that enhance biocompatibility. This process allows for the attachment of bioactive compounds such as growth factors, antimicrobial agents, or anti-inflammatory drugs to the surface of medical devices. The functionalized surfaces can promote desired biological responses while preventing adverse reactions, leading to improved performance of biomedical devices in clinical applications.Expand Specific Solutions05 Plasma surface modification for reduced protein adsorption and biofouling
Plasma treatment can modify surfaces to reduce non-specific protein adsorption and biofouling, which are critical for certain biomedical applications such as biosensors, diagnostic devices, and blood-contacting materials. By creating hydrophilic surfaces or grafting anti-fouling polymers through plasma processes, materials can resist protein adhesion and subsequent microbial colonization. This modification extends the functional lifetime of biomedical devices and reduces the risk of infection or inflammatory responses.Expand Specific Solutions
Leading Companies in Plasma Surface Treatment
Plasma surface treatment for biocompatibility is currently in a growth phase, with the global market expanding rapidly due to increasing demand in medical implants and devices. The market size is projected to reach significant value as healthcare sectors increasingly adopt this technology for improved patient outcomes. Technologically, the field shows varying maturity levels across applications, with companies like Becton, Dickinson & Co. and W.L. Gore & Associates leading commercial implementation, while research institutions such as Fraunhofer-Gesellschaft and Tsinghua University drive innovation. Emerging players like Bio-Gate AG and Plasmapp Co. Ltd. are developing specialized applications, while established medical device manufacturers including DePuy Synthes and Covidien integrate plasma treatments into their product lines, creating a competitive landscape balanced between established corporations and innovative startups.
DePuy Synthes Products, Inc.
Technical Solution: DePuy Synthes has developed advanced plasma surface treatment technologies specifically for orthopedic implants. Their process involves using low-temperature plasma modification to create nano-textured surfaces on titanium and other metallic implants. This treatment alters the surface chemistry and topography without affecting the bulk material properties. The plasma process introduces functional groups like hydroxyl, carboxyl, and amine groups that enhance protein adsorption and subsequent cell attachment. Their proprietary plasma treatment creates a hydrophilic surface that improves osseointegration by promoting osteoblast adhesion, proliferation, and differentiation. Clinical studies have shown that their plasma-treated implants achieve faster bone integration with stronger mechanical fixation compared to conventional implants, reducing healing time by approximately 30-40%.
Strengths: Highly specialized for orthopedic applications with proven clinical outcomes; creates consistent nano-textured surfaces that enhance cell attachment without compromising mechanical properties. Weaknesses: Process requires specialized equipment and controlled environments; treatment may have limited shelf-life requiring special packaging and storage conditions.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. has pioneered plasma-based surface modification techniques for medical devices and diagnostic products. Their approach utilizes radio frequency (RF) plasma treatment to modify polymer surfaces of catheters, needles, and blood collection devices. The process involves exposing the medical device surfaces to ionized gas (plasma) containing oxygen, nitrogen, or argon under controlled pressure and energy conditions. This creates functional groups on the surface that enhance wettability and biocompatibility without altering bulk material properties. BD's plasma treatment significantly reduces protein adsorption and platelet adhesion, minimizing thrombogenicity in blood-contacting devices. Their technology has demonstrated a reduction in bacterial adhesion by up to 90% compared to untreated surfaces, which is crucial for infection prevention. The company has integrated this plasma treatment into their manufacturing processes for various medical products, including vascular access devices and diagnostic equipment.
Strengths: Scalable technology integrated into high-volume manufacturing processes; provides consistent surface modification across complex geometries; reduces thrombogenicity and bacterial adhesion. Weaknesses: Process parameters must be precisely controlled for each material type; treatment effects may diminish over time for certain polymers requiring additional stabilization strategies.
Key Patents in Plasma-Enhanced Biocompatibility
Surface modification for improving biocompatibility
PatentInactiveUS7589070B2
Innovation
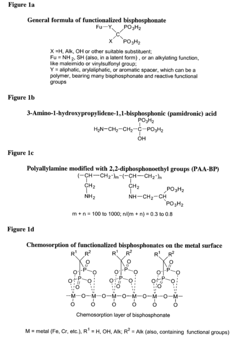
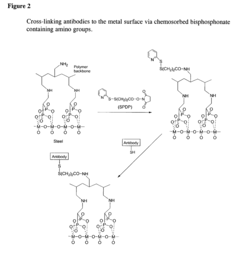
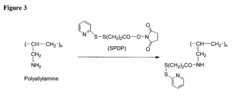
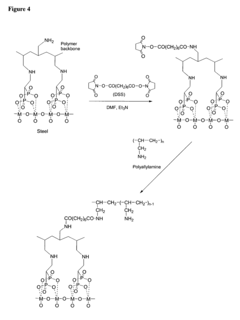
- A surface modification of metal supports using aminobisphosphonates and polyamines to chemically coordinate with metal surfaces, allowing for the direct attachment of biologically active molecules like antibodies and nucleic acids without polymer coatings, enhancing biocompatibility and retention at the delivery site.
Regulatory Framework for Medical Surface Treatments
The regulatory landscape governing plasma surface treatments for medical devices is complex and multifaceted, requiring manufacturers to navigate various international and regional frameworks. In the United States, the Food and Drug Administration (FDA) regulates medical devices through the 510(k) clearance process, where surface-treated devices must demonstrate substantial equivalence to predicate devices. For novel plasma treatments that significantly alter biocompatibility profiles, more rigorous Premarket Approval (PMA) may be required, including comprehensive biocompatibility testing according to ISO 10993 standards.
The European Union has implemented the Medical Device Regulation (MDR 2017/745), which places stringent requirements on surface-treated medical devices. Manufacturers must provide detailed documentation on plasma treatment processes, validate their consistency, and demonstrate that the treatment enhances rather than compromises biocompatibility. The regulation specifically addresses surface modifications in Annex I, requiring thorough risk assessment of potential leachables and degradation products.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for surface-modified medical devices, with particular attention to plasma treatments that interact with biological tissues. These guidelines mandate stability studies to ensure the durability of plasma-treated surfaces under physiological conditions.
International standards play a crucial role in harmonizing regulatory approaches. ISO 10993-1:2018 provides a framework for biological evaluation of medical devices, with specific considerations for surface characteristics. ASTM F1841 addresses standard practice for assessment of surface-modified materials, while ASTM F2459 specifically covers gamma irradiation effects on surfaces—relevant when plasma treatments are combined with sterilization processes.
Regulatory bodies increasingly require manufacturers to implement robust quality management systems for plasma treatment processes. This includes validation protocols, process controls, and monitoring systems to ensure consistency in surface treatment outcomes. Documentation must demonstrate that the plasma treatment parameters are optimized for enhancing biocompatibility without introducing new risks.
Recent regulatory trends indicate a shift toward more personalized approaches, where plasma surface treatments may be tailored to specific patient populations. This emerging paradigm presents new regulatory challenges, as authorities develop frameworks to evaluate customized surface treatments while maintaining safety standards. Manufacturers are advised to engage in early dialogue with regulatory bodies when developing novel plasma surface treatment technologies.
The European Union has implemented the Medical Device Regulation (MDR 2017/745), which places stringent requirements on surface-treated medical devices. Manufacturers must provide detailed documentation on plasma treatment processes, validate their consistency, and demonstrate that the treatment enhances rather than compromises biocompatibility. The regulation specifically addresses surface modifications in Annex I, requiring thorough risk assessment of potential leachables and degradation products.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for surface-modified medical devices, with particular attention to plasma treatments that interact with biological tissues. These guidelines mandate stability studies to ensure the durability of plasma-treated surfaces under physiological conditions.
International standards play a crucial role in harmonizing regulatory approaches. ISO 10993-1:2018 provides a framework for biological evaluation of medical devices, with specific considerations for surface characteristics. ASTM F1841 addresses standard practice for assessment of surface-modified materials, while ASTM F2459 specifically covers gamma irradiation effects on surfaces—relevant when plasma treatments are combined with sterilization processes.
Regulatory bodies increasingly require manufacturers to implement robust quality management systems for plasma treatment processes. This includes validation protocols, process controls, and monitoring systems to ensure consistency in surface treatment outcomes. Documentation must demonstrate that the plasma treatment parameters are optimized for enhancing biocompatibility without introducing new risks.
Recent regulatory trends indicate a shift toward more personalized approaches, where plasma surface treatments may be tailored to specific patient populations. This emerging paradigm presents new regulatory challenges, as authorities develop frameworks to evaluate customized surface treatments while maintaining safety standards. Manufacturers are advised to engage in early dialogue with regulatory bodies when developing novel plasma surface treatment technologies.
Sustainability Aspects of Plasma Processing
Plasma surface treatment technologies, while offering significant advantages for biocompatibility enhancement, must be evaluated through a sustainability lens. The environmental impact of plasma processing is relatively favorable compared to conventional chemical treatments, as it typically requires lower energy consumption and produces fewer hazardous waste streams. Atmospheric pressure plasma systems, in particular, demonstrate improved energy efficiency by eliminating the need for vacuum systems that consume substantial power.
The chemical footprint of plasma processing presents another sustainability advantage. Unlike traditional surface modification methods that often rely on environmentally harmful solvents and chemicals, plasma treatments generally utilize inert gases or air as process media. This significantly reduces toxic emissions and eliminates the need for chemical waste disposal, addressing growing environmental regulations and corporate sustainability goals in the medical device industry.
Resource efficiency represents a key sustainability benefit of plasma technology. The process requires minimal consumables beyond process gases and electricity, with no water consumption in most applications. Additionally, plasma treatments can extend product lifecycles by improving the durability and performance of biomedical implants, thereby reducing replacement frequency and associated resource demands.
From a circular economy perspective, plasma-treated biomedical devices potentially offer improved end-of-life management. The thin surface modifications typically do not interfere with material recycling processes, unlike bulk material treatments or coatings that may complicate material recovery. This characteristic aligns with emerging regulatory frameworks emphasizing product lifecycle management and material recoverability.
The scalability of plasma technology further enhances its sustainability profile. Modern plasma systems can be designed for high-throughput manufacturing while maintaining energy efficiency, making the technology viable for mass production of biocompatible medical devices. Recent innovations in atmospheric plasma systems have reduced the physical footprint and energy requirements of processing equipment, allowing for more compact and efficient manufacturing facilities.
As healthcare systems increasingly incorporate sustainability metrics into procurement decisions, the environmental advantages of plasma-treated biomedical products may provide market differentiation. Forward-thinking manufacturers are already highlighting the reduced environmental impact of plasma processing in their corporate sustainability reporting, recognizing that eco-friendly production methods are becoming a competitive advantage in the biomedical sector.
The chemical footprint of plasma processing presents another sustainability advantage. Unlike traditional surface modification methods that often rely on environmentally harmful solvents and chemicals, plasma treatments generally utilize inert gases or air as process media. This significantly reduces toxic emissions and eliminates the need for chemical waste disposal, addressing growing environmental regulations and corporate sustainability goals in the medical device industry.
Resource efficiency represents a key sustainability benefit of plasma technology. The process requires minimal consumables beyond process gases and electricity, with no water consumption in most applications. Additionally, plasma treatments can extend product lifecycles by improving the durability and performance of biomedical implants, thereby reducing replacement frequency and associated resource demands.
From a circular economy perspective, plasma-treated biomedical devices potentially offer improved end-of-life management. The thin surface modifications typically do not interfere with material recycling processes, unlike bulk material treatments or coatings that may complicate material recovery. This characteristic aligns with emerging regulatory frameworks emphasizing product lifecycle management and material recoverability.
The scalability of plasma technology further enhances its sustainability profile. Modern plasma systems can be designed for high-throughput manufacturing while maintaining energy efficiency, making the technology viable for mass production of biocompatible medical devices. Recent innovations in atmospheric plasma systems have reduced the physical footprint and energy requirements of processing equipment, allowing for more compact and efficient manufacturing facilities.
As healthcare systems increasingly incorporate sustainability metrics into procurement decisions, the environmental advantages of plasma-treated biomedical products may provide market differentiation. Forward-thinking manufacturers are already highlighting the reduced environmental impact of plasma processing in their corporate sustainability reporting, recognizing that eco-friendly production methods are becoming a competitive advantage in the biomedical sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!