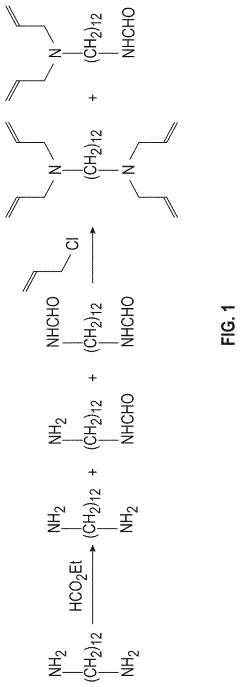
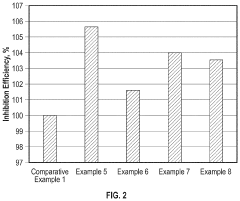
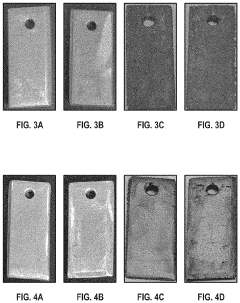
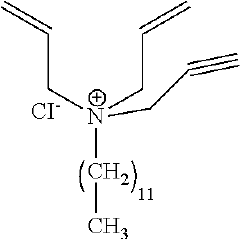
Hydrochloric Acid Corrosion Control: Best Techniques
JUL 1, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Corrosion Background
Hydrochloric acid (HCl) corrosion has been a significant challenge in various industries for decades. This highly corrosive substance, commonly used in chemical processing, oil and gas production, and metal treatment, poses severe risks to equipment integrity and operational safety. The history of HCl corrosion control dates back to the early 20th century when industrial applications of hydrochloric acid became widespread.
Initially, the primary focus was on material selection, with the development of corrosion-resistant alloys such as stainless steel and nickel-based alloys. However, these materials proved to be costly and not always suitable for all applications. This led to the exploration of alternative methods for corrosion control, including the use of inhibitors and protective coatings.
The 1950s and 1960s saw significant advancements in corrosion inhibitor technology. Organic compounds, such as amines and imidazolines, were discovered to be effective in reducing HCl corrosion rates. These inhibitors work by forming a protective film on metal surfaces, preventing direct contact between the acid and the metal.
In the following decades, research focused on understanding the mechanisms of HCl corrosion at a molecular level. This knowledge led to the development of more sophisticated inhibitor formulations and the introduction of synergistic inhibitor blends, which combine multiple compounds to enhance overall protection.
The oil and gas industry has been a major driver in HCl corrosion control research, particularly in the context of well stimulation and acidizing operations. The need for effective corrosion mitigation in high-temperature, high-pressure environments has pushed the boundaries of inhibitor technology and material science.
Recent years have seen a shift towards environmentally friendly corrosion control methods. This includes the development of green inhibitors derived from natural sources and the exploration of nano-materials for corrosion protection. Additionally, advanced monitoring techniques, such as real-time corrosion rate measurements and predictive modeling, have become integral to modern corrosion management strategies.
Despite these advancements, HCl corrosion remains a persistent challenge. The increasing demand for more efficient and cost-effective processes, coupled with stricter environmental regulations, continues to drive innovation in this field. Current research focuses on developing multi-functional inhibitors, improving the durability of protective coatings, and leveraging artificial intelligence for predictive corrosion management.
Initially, the primary focus was on material selection, with the development of corrosion-resistant alloys such as stainless steel and nickel-based alloys. However, these materials proved to be costly and not always suitable for all applications. This led to the exploration of alternative methods for corrosion control, including the use of inhibitors and protective coatings.
The 1950s and 1960s saw significant advancements in corrosion inhibitor technology. Organic compounds, such as amines and imidazolines, were discovered to be effective in reducing HCl corrosion rates. These inhibitors work by forming a protective film on metal surfaces, preventing direct contact between the acid and the metal.
In the following decades, research focused on understanding the mechanisms of HCl corrosion at a molecular level. This knowledge led to the development of more sophisticated inhibitor formulations and the introduction of synergistic inhibitor blends, which combine multiple compounds to enhance overall protection.
The oil and gas industry has been a major driver in HCl corrosion control research, particularly in the context of well stimulation and acidizing operations. The need for effective corrosion mitigation in high-temperature, high-pressure environments has pushed the boundaries of inhibitor technology and material science.
Recent years have seen a shift towards environmentally friendly corrosion control methods. This includes the development of green inhibitors derived from natural sources and the exploration of nano-materials for corrosion protection. Additionally, advanced monitoring techniques, such as real-time corrosion rate measurements and predictive modeling, have become integral to modern corrosion management strategies.
Despite these advancements, HCl corrosion remains a persistent challenge. The increasing demand for more efficient and cost-effective processes, coupled with stricter environmental regulations, continues to drive innovation in this field. Current research focuses on developing multi-functional inhibitors, improving the durability of protective coatings, and leveraging artificial intelligence for predictive corrosion management.
Market Analysis
The market for hydrochloric acid corrosion control techniques has been experiencing steady growth due to the increasing demand across various industries. The oil and gas sector remains the primary driver, accounting for a significant portion of the market share. This industry heavily relies on hydrochloric acid for well stimulation and cleaning processes, creating a constant need for effective corrosion control solutions.
Chemical processing and manufacturing industries also contribute substantially to the market demand. As these sectors continue to expand, particularly in emerging economies, the need for corrosion control techniques is expected to rise proportionally. The power generation industry, especially in regions with aging infrastructure, presents another key market segment for hydrochloric acid corrosion control.
Recent market trends indicate a shift towards more environmentally friendly and cost-effective solutions. This has led to increased research and development efforts in green corrosion inhibitors and advanced coating technologies. The market is also witnessing a growing preference for integrated corrosion management systems that offer comprehensive protection across various industrial applications.
Geographically, North America and Europe currently dominate the market due to stringent environmental regulations and the presence of well-established industries. However, the Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in infrastructure development.
The market landscape is characterized by a mix of large multinational corporations and specialized regional players. Key market players are focusing on product innovation and strategic partnerships to gain a competitive edge. There is also a noticeable trend towards mergers and acquisitions as companies seek to expand their product portfolios and geographical presence.
Challenges in the market include the high initial costs associated with advanced corrosion control techniques and the complexity of implementing these solutions in existing industrial setups. However, the long-term cost savings and operational benefits offered by effective corrosion control are expected to outweigh these initial hurdles.
Looking ahead, the market for hydrochloric acid corrosion control techniques is projected to maintain its growth trajectory. Factors such as increasing industrial activities, stringent safety regulations, and the need for extending the lifespan of industrial equipment are expected to drive market expansion. Additionally, the growing focus on sustainable practices and the development of smart corrosion monitoring systems are likely to create new opportunities in this market segment.
Chemical processing and manufacturing industries also contribute substantially to the market demand. As these sectors continue to expand, particularly in emerging economies, the need for corrosion control techniques is expected to rise proportionally. The power generation industry, especially in regions with aging infrastructure, presents another key market segment for hydrochloric acid corrosion control.
Recent market trends indicate a shift towards more environmentally friendly and cost-effective solutions. This has led to increased research and development efforts in green corrosion inhibitors and advanced coating technologies. The market is also witnessing a growing preference for integrated corrosion management systems that offer comprehensive protection across various industrial applications.
Geographically, North America and Europe currently dominate the market due to stringent environmental regulations and the presence of well-established industries. However, the Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in infrastructure development.
The market landscape is characterized by a mix of large multinational corporations and specialized regional players. Key market players are focusing on product innovation and strategic partnerships to gain a competitive edge. There is also a noticeable trend towards mergers and acquisitions as companies seek to expand their product portfolios and geographical presence.
Challenges in the market include the high initial costs associated with advanced corrosion control techniques and the complexity of implementing these solutions in existing industrial setups. However, the long-term cost savings and operational benefits offered by effective corrosion control are expected to outweigh these initial hurdles.
Looking ahead, the market for hydrochloric acid corrosion control techniques is projected to maintain its growth trajectory. Factors such as increasing industrial activities, stringent safety regulations, and the need for extending the lifespan of industrial equipment are expected to drive market expansion. Additionally, the growing focus on sustainable practices and the development of smart corrosion monitoring systems are likely to create new opportunities in this market segment.
Current Challenges
Hydrochloric acid corrosion control presents several significant challenges in various industrial applications. One of the primary issues is the aggressive nature of hydrochloric acid, which can rapidly deteriorate metal surfaces, leading to equipment failure and potential safety hazards. The corrosion rate is particularly high in elevated temperatures and concentrations, making it difficult to maintain the integrity of processing equipment in chemical plants, oil refineries, and other industrial facilities.
Another challenge lies in the selection of appropriate materials for construction and maintenance. While some materials, such as high-nickel alloys and certain plastics, offer better resistance to hydrochloric acid, they are often expensive and may not be suitable for all applications. This creates a constant trade-off between cost-effectiveness and corrosion resistance, forcing engineers to make difficult decisions in material selection.
The unpredictable nature of corrosion in hydrochloric acid environments also poses a significant challenge. Factors such as temperature fluctuations, presence of impurities, and varying acid concentrations can dramatically affect corrosion rates, making it difficult to accurately predict and prevent corrosion-related issues. This unpredictability necessitates frequent inspections and maintenance, which can be both time-consuming and costly.
Furthermore, the use of traditional corrosion inhibitors in hydrochloric acid systems is often limited due to their ineffectiveness in highly acidic environments or their potential to interfere with industrial processes. Developing effective, environmentally friendly, and process-compatible inhibitors remains an ongoing challenge in the field of corrosion control.
The disposal of hydrochloric acid and its byproducts also presents environmental and regulatory challenges. Strict environmental regulations require proper treatment and disposal of acid waste, which can be complex and expensive. Balancing the need for effective corrosion control with environmental responsibility adds another layer of complexity to the management of hydrochloric acid systems.
Lastly, the lack of standardized testing methods for evaluating the performance of corrosion control techniques in hydrochloric acid environments hinders the development and adoption of new technologies. This gap in standardization makes it difficult for industries to compare different solutions and make informed decisions about corrosion control strategies.
Another challenge lies in the selection of appropriate materials for construction and maintenance. While some materials, such as high-nickel alloys and certain plastics, offer better resistance to hydrochloric acid, they are often expensive and may not be suitable for all applications. This creates a constant trade-off between cost-effectiveness and corrosion resistance, forcing engineers to make difficult decisions in material selection.
The unpredictable nature of corrosion in hydrochloric acid environments also poses a significant challenge. Factors such as temperature fluctuations, presence of impurities, and varying acid concentrations can dramatically affect corrosion rates, making it difficult to accurately predict and prevent corrosion-related issues. This unpredictability necessitates frequent inspections and maintenance, which can be both time-consuming and costly.
Furthermore, the use of traditional corrosion inhibitors in hydrochloric acid systems is often limited due to their ineffectiveness in highly acidic environments or their potential to interfere with industrial processes. Developing effective, environmentally friendly, and process-compatible inhibitors remains an ongoing challenge in the field of corrosion control.
The disposal of hydrochloric acid and its byproducts also presents environmental and regulatory challenges. Strict environmental regulations require proper treatment and disposal of acid waste, which can be complex and expensive. Balancing the need for effective corrosion control with environmental responsibility adds another layer of complexity to the management of hydrochloric acid systems.
Lastly, the lack of standardized testing methods for evaluating the performance of corrosion control techniques in hydrochloric acid environments hinders the development and adoption of new technologies. This gap in standardization makes it difficult for industries to compare different solutions and make informed decisions about corrosion control strategies.
Existing Control Methods
01 Corrosion-resistant coatings
Various coatings can be applied to surfaces to protect against hydrochloric acid corrosion. These coatings may include specialized polymers, ceramic materials, or composite layers that create a barrier between the acid and the underlying substrate. The coatings are designed to withstand the aggressive nature of hydrochloric acid and prevent or significantly reduce corrosion damage.- Corrosion-resistant coatings: Various coatings can be applied to surfaces to protect against hydrochloric acid corrosion. These coatings may include polymer-based materials, ceramic composites, or specialized metal alloys that form a protective barrier between the acid and the underlying substrate. The coatings are designed to withstand the aggressive nature of hydrochloric acid and prevent or significantly reduce corrosion damage.
- Corrosion inhibitors: Chemical additives known as corrosion inhibitors can be used to mitigate the corrosive effects of hydrochloric acid. These inhibitors work by forming a protective film on metal surfaces or by altering the chemical properties of the acid solution. They can significantly reduce the rate of corrosion and extend the lifespan of equipment exposed to hydrochloric acid.
- Material selection and engineering: Choosing appropriate materials that are inherently resistant to hydrochloric acid corrosion is crucial. This may involve selecting specific grades of stainless steel, titanium alloys, or other corrosion-resistant metals. Additionally, engineering solutions such as cathodic protection systems or the use of sacrificial anodes can be implemented to protect against hydrochloric acid corrosion in various industrial applications.
- Surface treatment techniques: Various surface treatment methods can be employed to enhance the corrosion resistance of materials exposed to hydrochloric acid. These techniques may include passivation, electropolishing, or the application of conversion coatings. Such treatments modify the surface properties of the material, creating a more corrosion-resistant layer that can better withstand the aggressive nature of hydrochloric acid.
- Monitoring and maintenance strategies: Implementing effective monitoring and maintenance strategies is essential for managing hydrochloric acid corrosion. This may involve regular inspections, the use of corrosion sensors, and the development of predictive maintenance schedules. By closely monitoring corrosion rates and implementing timely maintenance procedures, the impact of hydrochloric acid corrosion can be minimized, and the longevity of equipment and structures can be extended.
02 Corrosion inhibitors
Chemical additives known as corrosion inhibitors can be used to mitigate the effects of hydrochloric acid corrosion. These inhibitors work by forming a protective film on metal surfaces or by altering the chemical properties of the acid to reduce its corrosive nature. Different types of inhibitors may be used depending on the specific application and environmental conditions.Expand Specific Solutions03 Material selection and treatment
Choosing appropriate materials that are inherently resistant to hydrochloric acid corrosion is crucial. This may involve selecting specific alloys, ceramics, or polymers that demonstrate high resistance to acid attack. Additionally, various heat treatments or surface modification techniques can be applied to enhance the corrosion resistance of materials exposed to hydrochloric acid.Expand Specific Solutions04 Cathodic protection systems
Cathodic protection techniques can be employed to prevent or reduce hydrochloric acid corrosion in certain applications. This method involves applying an electric current to the metal structure to be protected, making it the cathode in an electrochemical cell. This process can significantly reduce the rate of corrosion by altering the electrochemical potential of the metal surface.Expand Specific Solutions05 Monitoring and maintenance strategies
Implementing effective monitoring and maintenance strategies is essential for managing hydrochloric acid corrosion. This may include regular inspections, the use of corrosion sensors, and the development of predictive maintenance schedules. Advanced monitoring techniques can help detect early signs of corrosion, allowing for timely interventions and preventing catastrophic failures in industrial equipment and structures exposed to hydrochloric acid.Expand Specific Solutions
Key Industry Players
The hydrochloric acid corrosion control market is in a mature stage, with established players and proven technologies. The global market size is estimated to be in the billions of dollars, driven by demand from industries such as oil and gas, chemical processing, and water treatment. Key players like ChemTreat, Ecolab, and Halliburton offer comprehensive solutions, while research institutions such as King Fahd University of Petroleum & Minerals contribute to technological advancements. The market is characterized by a mix of large multinational corporations and specialized chemical companies, with ongoing innovation focused on improving efficiency and environmental sustainability of corrosion control techniques.
ChemTreat, Inc.
Technical Solution: ChemTreat employs a multi-faceted approach to hydrochloric acid corrosion control. Their strategy includes the use of advanced corrosion inhibitors, pH control agents, and oxygen scavengers. The company has developed proprietary blends of organic and inorganic inhibitors that form protective films on metal surfaces, significantly reducing corrosion rates[1]. They also utilize innovative monitoring techniques, such as real-time corrosion rate measurement and predictive modeling, to optimize inhibitor dosing and maintain system integrity[2]. Additionally, ChemTreat implements customized treatment programs that consider specific process conditions, metallurgy, and environmental factors to provide tailored solutions for each application[3].
Strengths: Customized solutions, advanced monitoring techniques, and proprietary inhibitor blends. Weaknesses: Potentially higher costs due to specialized treatments and ongoing monitoring requirements.
Halliburton Energy Services, Inc.
Technical Solution: Halliburton's approach to hydrochloric acid corrosion control focuses on innovative chemical solutions and advanced application techniques. They have developed a range of high-performance corrosion inhibitors specifically designed for high-temperature and high-pressure environments encountered in oil and gas operations[1]. Their technology includes novel organic inhibitors that form tenacious protective films on metal surfaces, even under extreme conditions[2]. Halliburton also employs smart delivery systems that ensure optimal inhibitor distribution throughout the system, maximizing protection while minimizing chemical usage[3]. Furthermore, they have pioneered the use of nanotechnology in corrosion inhibition, developing nanoparticle-based inhibitors that offer superior surface coverage and protection[4].
Strengths: Specialized solutions for extreme conditions, advanced delivery systems, and cutting-edge nanotechnology. Weaknesses: Potentially limited applicability outside of oil and gas industry, higher costs associated with advanced technologies.
Innovative Technologies
Corrosion inhibiting acid mixture containing monoamine / diamine and method of inhibiting corrosion in acid treatment
PatentActiveUS20230227714A1
Innovation
- An acid mixture comprising hydrochloric acid, a monoamine corrosion inhibitor, and at least one of a diamine corrosion inhibitor and an acid additive, with specific concentrations and combinations to inhibit corrosion in steel parts of the acidic fluid circulation system, maintaining the monoamine corrosion inhibitor in a range of 10 ppm to 400 ppm and the diamine corrosion inhibitor in a similar range, along with acid additives and intensifiers to enhance corrosion inhibition properties.
Process for treating stainless steel surfaces
PatentInactiveEP0814180A1
Innovation
- The method employs hydrochloric acid as the sole acid in a spray pickling process with electrolytic and immersion treatments, eliminating mechanical pretreatment and using a regenerable acid that does not produce waste water, while ensuring a smooth surface finish.
Environmental Regulations
Environmental regulations play a crucial role in shaping the practices and technologies used for hydrochloric acid corrosion control. These regulations are designed to protect human health and the environment from the potential hazards associated with the use and disposal of hydrochloric acid and its byproducts.
In many countries, strict guidelines govern the handling, storage, and disposal of hydrochloric acid. For instance, the United States Environmental Protection Agency (EPA) classifies hydrochloric acid as a hazardous substance under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). This classification imposes specific reporting requirements for spills and releases, as well as mandates for proper disposal methods.
The Clean Air Act also regulates emissions of hydrochloric acid, as it is considered a hazardous air pollutant. Industrial facilities using or producing hydrochloric acid must implement stringent control measures to minimize air emissions, often requiring the installation of scrubbers or other air pollution control devices.
Water quality regulations, such as the Clean Water Act in the United States, set limits on the discharge of hydrochloric acid and its derivatives into water bodies. These regulations necessitate the implementation of wastewater treatment systems and monitoring protocols to ensure compliance with effluent standards.
Occupational health and safety regulations, like those enforced by the Occupational Safety and Health Administration (OSHA), mandate specific workplace practices for handling hydrochloric acid. These include requirements for personal protective equipment, emergency response procedures, and employee training programs.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes additional requirements on the manufacture, import, and use of hydrochloric acid. This includes comprehensive safety assessments and the implementation of risk management measures throughout the supply chain.
As environmental concerns continue to grow, regulations are becoming increasingly stringent. Many jurisdictions are adopting more comprehensive lifecycle management approaches, focusing not only on the immediate impacts of hydrochloric acid use but also on long-term environmental sustainability.
These evolving regulations are driving innovation in corrosion control techniques. Industries are increasingly seeking environmentally friendly alternatives to traditional corrosion control methods, such as green inhibitors and advanced materials that reduce the need for hydrochloric acid in various processes.
Compliance with these regulations often requires significant investment in technology and infrastructure. However, it also presents opportunities for companies to differentiate themselves through superior environmental performance and sustainable practices in hydrochloric acid corrosion control.
In many countries, strict guidelines govern the handling, storage, and disposal of hydrochloric acid. For instance, the United States Environmental Protection Agency (EPA) classifies hydrochloric acid as a hazardous substance under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). This classification imposes specific reporting requirements for spills and releases, as well as mandates for proper disposal methods.
The Clean Air Act also regulates emissions of hydrochloric acid, as it is considered a hazardous air pollutant. Industrial facilities using or producing hydrochloric acid must implement stringent control measures to minimize air emissions, often requiring the installation of scrubbers or other air pollution control devices.
Water quality regulations, such as the Clean Water Act in the United States, set limits on the discharge of hydrochloric acid and its derivatives into water bodies. These regulations necessitate the implementation of wastewater treatment systems and monitoring protocols to ensure compliance with effluent standards.
Occupational health and safety regulations, like those enforced by the Occupational Safety and Health Administration (OSHA), mandate specific workplace practices for handling hydrochloric acid. These include requirements for personal protective equipment, emergency response procedures, and employee training programs.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes additional requirements on the manufacture, import, and use of hydrochloric acid. This includes comprehensive safety assessments and the implementation of risk management measures throughout the supply chain.
As environmental concerns continue to grow, regulations are becoming increasingly stringent. Many jurisdictions are adopting more comprehensive lifecycle management approaches, focusing not only on the immediate impacts of hydrochloric acid use but also on long-term environmental sustainability.
These evolving regulations are driving innovation in corrosion control techniques. Industries are increasingly seeking environmentally friendly alternatives to traditional corrosion control methods, such as green inhibitors and advanced materials that reduce the need for hydrochloric acid in various processes.
Compliance with these regulations often requires significant investment in technology and infrastructure. However, it also presents opportunities for companies to differentiate themselves through superior environmental performance and sustainable practices in hydrochloric acid corrosion control.
Economic Impact Assessment
The economic impact of hydrochloric acid corrosion control techniques extends far beyond the immediate costs of implementation. Effective corrosion control measures can significantly reduce maintenance expenses, extend equipment lifespan, and minimize production downtime, resulting in substantial long-term savings for industries reliant on hydrochloric acid processes.
In the chemical processing industry, where hydrochloric acid is widely used, the economic benefits of advanced corrosion control techniques are particularly pronounced. Companies implementing state-of-the-art corrosion prevention methods have reported reductions in equipment replacement costs of up to 30% over a five-year period. This translates to millions of dollars in savings for large-scale operations, improving overall profitability and competitiveness.
The oil and gas sector, another major user of hydrochloric acid, has seen similar economic advantages. Offshore platforms and refineries utilizing cutting-edge corrosion control technologies have experienced increased operational efficiency, with some facilities reporting a 15-20% decrease in unplanned shutdowns. This improvement in reliability not only reduces direct maintenance costs but also enhances production output, contributing to higher revenues.
Moreover, the economic impact extends to the corrosion control industry itself. The global market for corrosion inhibitors is projected to reach $9.2 billion by 2026, with a compound annual growth rate of 4.5%. This growth is largely driven by the demand for advanced solutions in hydrochloric acid applications, creating new opportunities for chemical manufacturers, materials scientists, and engineering firms specializing in corrosion prevention.
The adoption of innovative corrosion control techniques also has broader economic implications for national economies. In the United States alone, the total annual cost of corrosion across all industries is estimated at $276 billion. By implementing more effective corrosion control measures, particularly in hydrochloric acid environments, this figure could be significantly reduced, freeing up resources for investment in other areas of the economy.
Furthermore, the economic benefits of improved corrosion control extend to environmental protection and workplace safety. By reducing the risk of equipment failure and chemical leaks, companies can avoid costly environmental clean-up operations and potential regulatory fines. Additionally, enhanced safety measures contribute to lower insurance premiums and reduced worker compensation claims, further improving the bottom line for businesses operating in hydrochloric acid-intensive industries.
In the chemical processing industry, where hydrochloric acid is widely used, the economic benefits of advanced corrosion control techniques are particularly pronounced. Companies implementing state-of-the-art corrosion prevention methods have reported reductions in equipment replacement costs of up to 30% over a five-year period. This translates to millions of dollars in savings for large-scale operations, improving overall profitability and competitiveness.
The oil and gas sector, another major user of hydrochloric acid, has seen similar economic advantages. Offshore platforms and refineries utilizing cutting-edge corrosion control technologies have experienced increased operational efficiency, with some facilities reporting a 15-20% decrease in unplanned shutdowns. This improvement in reliability not only reduces direct maintenance costs but also enhances production output, contributing to higher revenues.
Moreover, the economic impact extends to the corrosion control industry itself. The global market for corrosion inhibitors is projected to reach $9.2 billion by 2026, with a compound annual growth rate of 4.5%. This growth is largely driven by the demand for advanced solutions in hydrochloric acid applications, creating new opportunities for chemical manufacturers, materials scientists, and engineering firms specializing in corrosion prevention.
The adoption of innovative corrosion control techniques also has broader economic implications for national economies. In the United States alone, the total annual cost of corrosion across all industries is estimated at $276 billion. By implementing more effective corrosion control measures, particularly in hydrochloric acid environments, this figure could be significantly reduced, freeing up resources for investment in other areas of the economy.
Furthermore, the economic benefits of improved corrosion control extend to environmental protection and workplace safety. By reducing the risk of equipment failure and chemical leaks, companies can avoid costly environmental clean-up operations and potential regulatory fines. Additionally, enhanced safety measures contribute to lower insurance premiums and reduced worker compensation claims, further improving the bottom line for businesses operating in hydrochloric acid-intensive industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!