How to Analyze Hydrochloric Acid's Effects on Materials?
JUL 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Corrosion Analysis Background and Objectives
The analysis of hydrochloric acid's effects on materials is a critical area of study in materials science and corrosion engineering. This research has far-reaching implications across various industries, including chemical processing, manufacturing, and infrastructure development. The primary objective of this investigation is to comprehensively understand the mechanisms by which hydrochloric acid (HCl) interacts with different materials, particularly metals and alloys, and to develop strategies for mitigating its corrosive effects.
Historically, the study of HCl corrosion has evolved alongside industrial advancements. As the use of hydrochloric acid became more prevalent in industrial processes during the 19th and 20th centuries, the need to understand and control its corrosive nature grew exponentially. This led to the development of corrosion science as a distinct field, with significant contributions from electrochemistry and materials science.
The current technological landscape demands a more sophisticated approach to analyzing HCl's effects. Modern industries require materials that can withstand increasingly harsh chemical environments while maintaining structural integrity and performance. This has driven research towards advanced analytical techniques, including in-situ monitoring, high-resolution microscopy, and computational modeling of corrosion processes.
One of the key trends in this field is the shift towards predictive modeling and real-time monitoring of corrosion. This involves integrating data from multiple sources, including electrochemical measurements, surface analysis, and environmental factors, to create comprehensive models of material degradation under HCl exposure. Such models aim to predict corrosion rates and failure modes more accurately, enabling proactive maintenance and material selection strategies.
Another significant trend is the development of novel materials and coatings specifically designed to resist HCl corrosion. This includes the exploration of advanced alloys, ceramic composites, and smart coatings that can adapt to changing environmental conditions. The goal is to create materials that not only resist corrosion but also maintain their functional properties in aggressive acidic environments.
The objectives of this technical research report are multifaceted. Firstly, it aims to provide a comprehensive overview of the current state of knowledge regarding HCl corrosion mechanisms across various material classes. Secondly, it seeks to evaluate the effectiveness of existing analytical techniques and identify areas where new methodologies are needed. Thirdly, the report will explore emerging technologies and materials that show promise in combating HCl corrosion, assessing their potential for industrial application.
By addressing these objectives, this report aims to bridge the gap between fundamental research and practical applications, providing valuable insights for industries grappling with HCl corrosion challenges. The ultimate goal is to contribute to the development of more resilient materials and more effective corrosion management strategies, thereby enhancing the safety, efficiency, and longevity of industrial processes and infrastructure exposed to hydrochloric acid.
Historically, the study of HCl corrosion has evolved alongside industrial advancements. As the use of hydrochloric acid became more prevalent in industrial processes during the 19th and 20th centuries, the need to understand and control its corrosive nature grew exponentially. This led to the development of corrosion science as a distinct field, with significant contributions from electrochemistry and materials science.
The current technological landscape demands a more sophisticated approach to analyzing HCl's effects. Modern industries require materials that can withstand increasingly harsh chemical environments while maintaining structural integrity and performance. This has driven research towards advanced analytical techniques, including in-situ monitoring, high-resolution microscopy, and computational modeling of corrosion processes.
One of the key trends in this field is the shift towards predictive modeling and real-time monitoring of corrosion. This involves integrating data from multiple sources, including electrochemical measurements, surface analysis, and environmental factors, to create comprehensive models of material degradation under HCl exposure. Such models aim to predict corrosion rates and failure modes more accurately, enabling proactive maintenance and material selection strategies.
Another significant trend is the development of novel materials and coatings specifically designed to resist HCl corrosion. This includes the exploration of advanced alloys, ceramic composites, and smart coatings that can adapt to changing environmental conditions. The goal is to create materials that not only resist corrosion but also maintain their functional properties in aggressive acidic environments.
The objectives of this technical research report are multifaceted. Firstly, it aims to provide a comprehensive overview of the current state of knowledge regarding HCl corrosion mechanisms across various material classes. Secondly, it seeks to evaluate the effectiveness of existing analytical techniques and identify areas where new methodologies are needed. Thirdly, the report will explore emerging technologies and materials that show promise in combating HCl corrosion, assessing their potential for industrial application.
By addressing these objectives, this report aims to bridge the gap between fundamental research and practical applications, providing valuable insights for industries grappling with HCl corrosion challenges. The ultimate goal is to contribute to the development of more resilient materials and more effective corrosion management strategies, thereby enhancing the safety, efficiency, and longevity of industrial processes and infrastructure exposed to hydrochloric acid.
Industrial Demand for HCl Resistance Studies
The industrial demand for hydrochloric acid (HCl) resistance studies has been steadily increasing across various sectors due to the widespread use of this corrosive substance in numerous applications. Industries such as chemical processing, metal treatment, oil and gas, and wastewater management heavily rely on materials that can withstand the aggressive nature of HCl. This demand is driven by the need to improve operational efficiency, reduce maintenance costs, and enhance safety measures in industrial processes involving HCl.
In the chemical processing industry, HCl resistance studies are crucial for designing and selecting appropriate materials for reactors, storage tanks, and piping systems. The ability to withstand HCl corrosion is essential for maintaining the integrity of equipment and preventing costly shutdowns. Similarly, in the metal treatment sector, where HCl is commonly used for pickling and surface cleaning, understanding the acid's effects on different materials is vital for optimizing processes and extending the lifespan of treatment equipment.
The oil and gas industry faces significant challenges related to HCl exposure, particularly in well stimulation and drilling operations. As exploration moves into more challenging environments, the demand for materials that can resist HCl under high pressure and temperature conditions has intensified. This has led to increased research and development efforts focused on advanced alloys and coatings capable of withstanding these harsh conditions.
Wastewater treatment facilities also require extensive HCl resistance studies to ensure the longevity and effectiveness of their infrastructure. The presence of HCl in industrial effluents necessitates the use of corrosion-resistant materials in treatment plants, pumps, and distribution systems. As environmental regulations become more stringent, the demand for improved HCl-resistant materials in this sector continues to grow.
The electronics industry, particularly in the manufacturing of semiconductors and printed circuit boards, relies heavily on HCl for etching processes. The demand for HCl resistance studies in this sector is driven by the need for precision and reliability in microelectronics production. As devices become smaller and more complex, the requirements for materials that can withstand HCl exposure while maintaining dimensional stability become increasingly critical.
Furthermore, the pharmaceutical and food processing industries require HCl-resistant materials for various applications, including drug synthesis and food-grade acid production. The stringent quality and safety standards in these sectors necessitate thorough studies on the interaction between HCl and materials used in production equipment and packaging.
The growing emphasis on sustainability and circular economy principles has also contributed to the demand for HCl resistance studies. Industries are seeking materials that not only resist corrosion but also offer recyclability and reduced environmental impact. This has led to research into bio-based and recyclable polymers with enhanced HCl resistance properties.
In the chemical processing industry, HCl resistance studies are crucial for designing and selecting appropriate materials for reactors, storage tanks, and piping systems. The ability to withstand HCl corrosion is essential for maintaining the integrity of equipment and preventing costly shutdowns. Similarly, in the metal treatment sector, where HCl is commonly used for pickling and surface cleaning, understanding the acid's effects on different materials is vital for optimizing processes and extending the lifespan of treatment equipment.
The oil and gas industry faces significant challenges related to HCl exposure, particularly in well stimulation and drilling operations. As exploration moves into more challenging environments, the demand for materials that can resist HCl under high pressure and temperature conditions has intensified. This has led to increased research and development efforts focused on advanced alloys and coatings capable of withstanding these harsh conditions.
Wastewater treatment facilities also require extensive HCl resistance studies to ensure the longevity and effectiveness of their infrastructure. The presence of HCl in industrial effluents necessitates the use of corrosion-resistant materials in treatment plants, pumps, and distribution systems. As environmental regulations become more stringent, the demand for improved HCl-resistant materials in this sector continues to grow.
The electronics industry, particularly in the manufacturing of semiconductors and printed circuit boards, relies heavily on HCl for etching processes. The demand for HCl resistance studies in this sector is driven by the need for precision and reliability in microelectronics production. As devices become smaller and more complex, the requirements for materials that can withstand HCl exposure while maintaining dimensional stability become increasingly critical.
Furthermore, the pharmaceutical and food processing industries require HCl-resistant materials for various applications, including drug synthesis and food-grade acid production. The stringent quality and safety standards in these sectors necessitate thorough studies on the interaction between HCl and materials used in production equipment and packaging.
The growing emphasis on sustainability and circular economy principles has also contributed to the demand for HCl resistance studies. Industries are seeking materials that not only resist corrosion but also offer recyclability and reduced environmental impact. This has led to research into bio-based and recyclable polymers with enhanced HCl resistance properties.
Current Challenges in HCl Corrosion Testing
The field of hydrochloric acid (HCl) corrosion testing faces several significant challenges that hinder accurate analysis and prediction of material degradation. One of the primary issues is the complexity of the corrosion process itself, which involves multiple variables and mechanisms. The interaction between HCl and various materials can be influenced by factors such as temperature, pressure, concentration, and the presence of other chemical species, making it difficult to isolate and study individual effects.
Standardization of testing procedures remains a persistent challenge in HCl corrosion testing. Different industries and research institutions often employ varied methodologies, leading to inconsistencies in results and difficulties in comparing data across studies. This lack of uniformity hampers the development of comprehensive databases and predictive models for material performance in HCl environments.
The simulation of real-world conditions in laboratory settings poses another significant hurdle. Many industrial applications involve dynamic environments with fluctuating temperatures, pressures, and chemical compositions. Replicating these conditions accurately in controlled experiments is often challenging, leading to potential discrepancies between laboratory results and actual field performance.
The time-dependent nature of corrosion processes further complicates testing procedures. Short-term tests may not accurately predict long-term material behavior, as corrosion mechanisms can evolve over extended periods. Accelerated testing methods, while useful for rapid screening, may not always correlate well with real-world degradation rates, necessitating careful interpretation and validation of results.
Material variability and surface conditions introduce additional complexities in HCl corrosion testing. Slight differences in composition, microstructure, or surface finish can significantly impact corrosion resistance, making it challenging to generalize results across material batches or processing conditions. This variability necessitates extensive testing and statistical analysis to ensure reliable conclusions.
The development of advanced monitoring techniques for in-situ corrosion measurement remains an ongoing challenge. While electrochemical methods provide valuable insights, they may not always be applicable or accurate in highly aggressive HCl environments. Non-destructive evaluation techniques capable of real-time corrosion monitoring in industrial settings are still limited, hindering the ability to detect and mitigate corrosion issues proactively.
Lastly, the environmental and safety concerns associated with HCl handling and disposal present logistical challenges in conducting extensive corrosion tests. Stringent safety protocols and waste management requirements can limit the scale and scope of experiments, particularly for long-term or large-scale studies.
Standardization of testing procedures remains a persistent challenge in HCl corrosion testing. Different industries and research institutions often employ varied methodologies, leading to inconsistencies in results and difficulties in comparing data across studies. This lack of uniformity hampers the development of comprehensive databases and predictive models for material performance in HCl environments.
The simulation of real-world conditions in laboratory settings poses another significant hurdle. Many industrial applications involve dynamic environments with fluctuating temperatures, pressures, and chemical compositions. Replicating these conditions accurately in controlled experiments is often challenging, leading to potential discrepancies between laboratory results and actual field performance.
The time-dependent nature of corrosion processes further complicates testing procedures. Short-term tests may not accurately predict long-term material behavior, as corrosion mechanisms can evolve over extended periods. Accelerated testing methods, while useful for rapid screening, may not always correlate well with real-world degradation rates, necessitating careful interpretation and validation of results.
Material variability and surface conditions introduce additional complexities in HCl corrosion testing. Slight differences in composition, microstructure, or surface finish can significantly impact corrosion resistance, making it challenging to generalize results across material batches or processing conditions. This variability necessitates extensive testing and statistical analysis to ensure reliable conclusions.
The development of advanced monitoring techniques for in-situ corrosion measurement remains an ongoing challenge. While electrochemical methods provide valuable insights, they may not always be applicable or accurate in highly aggressive HCl environments. Non-destructive evaluation techniques capable of real-time corrosion monitoring in industrial settings are still limited, hindering the ability to detect and mitigate corrosion issues proactively.
Lastly, the environmental and safety concerns associated with HCl handling and disposal present logistical challenges in conducting extensive corrosion tests. Stringent safety protocols and waste management requirements can limit the scale and scope of experiments, particularly for long-term or large-scale studies.
Existing HCl Corrosion Analysis Methodologies
01 Corrosive effects on materials
Hydrochloric acid has significant corrosive effects on various materials, particularly metals. It can cause rapid deterioration of metal surfaces, leading to structural weakening and potential equipment failure. This property is both a challenge in industrial settings and a useful feature in certain applications, such as metal cleaning and etching processes.- Corrosive effects on materials: Hydrochloric acid has significant corrosive effects on various materials, particularly metals and certain types of plastics. It can cause rapid deterioration of surfaces, leading to structural weakening and potential equipment failure. This property is both a challenge in handling and storage, and a useful feature in certain industrial cleaning and etching processes.
- Industrial applications: Hydrochloric acid is widely used in various industrial processes, including metal treatment, chemical synthesis, and pH regulation. It plays a crucial role in the production of chlorides, water treatment, and as a catalyst in organic synthesis reactions. Its strong acidic properties make it valuable in processes such as steel pickling and oil well acidizing.
- Environmental and safety considerations: The use of hydrochloric acid requires strict safety measures due to its corrosive nature and potential for harmful fumes. Proper handling, storage, and disposal are essential to prevent environmental contamination and ensure worker safety. Specialized equipment and neutralization techniques are often necessary when working with this acid.
- Biological and health effects: Exposure to hydrochloric acid can have severe health implications, including skin burns, respiratory irritation, and eye damage. In industrial settings, proper protective equipment is crucial. However, in controlled medical applications, dilute hydrochloric acid solutions can be used in treatments related to digestive health and pH balance.
- Neutralization and waste treatment: Effective neutralization and treatment of hydrochloric acid waste is crucial in industrial processes. This involves careful pH adjustment, often using bases like sodium hydroxide, and specialized waste treatment systems. Proper neutralization not only ensures environmental compliance but also prevents damage to drainage systems and water treatment facilities.
02 Environmental and health impacts
The release of hydrochloric acid can have severe environmental and health consequences. It can cause respiratory irritation, skin burns, and eye damage in humans and animals. In the environment, it can alter soil and water pH, affecting ecosystems. Proper handling, storage, and disposal procedures are crucial to mitigate these risks.Expand Specific Solutions03 Industrial applications
Hydrochloric acid is widely used in various industrial processes. It plays a crucial role in chemical manufacturing, metal processing, and water treatment. Its ability to dissolve certain compounds makes it valuable in ore processing and the production of various chemicals. However, its corrosive nature necessitates specialized equipment and safety measures in these applications.Expand Specific Solutions04 Neutralization and waste treatment
The effects of hydrochloric acid can be mitigated through neutralization processes. This is particularly important in waste treatment and environmental remediation. Specialized equipment and procedures are employed to neutralize acidic waste streams, ensuring safe disposal and minimizing environmental impact. The neutralization process often involves the use of alkaline substances to balance the pH.Expand Specific Solutions05 Analytical and laboratory uses
In analytical chemistry and laboratory settings, hydrochloric acid serves various purposes. It is used in titrations, pH adjustments, and as a reagent in numerous chemical reactions. Its strong acidic properties make it valuable for dissolving samples and preparing solutions. However, its use requires careful handling and appropriate safety measures due to its corrosive nature.Expand Specific Solutions
Key Players in Corrosion Testing Industry
The analysis of hydrochloric acid's effects on materials is a mature field within the chemical industry, currently in a stable growth phase. The market size for this research area is substantial, driven by diverse applications across industries such as manufacturing, construction, and environmental protection. Technologically, the field is well-developed, with companies like Fluid Energy Group Ltd., Dow Global Technologies LLC, and Henkel AG & Co. KGaA leading in innovation. These firms have established advanced methodologies for material testing and corrosion analysis. However, there's ongoing research to improve precision and develop more resistant materials, particularly in sectors like semiconductor manufacturing, where GlobalFoundries U.S., Inc. is a key player.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed advanced analytical techniques for studying hydrochloric acid's effects on materials. Their approach combines spectroscopic methods with electrochemical measurements to provide real-time data on material degradation[1]. They utilize Raman spectroscopy to monitor chemical changes in materials exposed to HCl, while simultaneously employing electrochemical impedance spectroscopy to assess corrosion rates[2]. This multi-modal analysis allows for a comprehensive understanding of both chemical and physical effects of HCl on various substrates. Additionally, Dow has implemented machine learning algorithms to predict long-term material performance based on short-term exposure data, enhancing the efficiency of their analysis process[3].
Strengths: Comprehensive multi-modal analysis, real-time data collection, and predictive modeling capabilities. Weaknesses: May require specialized equipment and expertise, potentially limiting widespread adoption.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed a novel approach to analyze hydrochloric acid's effects on materials, focusing particularly on adhesives and surface treatments. Their method involves a combination of accelerated aging tests and advanced imaging techniques[1]. They use atomic force microscopy (AFM) to examine surface changes at the nanoscale, providing insights into early stages of material degradation[2]. Henkel also employs Fourier-transform infrared spectroscopy (FTIR) to identify chemical changes in materials exposed to HCl. To simulate long-term exposure, they have designed custom environmental chambers that can precisely control temperature, humidity, and HCl concentration[3]. This allows for rapid assessment of material performance under various conditions. Furthermore, Henkel has developed proprietary software that integrates data from multiple analytical techniques to provide a holistic view of material degradation processes.
Strengths: High-resolution analysis at nanoscale, ability to simulate long-term exposure in accelerated tests. Weaknesses: May be more focused on specific industries (adhesives, surface treatments) rather than providing a universal solution.
Innovative Approaches in HCl Effects Evaluation
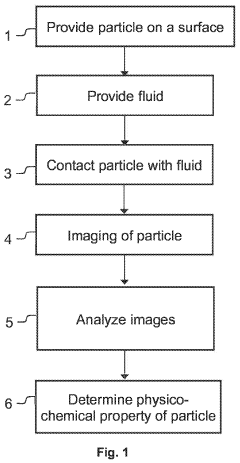
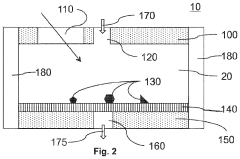
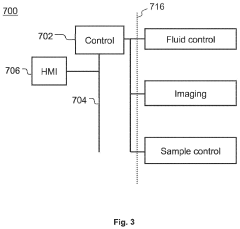
Method and device for physicochemical characterization of materials
PatentActiveUS20220003737A1
Innovation
- A method involving particles on a surface interacting with a fluid, where changes are detected and analyzed to correlate with physicochemical properties, allowing for real-time measurement of properties like solubility and lipophilicity at the nanogram scale without mechanical fixation, using conventional optical microscopy and automated data analysis.
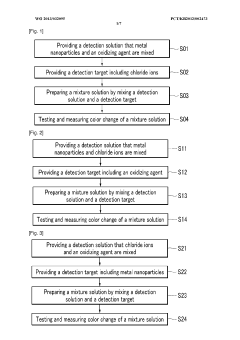
Detection method using colorimetric analysis
PatentWO2013032095A1
Innovation
- A colorimetric detection method using metal nanoparticles, specifically gold or silver nanoparticles, in combination with an oxidizing agent like nitric acid or hydrogen peroxide, to detect chloride ions in water samples, where the color change indicates the presence and concentration of hydrochloric acid.
Environmental Impact of HCl Corrosion Testing
The environmental impact of hydrochloric acid (HCl) corrosion testing is a critical consideration in materials science and engineering. As industries continue to rely on corrosion-resistant materials, the need for accurate and comprehensive testing methods grows. However, these tests often involve the use of highly corrosive substances, such as HCl, which can have significant environmental implications if not properly managed.
HCl corrosion testing typically involves exposing materials to concentrated acid solutions, simulating extreme conditions to evaluate material performance. While these tests provide valuable data on material durability, they also generate hazardous waste that requires careful handling and disposal. The acidic effluents from these tests can potentially contaminate soil and water sources if released into the environment without proper treatment.
One of the primary environmental concerns associated with HCl corrosion testing is the potential for acid rain formation. When HCl vapors are released into the atmosphere, they can combine with water vapor to form hydrochloric acid droplets. These droplets can fall as acid rain, causing damage to vegetation, aquatic ecosystems, and infrastructure. To mitigate this risk, laboratories and testing facilities must implement robust vapor capture and neutralization systems.
Soil contamination is another significant environmental risk posed by HCl corrosion testing. Accidental spills or improper disposal of acid solutions can lead to soil acidification, altering its chemical composition and potentially rendering it unsuitable for plant growth. This can have far-reaching consequences for local ecosystems and agricultural productivity.
Water pollution is a third major concern associated with HCl corrosion testing. If acidic waste from these tests enters water bodies, it can dramatically lower the pH, causing harm to aquatic life and disrupting entire ecosystems. Additionally, the increased acidity can mobilize heavy metals present in sediments, further exacerbating water quality issues.
To address these environmental challenges, researchers and industry professionals are developing more sustainable approaches to corrosion testing. These include the use of less hazardous alternatives to HCl, such as organic acids or electrochemical methods that minimize waste generation. Additionally, advanced waste treatment technologies are being implemented to neutralize and safely dispose of acidic effluents from corrosion tests.
Regulatory bodies worldwide are also taking steps to minimize the environmental impact of HCl corrosion testing. Stricter guidelines for waste management, emissions control, and safety protocols are being enforced to ensure that these essential tests can be conducted without compromising environmental integrity. As the field of materials science continues to evolve, balancing the need for rigorous corrosion testing with environmental stewardship remains a key challenge for researchers and industry professionals alike.
HCl corrosion testing typically involves exposing materials to concentrated acid solutions, simulating extreme conditions to evaluate material performance. While these tests provide valuable data on material durability, they also generate hazardous waste that requires careful handling and disposal. The acidic effluents from these tests can potentially contaminate soil and water sources if released into the environment without proper treatment.
One of the primary environmental concerns associated with HCl corrosion testing is the potential for acid rain formation. When HCl vapors are released into the atmosphere, they can combine with water vapor to form hydrochloric acid droplets. These droplets can fall as acid rain, causing damage to vegetation, aquatic ecosystems, and infrastructure. To mitigate this risk, laboratories and testing facilities must implement robust vapor capture and neutralization systems.
Soil contamination is another significant environmental risk posed by HCl corrosion testing. Accidental spills or improper disposal of acid solutions can lead to soil acidification, altering its chemical composition and potentially rendering it unsuitable for plant growth. This can have far-reaching consequences for local ecosystems and agricultural productivity.
Water pollution is a third major concern associated with HCl corrosion testing. If acidic waste from these tests enters water bodies, it can dramatically lower the pH, causing harm to aquatic life and disrupting entire ecosystems. Additionally, the increased acidity can mobilize heavy metals present in sediments, further exacerbating water quality issues.
To address these environmental challenges, researchers and industry professionals are developing more sustainable approaches to corrosion testing. These include the use of less hazardous alternatives to HCl, such as organic acids or electrochemical methods that minimize waste generation. Additionally, advanced waste treatment technologies are being implemented to neutralize and safely dispose of acidic effluents from corrosion tests.
Regulatory bodies worldwide are also taking steps to minimize the environmental impact of HCl corrosion testing. Stricter guidelines for waste management, emissions control, and safety protocols are being enforced to ensure that these essential tests can be conducted without compromising environmental integrity. As the field of materials science continues to evolve, balancing the need for rigorous corrosion testing with environmental stewardship remains a key challenge for researchers and industry professionals alike.
Safety Protocols for HCl Handling in Labs
Handling hydrochloric acid (HCl) in laboratory settings requires strict adherence to safety protocols to protect personnel and maintain a secure working environment. Proper safety measures begin with comprehensive risk assessment and the implementation of engineering controls. Laboratories should be equipped with fume hoods or local exhaust ventilation systems to minimize exposure to HCl vapors. These systems should be regularly inspected and maintained to ensure optimal performance.
Personal protective equipment (PPE) is crucial when working with HCl. Laboratory personnel must wear appropriate chemical-resistant gloves, such as those made from neoprene or butyl rubber. Eye protection in the form of safety goggles or a face shield is essential to guard against splashes. Additionally, a lab coat or chemical-resistant apron should be worn to protect skin and clothing from potential spills or splashes.
Proper storage of HCl is vital to prevent accidents and maintain the integrity of the acid. HCl should be stored in a cool, dry, well-ventilated area, away from incompatible materials such as metals, alkalis, and oxidizing agents. Containers should be kept tightly closed when not in use and regularly inspected for signs of damage or leakage.
Emergency response procedures must be clearly established and communicated to all laboratory personnel. This includes the location and proper use of eyewash stations and safety showers, which should be easily accessible and tested regularly. Spill control kits specifically designed for acid spills should be readily available, and staff should be trained in their use.
Training is a critical component of HCl safety protocols. All personnel working with or around HCl must receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be documented and refreshed periodically to ensure ongoing competence and awareness.
Waste disposal procedures for HCl and HCl-contaminated materials must comply with local regulations and environmental standards. Neutralization of waste HCl should be performed carefully, typically using a base such as sodium bicarbonate, before disposal. Proper labeling and segregation of waste containers are essential to prevent accidental mixing with incompatible substances.
Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing documentation of training and incident reports.
In the event of exposure, clear emergency procedures must be in place. This includes immediate flushing of affected areas with copious amounts of water and seeking medical attention as necessary. Incident reporting and investigation protocols should be established to learn from any accidents or near-misses and prevent future occurrences.
Personal protective equipment (PPE) is crucial when working with HCl. Laboratory personnel must wear appropriate chemical-resistant gloves, such as those made from neoprene or butyl rubber. Eye protection in the form of safety goggles or a face shield is essential to guard against splashes. Additionally, a lab coat or chemical-resistant apron should be worn to protect skin and clothing from potential spills or splashes.
Proper storage of HCl is vital to prevent accidents and maintain the integrity of the acid. HCl should be stored in a cool, dry, well-ventilated area, away from incompatible materials such as metals, alkalis, and oxidizing agents. Containers should be kept tightly closed when not in use and regularly inspected for signs of damage or leakage.
Emergency response procedures must be clearly established and communicated to all laboratory personnel. This includes the location and proper use of eyewash stations and safety showers, which should be easily accessible and tested regularly. Spill control kits specifically designed for acid spills should be readily available, and staff should be trained in their use.
Training is a critical component of HCl safety protocols. All personnel working with or around HCl must receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be documented and refreshed periodically to ensure ongoing competence and awareness.
Waste disposal procedures for HCl and HCl-contaminated materials must comply with local regulations and environmental standards. Neutralization of waste HCl should be performed carefully, typically using a base such as sodium bicarbonate, before disposal. Proper labeling and segregation of waste containers are essential to prevent accidental mixing with incompatible substances.
Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing documentation of training and incident reports.
In the event of exposure, clear emergency procedures must be in place. This includes immediate flushing of affected areas with copious amounts of water and seeking medical attention as necessary. Incident reporting and investigation protocols should be established to learn from any accidents or near-misses and prevent future occurrences.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!