Implementing Standards in Plasma Treatment of Medical Equipment
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasma Treatment Standards Evolution and Objectives
Plasma treatment for medical equipment has evolved significantly over the past several decades, transforming from experimental applications to standardized industrial processes. The technology first emerged in the 1970s as researchers discovered plasma's ability to sterilize surfaces without the heat damage associated with traditional methods. By the 1990s, low-temperature plasma sterilization began gaining traction in healthcare settings, particularly for heat-sensitive instruments.
The evolution of plasma treatment standards has been driven by increasing concerns about healthcare-associated infections and the limitations of conventional sterilization methods. Early standards focused primarily on efficacy against common pathogens, with minimal consideration for material compatibility or process validation. As the technology matured, standards expanded to address these gaps, incorporating parameters for plasma generation, exposure time, and quality control measures.
A significant milestone occurred in the early 2000s when international organizations like ISO and AAMI began developing specific guidelines for plasma-based sterilization. These efforts culminated in standards such as ISO 14937, which established requirements for characterization, validation, and routine monitoring of sterilization processes, including those utilizing plasma technology.
Recent developments have focused on harmonizing global standards to facilitate international trade and ensure consistent safety levels worldwide. The emergence of novel medical materials and increasingly complex device designs has necessitated continuous refinement of plasma treatment protocols and corresponding standards. Additionally, growing awareness of environmental impacts has prompted the inclusion of sustainability criteria in newer standards.
The primary objective of current plasma treatment standards is to ensure effective microbial inactivation while preserving the integrity and functionality of treated medical equipment. This includes establishing minimum requirements for bioburden reduction, defining acceptable residual levels of process chemicals, and specifying validation methodologies that manufacturers must follow.
Another critical goal is to standardize testing protocols that can reliably demonstrate the efficacy of plasma treatments across different equipment types and microbial challenges. This standardization enables meaningful comparisons between different plasma systems and facilitates regulatory approval processes globally.
Looking forward, emerging objectives include developing standards for specialized applications such as plasma-activated water for disinfection, atmospheric pressure plasma for in-situ treatment, and combined plasma-based processes that integrate cleaning and sterilization. There is also growing interest in establishing standards for real-time monitoring systems that can verify plasma treatment effectiveness without relying solely on biological indicators or process parameters.
The evolution of plasma treatment standards has been driven by increasing concerns about healthcare-associated infections and the limitations of conventional sterilization methods. Early standards focused primarily on efficacy against common pathogens, with minimal consideration for material compatibility or process validation. As the technology matured, standards expanded to address these gaps, incorporating parameters for plasma generation, exposure time, and quality control measures.
A significant milestone occurred in the early 2000s when international organizations like ISO and AAMI began developing specific guidelines for plasma-based sterilization. These efforts culminated in standards such as ISO 14937, which established requirements for characterization, validation, and routine monitoring of sterilization processes, including those utilizing plasma technology.
Recent developments have focused on harmonizing global standards to facilitate international trade and ensure consistent safety levels worldwide. The emergence of novel medical materials and increasingly complex device designs has necessitated continuous refinement of plasma treatment protocols and corresponding standards. Additionally, growing awareness of environmental impacts has prompted the inclusion of sustainability criteria in newer standards.
The primary objective of current plasma treatment standards is to ensure effective microbial inactivation while preserving the integrity and functionality of treated medical equipment. This includes establishing minimum requirements for bioburden reduction, defining acceptable residual levels of process chemicals, and specifying validation methodologies that manufacturers must follow.
Another critical goal is to standardize testing protocols that can reliably demonstrate the efficacy of plasma treatments across different equipment types and microbial challenges. This standardization enables meaningful comparisons between different plasma systems and facilitates regulatory approval processes globally.
Looking forward, emerging objectives include developing standards for specialized applications such as plasma-activated water for disinfection, atmospheric pressure plasma for in-situ treatment, and combined plasma-based processes that integrate cleaning and sterilization. There is also growing interest in establishing standards for real-time monitoring systems that can verify plasma treatment effectiveness without relying solely on biological indicators or process parameters.
Market Demand for Sterilized Medical Equipment
The global market for sterilized medical equipment has experienced substantial growth in recent years, driven by increasing healthcare expenditures, rising surgical procedures, and growing awareness about hospital-acquired infections (HAIs). The market value reached approximately $8.5 billion in 2022 and is projected to grow at a compound annual growth rate of 7.2% through 2030, reflecting the essential nature of sterilization technologies in healthcare settings.
Healthcare facilities worldwide are facing mounting pressure to enhance their sterilization protocols due to the rising incidence of HAIs, which affect millions of patients annually and result in significant healthcare costs. According to the World Health Organization, at any given time, about 7% of patients in developed countries and 10% in developing countries will acquire at least one HAI during their hospital stay. This alarming statistic has intensified the demand for more effective sterilization methods, including advanced plasma treatment technologies.
The COVID-19 pandemic has further accelerated market growth, creating unprecedented demand for sterilized medical equipment. Healthcare facilities have implemented stricter infection control measures, and regulatory bodies have heightened their scrutiny of sterilization practices. This shift has created a substantial market opportunity for plasma sterilization technologies, which offer advantages over traditional methods such as ethylene oxide and steam sterilization.
Surgical instruments represent the largest segment in the sterilized medical equipment market, accounting for approximately 35% of the total market share. The increasing volume of surgical procedures, particularly minimally invasive surgeries requiring specialized instruments, has driven demand for effective sterilization solutions that can handle complex device geometries without causing damage.
Emerging economies in Asia-Pacific and Latin America present significant growth opportunities due to expanding healthcare infrastructure, increasing medical tourism, and growing adoption of advanced sterilization technologies. These regions are expected to witness the highest growth rates in the coming years, with China and India leading the expansion.
The market is also being shaped by evolving end-user preferences, with a growing emphasis on environmentally friendly sterilization methods. Traditional chemical sterilization techniques face increasing scrutiny due to environmental concerns and workplace safety issues. Plasma treatment, as a more environmentally sustainable alternative, is gaining traction among healthcare facilities seeking to reduce their ecological footprint while maintaining rigorous sterilization standards.
Technological advancements in plasma sterilization equipment, including improved process control, reduced cycle times, and enhanced material compatibility, are further driving market adoption. Healthcare providers are increasingly recognizing the total cost benefits of plasma sterilization, including reduced processing times, extended equipment lifespan, and decreased environmental compliance costs.
Healthcare facilities worldwide are facing mounting pressure to enhance their sterilization protocols due to the rising incidence of HAIs, which affect millions of patients annually and result in significant healthcare costs. According to the World Health Organization, at any given time, about 7% of patients in developed countries and 10% in developing countries will acquire at least one HAI during their hospital stay. This alarming statistic has intensified the demand for more effective sterilization methods, including advanced plasma treatment technologies.
The COVID-19 pandemic has further accelerated market growth, creating unprecedented demand for sterilized medical equipment. Healthcare facilities have implemented stricter infection control measures, and regulatory bodies have heightened their scrutiny of sterilization practices. This shift has created a substantial market opportunity for plasma sterilization technologies, which offer advantages over traditional methods such as ethylene oxide and steam sterilization.
Surgical instruments represent the largest segment in the sterilized medical equipment market, accounting for approximately 35% of the total market share. The increasing volume of surgical procedures, particularly minimally invasive surgeries requiring specialized instruments, has driven demand for effective sterilization solutions that can handle complex device geometries without causing damage.
Emerging economies in Asia-Pacific and Latin America present significant growth opportunities due to expanding healthcare infrastructure, increasing medical tourism, and growing adoption of advanced sterilization technologies. These regions are expected to witness the highest growth rates in the coming years, with China and India leading the expansion.
The market is also being shaped by evolving end-user preferences, with a growing emphasis on environmentally friendly sterilization methods. Traditional chemical sterilization techniques face increasing scrutiny due to environmental concerns and workplace safety issues. Plasma treatment, as a more environmentally sustainable alternative, is gaining traction among healthcare facilities seeking to reduce their ecological footprint while maintaining rigorous sterilization standards.
Technological advancements in plasma sterilization equipment, including improved process control, reduced cycle times, and enhanced material compatibility, are further driving market adoption. Healthcare providers are increasingly recognizing the total cost benefits of plasma sterilization, including reduced processing times, extended equipment lifespan, and decreased environmental compliance costs.
Current Plasma Technology Landscape and Barriers
Plasma treatment technology for medical equipment has evolved significantly over the past two decades, with major advancements in both low-temperature and atmospheric pressure plasma systems. Currently, the global landscape features diverse technological approaches including direct plasma treatment, plasma-activated media, and plasma-enhanced chemical vapor deposition. These technologies operate across different pressure regimes, from vacuum to atmospheric conditions, each offering distinct advantages for medical device sterilization and surface modification.
Despite these advancements, the field faces substantial barriers to widespread implementation. A primary challenge is the lack of standardized protocols for plasma treatment parameters. Treatment times, power settings, gas compositions, and pressure conditions vary widely across manufacturers and research institutions, creating inconsistency in outcomes and complicating regulatory approval processes. This absence of harmonized standards impedes technology transfer from laboratory settings to industrial applications.
Technical limitations present additional obstacles. Current plasma systems often struggle with treating complex geometries and internal surfaces of medical devices, particularly those with narrow lumens or intricate designs. The plasma's penetration depth remains limited, resulting in incomplete treatment of certain device configurations. Furthermore, the stability and reproducibility of plasma treatments across different batches and production scales represent ongoing challenges for quality assurance.
Material compatibility issues constitute another significant barrier. Certain polymers commonly used in medical devices experience degradation when exposed to plasma, including surface cracking, discoloration, or alterations in mechanical properties. This restricts the applicability of plasma treatments across the full spectrum of medical equipment materials and necessitates careful optimization for each specific material-plasma combination.
From a regulatory perspective, the plasma treatment of medical equipment faces substantial hurdles. Current regulatory frameworks in major markets like the US, EU, and Japan lack specific guidelines for plasma-treated medical devices. Manufacturers must navigate complex approval pathways with limited precedent, often requiring extensive validation studies to demonstrate both efficacy and safety. The absence of recognized standards complicates the regulatory submission process and extends time-to-market.
Economic considerations further constrain adoption. The initial capital investment for advanced plasma systems remains high, particularly for systems capable of treating diverse medical equipment at industrial scales. Operating costs, including specialized gases, maintenance, and energy consumption, can be prohibitive for smaller manufacturers. Without clear standardization, companies hesitate to make these investments due to uncertainty about future regulatory requirements and technology obsolescence.
Despite these advancements, the field faces substantial barriers to widespread implementation. A primary challenge is the lack of standardized protocols for plasma treatment parameters. Treatment times, power settings, gas compositions, and pressure conditions vary widely across manufacturers and research institutions, creating inconsistency in outcomes and complicating regulatory approval processes. This absence of harmonized standards impedes technology transfer from laboratory settings to industrial applications.
Technical limitations present additional obstacles. Current plasma systems often struggle with treating complex geometries and internal surfaces of medical devices, particularly those with narrow lumens or intricate designs. The plasma's penetration depth remains limited, resulting in incomplete treatment of certain device configurations. Furthermore, the stability and reproducibility of plasma treatments across different batches and production scales represent ongoing challenges for quality assurance.
Material compatibility issues constitute another significant barrier. Certain polymers commonly used in medical devices experience degradation when exposed to plasma, including surface cracking, discoloration, or alterations in mechanical properties. This restricts the applicability of plasma treatments across the full spectrum of medical equipment materials and necessitates careful optimization for each specific material-plasma combination.
From a regulatory perspective, the plasma treatment of medical equipment faces substantial hurdles. Current regulatory frameworks in major markets like the US, EU, and Japan lack specific guidelines for plasma-treated medical devices. Manufacturers must navigate complex approval pathways with limited precedent, often requiring extensive validation studies to demonstrate both efficacy and safety. The absence of recognized standards complicates the regulatory submission process and extends time-to-market.
Economic considerations further constrain adoption. The initial capital investment for advanced plasma systems remains high, particularly for systems capable of treating diverse medical equipment at industrial scales. Operating costs, including specialized gases, maintenance, and energy consumption, can be prohibitive for smaller manufacturers. Without clear standardization, companies hesitate to make these investments due to uncertainty about future regulatory requirements and technology obsolescence.
Current Plasma Sterilization Implementation Methods
01 Plasma treatment systems for semiconductor processing
Plasma treatment systems are widely used in semiconductor manufacturing for etching, deposition, and surface modification. These systems typically include plasma chambers, RF power sources, and gas delivery components designed to create controlled plasma environments. Advanced systems incorporate features for uniform plasma distribution, temperature control, and precise process parameter management to enhance semiconductor device fabrication quality and efficiency.- Plasma processing chambers and apparatus design: Various designs of plasma processing chambers and apparatus are used for efficient plasma treatment. These include specialized electrode configurations, gas distribution systems, and chamber geometries that optimize plasma generation and uniformity. The designs focus on improving process control, reducing contamination, and enhancing treatment effectiveness for different substrate types and applications.
- Plasma generation and power control methods: Different methods for generating and controlling plasma are employed in treatment processes. These include various power delivery systems, frequency modulation techniques, and pulsed plasma generation. Advanced control systems regulate power parameters to achieve specific plasma characteristics, ensuring optimal treatment conditions while minimizing damage to sensitive substrates.
- Surface modification and etching applications: Plasma treatment is widely used for surface modification and etching of various materials. The process can alter surface properties such as wettability, adhesion, and biocompatibility. Controlled plasma etching enables precise material removal for semiconductor fabrication, microelectronics, and nanotechnology applications. Different gas compositions and process parameters are used to achieve specific surface modifications.
- Semiconductor processing and wafer treatment: Specialized plasma treatment processes are developed for semiconductor manufacturing. These include wafer cleaning, oxide removal, surface activation, and thin film deposition. The treatments are designed to meet the demanding requirements of advanced semiconductor fabrication, with precise control over plasma parameters to ensure uniform processing across large wafers while minimizing damage to sensitive device structures.
- Novel plasma treatment technologies and applications: Emerging plasma treatment technologies expand the application scope beyond traditional uses. These include atmospheric pressure plasma systems, cold plasma for heat-sensitive materials, and specialized treatments for medical devices, textiles, and food packaging. Novel approaches combine plasma with other processes or use innovative gas mixtures to achieve unique surface properties or treatment effects not possible with conventional methods.
02 Plasma chamber design and configuration
Specialized plasma chamber designs focus on optimizing plasma distribution, energy efficiency, and process control. These chambers may incorporate unique electrode configurations, gas flow patterns, and thermal management systems. Innovations in chamber design include features for reducing contamination, enhancing plasma stability, and accommodating various substrate sizes and types, which are critical for consistent treatment results across different applications.Expand Specific Solutions03 Plasma treatment for surface modification
Plasma treatments are employed to modify surface properties of various materials, including polymers, metals, and ceramics. These treatments can enhance adhesion, wettability, biocompatibility, and other surface characteristics without altering bulk material properties. The process typically involves exposing materials to reactive plasma species that can functionalize surfaces, remove contaminants, or create specific surface textures for improved performance in subsequent manufacturing steps.Expand Specific Solutions04 Advanced plasma control and monitoring systems
Modern plasma treatment systems incorporate sophisticated control and monitoring technologies to ensure process stability and reproducibility. These systems may include real-time plasma diagnostics, automated feedback control loops, and advanced software interfaces. Innovations focus on precise power delivery, gas flow control, pressure management, and temperature regulation to maintain optimal plasma conditions throughout treatment cycles, resulting in improved process yields and product quality.Expand Specific Solutions05 Specialized plasma applications and techniques
Beyond standard applications, plasma treatment has been developed for specialized uses including atmospheric pressure plasma processing, selective area treatment, and integration with other manufacturing processes. These techniques may employ unique gas mixtures, pulsed plasma generation, or specialized delivery systems to achieve specific treatment outcomes. Applications range from nanomaterial synthesis to medical device sterilization, with each requiring tailored plasma parameters and treatment protocols to optimize results.Expand Specific Solutions
Key Industry Players and Competitive Analysis
The plasma treatment of medical equipment market is currently in a growth phase, characterized by increasing adoption of advanced sterilization technologies. The global market size is expanding rapidly, driven by stringent regulatory requirements for infection control and growing healthcare infrastructure. Technologically, the field is maturing with established players like Nordson Corp. and Tokyo Electron leading innovation in industrial applications, while specialized companies such as Plasmapp Co. Ltd. and relyon plasma GmbH focus on medical-specific solutions. Large electronics conglomerates including Samsung, Panasonic, and Canon are leveraging their expertise in plasma technology to enter this high-value sector. Research institutions like Korea Basic Science Institute and University of Edinburgh are advancing fundamental plasma science, creating opportunities for next-generation applications in medical equipment sterilization.
Nordson Corp.
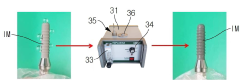
Technical Solution: Nordson has developed advanced plasma treatment systems specifically designed for medical equipment sterilization that comply with ISO 13485 and FDA requirements. Their MARCH MediSURE platform utilizes low-temperature gas plasma technology that achieves a 6-log reduction of bacterial spores while preserving the integrity of heat-sensitive medical devices. The system incorporates real-time monitoring with parametric release capabilities that document critical process parameters including gas concentration, pressure, temperature, and exposure time. Nordson's plasma treatment technology employs radio frequency (RF) excitation to create reactive species that effectively eliminate microorganisms while maintaining material compatibility with polymers, metals, and complex medical device geometries. Their systems feature validated cycles for various medical equipment categories with traceability features that support regulatory compliance.
Strengths: Comprehensive regulatory compliance with medical device standards, validated protocols for different device types, and material compatibility with diverse medical equipment. Weaknesses: Higher implementation costs compared to traditional methods and requires specialized technical expertise for operation and maintenance.
Plasmapp Co. Ltd.
Technical Solution: Plasmapp has pioneered a proprietary low-temperature atmospheric pressure plasma sterilization technology specifically for medical equipment that meets ISO 14937 standards. Their STERLINK platform utilizes a unique dual-mode plasma generation system that combines direct and indirect plasma treatment, achieving sterilization at temperatures below 50°C. The technology employs hydrogen peroxide as a precursor that, when activated by plasma, creates highly reactive oxygen species capable of penetrating complex medical device geometries. Plasmapp's systems incorporate real-time monitoring with integrated biological indicators that verify sterilization efficacy. Their plasma chambers are designed with specialized gas distribution systems that ensure uniform treatment across various medical equipment types, from surgical instruments to endoscopes. The company has developed standardized protocols that have been validated through third-party testing to demonstrate consistent 6-log reduction of resistant bacterial spores.
Strengths: Low-temperature operation preserves heat-sensitive devices, atmospheric pressure operation eliminates vacuum requirements, and dual-mode technology ensures thorough sterilization of complex geometries. Weaknesses: Relatively new technology with limited long-term clinical data and higher operational costs due to hydrogen peroxide consumption.
Critical Patents and Innovations in Plasma Treatment
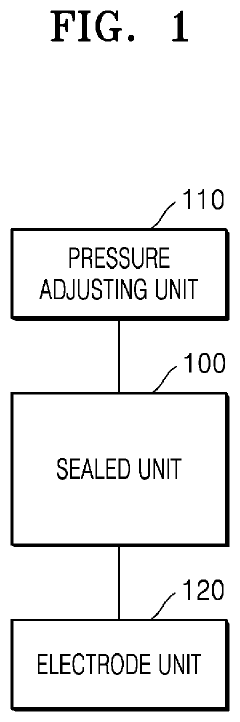
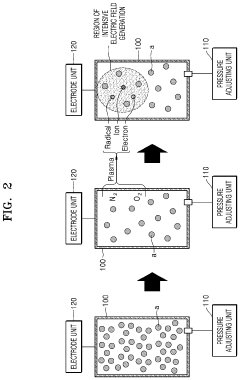
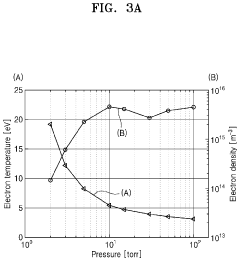
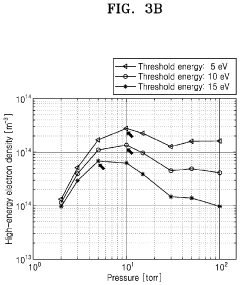
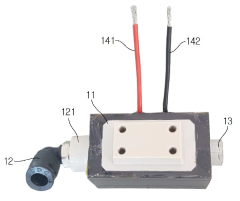
Plasma treatment apparatus
PatentPendingUS20240207473A1
Innovation
- A plasma treatment apparatus that generates stable plasma by discharging a low-pressure atmosphere without using a separate process gas, utilizing a sealed space with pressure control and electrode structures to improve surface treatment efficiency and safety, while reducing the generation of by-products and maintaining sterility.
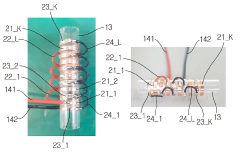
An Apparatus for Treating a Medical Material with a Plasma
PatentActiveKR1020200047062A
Innovation
- A medical plasma processing apparatus with a discharge module, induction tube, and discharge tube, featuring electrode ring groups for generating plasma, allowing easy and safe treatment of medical materials before procedures.
Regulatory Compliance Framework for Medical Devices
The regulatory landscape for plasma treatment of medical equipment is governed by a complex framework of international standards and national regulations. Medical device manufacturers must navigate these requirements to ensure their plasma-treated products meet safety, efficacy, and quality standards. The FDA in the United States, the European Medicines Agency (EMA) in Europe, and similar regulatory bodies worldwide have established specific guidelines for plasma sterilization processes, which differ significantly from traditional sterilization methods.
ISO 13485, the international standard for medical device quality management systems, provides the foundational framework for regulatory compliance in plasma treatment processes. This standard requires manufacturers to implement and maintain quality systems that ensure consistent production of safe medical devices. For plasma treatment specifically, ISO 14937 outlines requirements for the characterization of a sterilizing agent and the development, validation, and routine control of a sterilization process.
Regulatory bodies require extensive validation protocols for plasma sterilization processes. These typically include installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) to demonstrate that the plasma treatment consistently achieves the required sterility assurance level (SAL) of 10^-6 for medical devices. Documentation of these validation processes is critical for regulatory submissions and inspections.
Risk management frameworks, particularly ISO 14971, must be integrated into the plasma treatment process. Manufacturers must identify potential hazards associated with plasma treatment, such as material compatibility issues, residual processing agents, and process variability, and implement appropriate risk control measures.
Compliance with environmental regulations presents another layer of complexity. Many traditional sterilization methods use ethylene oxide or radiation, which have significant environmental and safety concerns. While plasma sterilization offers environmental advantages, manufacturers must still address regulations regarding energy consumption, waste management, and potential emissions from the plasma generation process.
Post-market surveillance requirements are increasingly stringent for medical devices, including those treated with plasma processes. Manufacturers must implement systems to monitor the performance of plasma-treated devices in clinical use and report adverse events to regulatory authorities. This includes maintaining traceability throughout the supply chain to facilitate recalls if necessary.
Emerging global harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline regulatory processes across multiple jurisdictions. However, significant regional variations in requirements for plasma-treated medical devices persist, necessitating tailored compliance strategies for different markets.
ISO 13485, the international standard for medical device quality management systems, provides the foundational framework for regulatory compliance in plasma treatment processes. This standard requires manufacturers to implement and maintain quality systems that ensure consistent production of safe medical devices. For plasma treatment specifically, ISO 14937 outlines requirements for the characterization of a sterilizing agent and the development, validation, and routine control of a sterilization process.
Regulatory bodies require extensive validation protocols for plasma sterilization processes. These typically include installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) to demonstrate that the plasma treatment consistently achieves the required sterility assurance level (SAL) of 10^-6 for medical devices. Documentation of these validation processes is critical for regulatory submissions and inspections.
Risk management frameworks, particularly ISO 14971, must be integrated into the plasma treatment process. Manufacturers must identify potential hazards associated with plasma treatment, such as material compatibility issues, residual processing agents, and process variability, and implement appropriate risk control measures.
Compliance with environmental regulations presents another layer of complexity. Many traditional sterilization methods use ethylene oxide or radiation, which have significant environmental and safety concerns. While plasma sterilization offers environmental advantages, manufacturers must still address regulations regarding energy consumption, waste management, and potential emissions from the plasma generation process.
Post-market surveillance requirements are increasingly stringent for medical devices, including those treated with plasma processes. Manufacturers must implement systems to monitor the performance of plasma-treated devices in clinical use and report adverse events to regulatory authorities. This includes maintaining traceability throughout the supply chain to facilitate recalls if necessary.
Emerging global harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline regulatory processes across multiple jurisdictions. However, significant regional variations in requirements for plasma-treated medical devices persist, necessitating tailored compliance strategies for different markets.
Environmental Impact Assessment of Plasma Technologies
Plasma treatment technologies for medical equipment sterilization, while highly effective, present several environmental considerations that must be carefully assessed. The primary environmental advantage of plasma sterilization is its significantly reduced chemical waste compared to traditional methods like ethylene oxide or glutaraldehyde. Most plasma systems utilize hydrogen peroxide or other compounds that decompose into oxygen and water, leaving minimal toxic residues. This represents a substantial improvement over conventional chemical sterilants that produce hazardous waste requiring specialized disposal protocols.
Energy consumption remains a notable environmental concern for plasma technologies. The generation and maintenance of plasma states require considerable electrical input, contributing to the carbon footprint of healthcare facilities. Recent advancements have improved energy efficiency by optimizing plasma generation cycles and implementing smart power management systems, yet further improvements are necessary to minimize environmental impact.
Air quality considerations are particularly relevant when evaluating plasma technologies. While modern systems are designed with effective containment mechanisms, potential emissions of ozone and nitrogen oxides during operation must be monitored. These byproducts, though minimal compared to chemical sterilization methods, can contribute to indoor air quality issues if ventilation systems are inadequate. Manufacturers have responded by incorporating advanced filtration systems and real-time monitoring capabilities.
Water usage in plasma systems is generally minimal compared to steam sterilization, representing an environmental advantage in water-stressed regions. However, the production of hydrogen peroxide and other precursor chemicals used in plasma generation does involve water-intensive industrial processes upstream in the supply chain. Life cycle assessments indicate that indirect water consumption should be considered when evaluating overall environmental impact.
Material compatibility aspects of plasma treatment also have environmental implications. The non-destructive nature of low-temperature plasma extends the usable life of medical devices, reducing waste from premature equipment disposal. This advantage is particularly significant for sophisticated electronic medical equipment that cannot withstand high-temperature sterilization methods.
End-of-life considerations for plasma sterilization equipment reveal challenges in recycling specialized components like plasma generators and vacuum systems. Manufacturers are increasingly adopting design-for-disassembly principles and establishing take-back programs to address these concerns, though comprehensive recycling infrastructure remains underdeveloped in many regions.
Energy consumption remains a notable environmental concern for plasma technologies. The generation and maintenance of plasma states require considerable electrical input, contributing to the carbon footprint of healthcare facilities. Recent advancements have improved energy efficiency by optimizing plasma generation cycles and implementing smart power management systems, yet further improvements are necessary to minimize environmental impact.
Air quality considerations are particularly relevant when evaluating plasma technologies. While modern systems are designed with effective containment mechanisms, potential emissions of ozone and nitrogen oxides during operation must be monitored. These byproducts, though minimal compared to chemical sterilization methods, can contribute to indoor air quality issues if ventilation systems are inadequate. Manufacturers have responded by incorporating advanced filtration systems and real-time monitoring capabilities.
Water usage in plasma systems is generally minimal compared to steam sterilization, representing an environmental advantage in water-stressed regions. However, the production of hydrogen peroxide and other precursor chemicals used in plasma generation does involve water-intensive industrial processes upstream in the supply chain. Life cycle assessments indicate that indirect water consumption should be considered when evaluating overall environmental impact.
Material compatibility aspects of plasma treatment also have environmental implications. The non-destructive nature of low-temperature plasma extends the usable life of medical devices, reducing waste from premature equipment disposal. This advantage is particularly significant for sophisticated electronic medical equipment that cannot withstand high-temperature sterilization methods.
End-of-life considerations for plasma sterilization equipment reveal challenges in recycling specialized components like plasma generators and vacuum systems. Manufacturers are increasingly adopting design-for-disassembly principles and establishing take-back programs to address these concerns, though comprehensive recycling infrastructure remains underdeveloped in many regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!