Cutting-Edge Hydrochloric Acid Manufacturing Techniques
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Manufacturing Evolution and Objectives
Hydrochloric acid (HCl) manufacturing has undergone significant evolution since its inception in the early 19th century. The journey from small-scale production to modern industrial processes reflects the growing demand for this versatile chemical across various sectors. Initially, HCl was primarily a by-product of the Leblanc process for soda ash production. However, as its applications expanded, dedicated manufacturing techniques emerged to meet the increasing demand.
The evolution of HCl manufacturing techniques has been driven by the need for higher efficiency, improved safety, and reduced environmental impact. Early methods often involved the direct reaction of sulfuric acid with sodium chloride, which was both inefficient and environmentally problematic. The introduction of the chlor-alkali process in the mid-20th century marked a significant milestone, as it allowed for the co-production of chlorine, sodium hydroxide, and hydrogen, with HCl as a valuable by-product.
In recent decades, the focus has shifted towards developing more sustainable and cost-effective production methods. The synthesis of HCl from its elemental components, chlorine and hydrogen, has gained prominence due to its higher purity output and better control over the production process. Additionally, the recovery and recycling of HCl from industrial waste streams have become increasingly important, aligning with circular economy principles and stricter environmental regulations.
The current objectives in HCl manufacturing research are multifaceted. Firstly, there is a strong emphasis on improving energy efficiency across all production stages, from raw material processing to final product purification. This includes the development of novel catalysts and reactor designs that can operate at lower temperatures and pressures while maintaining high yields. Secondly, researchers are exploring ways to minimize waste generation and maximize resource utilization, particularly in the context of by-product valorization and closed-loop manufacturing systems.
Another key objective is the enhancement of process safety and control. As HCl is a highly corrosive substance, advanced materials and containment technologies are being investigated to reduce the risk of leaks and equipment degradation. Furthermore, the integration of smart manufacturing principles, including real-time monitoring and predictive maintenance, is becoming increasingly important to optimize production and ensure consistent product quality.
Looking ahead, the future of HCl manufacturing is likely to be shaped by emerging technologies such as membrane separation techniques, advanced electrochemical processes, and potentially even biotechnological approaches. These cutting-edge methods aim to address the limitations of current production techniques while opening up new possibilities for on-demand, small-scale manufacturing that could revolutionize the industry's supply chain dynamics.
The evolution of HCl manufacturing techniques has been driven by the need for higher efficiency, improved safety, and reduced environmental impact. Early methods often involved the direct reaction of sulfuric acid with sodium chloride, which was both inefficient and environmentally problematic. The introduction of the chlor-alkali process in the mid-20th century marked a significant milestone, as it allowed for the co-production of chlorine, sodium hydroxide, and hydrogen, with HCl as a valuable by-product.
In recent decades, the focus has shifted towards developing more sustainable and cost-effective production methods. The synthesis of HCl from its elemental components, chlorine and hydrogen, has gained prominence due to its higher purity output and better control over the production process. Additionally, the recovery and recycling of HCl from industrial waste streams have become increasingly important, aligning with circular economy principles and stricter environmental regulations.
The current objectives in HCl manufacturing research are multifaceted. Firstly, there is a strong emphasis on improving energy efficiency across all production stages, from raw material processing to final product purification. This includes the development of novel catalysts and reactor designs that can operate at lower temperatures and pressures while maintaining high yields. Secondly, researchers are exploring ways to minimize waste generation and maximize resource utilization, particularly in the context of by-product valorization and closed-loop manufacturing systems.
Another key objective is the enhancement of process safety and control. As HCl is a highly corrosive substance, advanced materials and containment technologies are being investigated to reduce the risk of leaks and equipment degradation. Furthermore, the integration of smart manufacturing principles, including real-time monitoring and predictive maintenance, is becoming increasingly important to optimize production and ensure consistent product quality.
Looking ahead, the future of HCl manufacturing is likely to be shaped by emerging technologies such as membrane separation techniques, advanced electrochemical processes, and potentially even biotechnological approaches. These cutting-edge methods aim to address the limitations of current production techniques while opening up new possibilities for on-demand, small-scale manufacturing that could revolutionize the industry's supply chain dynamics.
Market Analysis for HCl Production
The global hydrochloric acid (HCl) market has shown steady growth in recent years, driven by increasing demand from various industries. The market size was valued at approximately 39.3 million metric tons in 2020 and is projected to reach 46.2 million metric tons by 2027, growing at a CAGR of 2.5% during the forecast period. This growth is primarily attributed to the expanding applications of HCl in diverse sectors such as steel pickling, oil well acidizing, and chemical manufacturing.
The steel industry remains the largest consumer of hydrochloric acid, accounting for nearly 40% of the global demand. The growing construction and automotive sectors in emerging economies are fueling the need for steel, consequently driving the demand for HCl in steel pickling processes. Additionally, the oil and gas industry's increasing use of HCl for well acidizing and enhanced oil recovery techniques is contributing significantly to market growth.
In terms of regional distribution, Asia-Pacific dominates the global HCl market, with China being the largest producer and consumer. The region's rapid industrialization, particularly in countries like India and Southeast Asian nations, is expected to maintain its leading position in the coming years. North America and Europe follow as significant markets, with steady demand from established industrial sectors.
The chemical manufacturing sector is another key driver of HCl demand, particularly in the production of PVC, MDI, and other chlorinated compounds. The growing use of these materials in construction, automotive, and consumer goods industries is indirectly boosting the HCl market. Furthermore, the water treatment industry's increasing adoption of HCl for pH adjustment and chlorine dioxide generation is opening new avenues for market expansion.
However, the market faces challenges such as environmental concerns and stringent regulations regarding HCl production and handling. This has led to a growing focus on developing more sustainable and eco-friendly manufacturing techniques. Recycling and recovery of HCl from industrial processes are gaining traction as companies strive to reduce their environmental footprint and operational costs.
The competitive landscape of the HCl market is characterized by the presence of several large multinational corporations and regional players. Key market participants are investing in research and development to improve production efficiency and explore new applications. Strategic partnerships and mergers are also observed as companies aim to strengthen their market position and expand their geographical presence.
The steel industry remains the largest consumer of hydrochloric acid, accounting for nearly 40% of the global demand. The growing construction and automotive sectors in emerging economies are fueling the need for steel, consequently driving the demand for HCl in steel pickling processes. Additionally, the oil and gas industry's increasing use of HCl for well acidizing and enhanced oil recovery techniques is contributing significantly to market growth.
In terms of regional distribution, Asia-Pacific dominates the global HCl market, with China being the largest producer and consumer. The region's rapid industrialization, particularly in countries like India and Southeast Asian nations, is expected to maintain its leading position in the coming years. North America and Europe follow as significant markets, with steady demand from established industrial sectors.
The chemical manufacturing sector is another key driver of HCl demand, particularly in the production of PVC, MDI, and other chlorinated compounds. The growing use of these materials in construction, automotive, and consumer goods industries is indirectly boosting the HCl market. Furthermore, the water treatment industry's increasing adoption of HCl for pH adjustment and chlorine dioxide generation is opening new avenues for market expansion.
However, the market faces challenges such as environmental concerns and stringent regulations regarding HCl production and handling. This has led to a growing focus on developing more sustainable and eco-friendly manufacturing techniques. Recycling and recovery of HCl from industrial processes are gaining traction as companies strive to reduce their environmental footprint and operational costs.
The competitive landscape of the HCl market is characterized by the presence of several large multinational corporations and regional players. Key market participants are investing in research and development to improve production efficiency and explore new applications. Strategic partnerships and mergers are also observed as companies aim to strengthen their market position and expand their geographical presence.
Current HCl Synthesis Challenges
The current landscape of hydrochloric acid (HCl) synthesis faces several significant challenges that hinder efficiency, sustainability, and cost-effectiveness. One of the primary issues is the environmental impact of traditional production methods. The Mannheim process, which involves the reaction of sodium chloride with sulfuric acid, generates substantial amounts of sodium sulfate as a by-product, leading to waste management concerns and reduced overall efficiency.
Energy consumption remains a critical challenge in HCl manufacturing. Many existing processes require high temperatures and pressures, resulting in substantial energy inputs and associated costs. This not only affects the economic viability of production but also contributes to the carbon footprint of the industry, raising concerns about long-term sustainability in an increasingly environmentally conscious market.
Raw material availability and cost fluctuations pose another significant challenge. The dependence on specific feedstocks, such as rock salt for the Mannheim process or methane for the methane chlorination process, exposes manufacturers to supply chain vulnerabilities and price volatility. This can lead to unpredictable production costs and potential supply disruptions, impacting the stability of HCl production.
Corrosion and equipment degradation present ongoing technical challenges in HCl synthesis. The highly corrosive nature of hydrochloric acid necessitates the use of specialized materials and frequent maintenance, increasing operational costs and potential safety risks. Developing more resistant materials or innovative reactor designs remains a key focus area for improving process reliability and reducing downtime.
Quality control and product purity are persistent challenges, particularly for high-grade HCl applications in electronics and pharmaceutical industries. Trace impurities can significantly impact the acid's performance in sensitive applications, necessitating advanced purification techniques that add complexity and cost to the production process.
The scaling of novel synthesis methods from laboratory to industrial scale presents a significant hurdle. Promising technologies, such as electrochemical HCl production or innovative catalytic processes, often face challenges in maintaining efficiency and economic viability when scaled up. Bridging this gap requires substantial investment in research and development, as well as pilot plant testing.
Regulatory compliance and safety standards continue to evolve, particularly concerning emissions and worker safety. Meeting these increasingly stringent requirements while maintaining competitive production costs poses a complex challenge for manufacturers. This necessitates ongoing innovation in process design, emissions control, and safety protocols.
Addressing these multifaceted challenges requires a holistic approach, combining technological innovation, process optimization, and strategic planning. The future of HCl synthesis lies in developing more sustainable, energy-efficient, and cost-effective production methods that can meet the growing global demand while minimizing environmental impact and ensuring product quality.
Energy consumption remains a critical challenge in HCl manufacturing. Many existing processes require high temperatures and pressures, resulting in substantial energy inputs and associated costs. This not only affects the economic viability of production but also contributes to the carbon footprint of the industry, raising concerns about long-term sustainability in an increasingly environmentally conscious market.
Raw material availability and cost fluctuations pose another significant challenge. The dependence on specific feedstocks, such as rock salt for the Mannheim process or methane for the methane chlorination process, exposes manufacturers to supply chain vulnerabilities and price volatility. This can lead to unpredictable production costs and potential supply disruptions, impacting the stability of HCl production.
Corrosion and equipment degradation present ongoing technical challenges in HCl synthesis. The highly corrosive nature of hydrochloric acid necessitates the use of specialized materials and frequent maintenance, increasing operational costs and potential safety risks. Developing more resistant materials or innovative reactor designs remains a key focus area for improving process reliability and reducing downtime.
Quality control and product purity are persistent challenges, particularly for high-grade HCl applications in electronics and pharmaceutical industries. Trace impurities can significantly impact the acid's performance in sensitive applications, necessitating advanced purification techniques that add complexity and cost to the production process.
The scaling of novel synthesis methods from laboratory to industrial scale presents a significant hurdle. Promising technologies, such as electrochemical HCl production or innovative catalytic processes, often face challenges in maintaining efficiency and economic viability when scaled up. Bridging this gap requires substantial investment in research and development, as well as pilot plant testing.
Regulatory compliance and safety standards continue to evolve, particularly concerning emissions and worker safety. Meeting these increasingly stringent requirements while maintaining competitive production costs poses a complex challenge for manufacturers. This necessitates ongoing innovation in process design, emissions control, and safety protocols.
Addressing these multifaceted challenges requires a holistic approach, combining technological innovation, process optimization, and strategic planning. The future of HCl synthesis lies in developing more sustainable, energy-efficient, and cost-effective production methods that can meet the growing global demand while minimizing environmental impact and ensuring product quality.
State-of-the-Art HCl Synthesis Methods
01 Production methods of hydrochloric acid
Various methods are employed to produce hydrochloric acid, including the reaction of chlorine with hydrogen, the chlorination of hydrocarbons, and as a byproduct in chemical processes. These production methods aim to optimize yield and purity while minimizing environmental impact.- Production methods of hydrochloric acid: Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.
- Purification and concentration techniques: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a catalyst in various reactions. It plays a crucial role in the production of pharmaceuticals, plastics, and other industrial chemicals.
- Safety and handling equipment: Specialized equipment and safety measures are essential for handling hydrochloric acid due to its corrosive nature. This includes acid-resistant storage tanks, protective gear, and emergency response systems to mitigate risks associated with spills or exposure.
- Environmental impact and waste management: Managing the environmental impact of hydrochloric acid involves proper disposal methods, neutralization techniques, and recycling processes. Efforts are made to minimize emissions and develop eco-friendly alternatives in industrial processes where possible.
02 Purification and concentration of hydrochloric acid
Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and increase the acid concentration for various industrial applications.Expand Specific Solutions03 Applications of hydrochloric acid in chemical processing
Hydrochloric acid is widely used in various chemical processes, including metal treatment, pH regulation, and as a reagent in organic synthesis. Its versatility makes it a crucial component in many industrial applications.Expand Specific Solutions04 Safety and handling of hydrochloric acid
Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of specialized equipment, protective gear, and storage solutions to minimize risks associated with its use.Expand Specific Solutions05 Environmental considerations and waste management
Managing the environmental impact of hydrochloric acid production and use involves developing sustainable practices, recycling methods, and proper disposal techniques. These efforts aim to reduce pollution and comply with environmental regulations.Expand Specific Solutions
Key HCl Manufacturers and Suppliers
The research on cutting-edge hydrochloric acid manufacturing techniques is in a mature phase, with established players dominating the market. The global hydrochloric acid market size is substantial, driven by diverse industrial applications. Technological advancements focus on improving efficiency and environmental sustainability. Key players like BASF Corp., Sumitomo Chemical Co., Ltd., and Shin-Etsu Chemical Co., Ltd. are at the forefront of innovation, investing in R&D to develop cleaner production methods and higher-purity products. Emerging companies such as Jiangyin Runma Electronic Material Co., Ltd. are also contributing to the field, particularly in high-purity electronic-grade hydrochloric acid production. The competitive landscape is characterized by a mix of large chemical conglomerates and specialized manufacturers, with ongoing efforts to enhance production processes and product quality.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has pioneered a cutting-edge hydrochloric acid manufacturing technique based on the chlor-alkali process with improved sustainability features. Their method utilizes a zero-gap membrane electrolysis cell design, which significantly reduces the inter-electrode gap, leading to lower electrical resistance and improved energy efficiency[2]. The company has also implemented advanced process control systems and real-time monitoring to optimize the production parameters. Additionally, Akzo Nobel has developed a novel approach to recover and recycle by-products, minimizing waste and improving overall resource utilization in the hydrochloric acid production process[4].
Strengths: Highly energy-efficient, reduced environmental footprint, improved process control. Weaknesses: Complex system design, potentially higher maintenance requirements.
BASF Corp.
Technical Solution: BASF has developed an innovative hydrochloric acid manufacturing technique using a membrane electrolysis process. This method produces high-purity hydrochloric acid by electrolyzing an aqueous solution of sodium chloride in a membrane cell[1]. The process involves the use of ion-selective membranes to separate the anode and cathode compartments, allowing for the efficient production of HCl without the need for additional purification steps. BASF's technique also incorporates advanced catalysts and electrode materials to enhance the electrolysis efficiency and reduce energy consumption[3].
Strengths: High purity product, energy-efficient process, reduced environmental impact. Weaknesses: Higher initial capital investment, potential membrane fouling issues over time.
Innovative HCl Production Patents
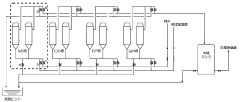
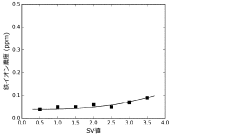
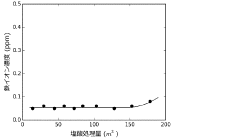
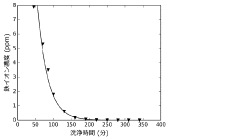
Production method and production apparatus for high purity hydrochloric acid
PatentActiveJP2016138017A
Innovation
- A method and apparatus using strongly basic anion exchange resins to selectively capture and remove iron ions from hydrochloric acid, employing multiple parallel columns for continuous operation and sequential regeneration to maintain efficiency.
Method for producing high-purity hydrochloric acid
PatentInactiveUS6793905B1
Innovation
- The process involves heating hydrogen chloride gas from hydrochloric acid with a content greater than 21% to pass through a retention column and demister made of fluorinated or perfluorinated polyolefin, followed by absorption in ultrapure water, with recycling and concentration control to achieve a 35-38% hydrochloric acid solution, using a vaporization plant and adiabatic absorption column system.
Environmental Impact of HCl Production
The production of hydrochloric acid (HCl) has significant environmental implications that must be carefully considered and managed. The primary environmental concerns associated with HCl manufacturing include air pollution, water contamination, and soil degradation. During the production process, potential emissions of chlorine gas, hydrogen chloride, and other hazardous substances can contribute to air quality issues if not properly controlled. These emissions may lead to the formation of acid rain, which can have far-reaching effects on ecosystems, infrastructure, and human health.
Water pollution is another critical environmental aspect of HCl production. Effluents from manufacturing facilities may contain traces of acid, heavy metals, and other contaminants. If not adequately treated, these discharges can harm aquatic ecosystems, disrupt the pH balance of water bodies, and potentially contaminate drinking water sources. Proper wastewater treatment and monitoring systems are essential to mitigate these risks and ensure compliance with environmental regulations.
Soil contamination is a potential long-term consequence of HCl production, particularly in areas surrounding manufacturing plants. Accidental spills, leaks, or improper disposal of acid and related chemicals can lead to soil acidification, reducing fertility and damaging vegetation. This can have cascading effects on local biodiversity and agricultural productivity.
The energy-intensive nature of HCl production also contributes to its environmental footprint. The process typically requires significant amounts of electricity and heat, often derived from fossil fuels, leading to greenhouse gas emissions and contributing to climate change. As such, improving energy efficiency and transitioning to renewable energy sources in HCl manufacturing are crucial steps towards reducing its overall environmental impact.
Waste management is another key environmental consideration. The production process generates various by-products and waste materials that require proper handling and disposal. Improper management of these wastes can lead to environmental contamination and pose risks to human health and safety.
To address these environmental challenges, cutting-edge HCl manufacturing techniques are focusing on several key areas. These include the development of closed-loop production systems to minimize emissions and waste, the implementation of advanced scrubbing technologies to capture and neutralize acid gases, and the adoption of more efficient catalysts and process designs to reduce energy consumption and raw material usage.
Furthermore, there is a growing emphasis on green chemistry principles in HCl production. This involves exploring alternative synthesis routes that use less hazardous reagents, produce fewer by-products, and operate under milder conditions. Such approaches not only reduce environmental impacts but also often lead to improved process economics and safety.
Water pollution is another critical environmental aspect of HCl production. Effluents from manufacturing facilities may contain traces of acid, heavy metals, and other contaminants. If not adequately treated, these discharges can harm aquatic ecosystems, disrupt the pH balance of water bodies, and potentially contaminate drinking water sources. Proper wastewater treatment and monitoring systems are essential to mitigate these risks and ensure compliance with environmental regulations.
Soil contamination is a potential long-term consequence of HCl production, particularly in areas surrounding manufacturing plants. Accidental spills, leaks, or improper disposal of acid and related chemicals can lead to soil acidification, reducing fertility and damaging vegetation. This can have cascading effects on local biodiversity and agricultural productivity.
The energy-intensive nature of HCl production also contributes to its environmental footprint. The process typically requires significant amounts of electricity and heat, often derived from fossil fuels, leading to greenhouse gas emissions and contributing to climate change. As such, improving energy efficiency and transitioning to renewable energy sources in HCl manufacturing are crucial steps towards reducing its overall environmental impact.
Waste management is another key environmental consideration. The production process generates various by-products and waste materials that require proper handling and disposal. Improper management of these wastes can lead to environmental contamination and pose risks to human health and safety.
To address these environmental challenges, cutting-edge HCl manufacturing techniques are focusing on several key areas. These include the development of closed-loop production systems to minimize emissions and waste, the implementation of advanced scrubbing technologies to capture and neutralize acid gases, and the adoption of more efficient catalysts and process designs to reduce energy consumption and raw material usage.
Furthermore, there is a growing emphasis on green chemistry principles in HCl production. This involves exploring alternative synthesis routes that use less hazardous reagents, produce fewer by-products, and operate under milder conditions. Such approaches not only reduce environmental impacts but also often lead to improved process economics and safety.
Safety Regulations in HCl Industry
The hydrochloric acid (HCl) industry is subject to stringent safety regulations due to the hazardous nature of the chemical. These regulations are designed to protect workers, the environment, and the general public from potential risks associated with HCl production, storage, transportation, and use.
At the manufacturing level, safety regulations mandate the implementation of robust process safety management systems. These systems include hazard identification and risk assessment protocols, as well as the establishment of comprehensive emergency response plans. Manufacturers are required to conduct regular safety audits and maintain detailed documentation of their safety procedures and incident reports.
Personal protective equipment (PPE) requirements are a critical component of safety regulations in the HCl industry. Workers must be provided with and trained in the use of appropriate PPE, including chemical-resistant suits, gloves, boots, and respiratory protection. The selection of PPE must be based on the specific hazards present in each work area and the tasks being performed.
Containment and storage regulations are equally important. HCl must be stored in corrosion-resistant containers, typically made of materials such as polyethylene or specially lined steel. Storage areas must be equipped with secondary containment systems to prevent spills from spreading, and ventilation systems to manage potential fumes. Regular inspections of storage facilities are mandated to ensure the integrity of containment systems.
Transportation of HCl is governed by strict regulations that vary depending on the mode of transport and the quantity being shipped. These regulations cover aspects such as container specifications, labeling requirements, and documentation. Drivers and handlers must receive specialized training in the safe transport of hazardous materials and emergency response procedures.
Environmental protection is a key focus of HCl industry regulations. Manufacturers must implement emission control technologies to minimize the release of HCl vapors into the atmosphere. Wastewater treatment systems are required to neutralize any acidic effluents before discharge. Regular monitoring and reporting of emissions and effluents are typically mandated by environmental agencies.
Occupational exposure limits for HCl are set by regulatory bodies to protect worker health. These limits specify the maximum allowable concentration of HCl in the air that workers can be exposed to over various time periods. Employers are required to conduct regular air quality monitoring and implement engineering controls or work practices to maintain exposure levels below these limits.
In recent years, there has been an increased focus on process safety information management within the HCl industry. This includes the development and maintenance of detailed process flow diagrams, piping and instrumentation diagrams, and material safety data sheets. These documents must be readily accessible to workers and emergency responders.
As research into cutting-edge HCl manufacturing techniques progresses, safety regulations are likely to evolve. Regulatory bodies will need to stay abreast of new technologies and potential hazards, updating their guidelines accordingly. This may include the development of new safety standards for novel production methods or the implementation of advanced monitoring technologies to enhance safety in HCl facilities.
At the manufacturing level, safety regulations mandate the implementation of robust process safety management systems. These systems include hazard identification and risk assessment protocols, as well as the establishment of comprehensive emergency response plans. Manufacturers are required to conduct regular safety audits and maintain detailed documentation of their safety procedures and incident reports.
Personal protective equipment (PPE) requirements are a critical component of safety regulations in the HCl industry. Workers must be provided with and trained in the use of appropriate PPE, including chemical-resistant suits, gloves, boots, and respiratory protection. The selection of PPE must be based on the specific hazards present in each work area and the tasks being performed.
Containment and storage regulations are equally important. HCl must be stored in corrosion-resistant containers, typically made of materials such as polyethylene or specially lined steel. Storage areas must be equipped with secondary containment systems to prevent spills from spreading, and ventilation systems to manage potential fumes. Regular inspections of storage facilities are mandated to ensure the integrity of containment systems.
Transportation of HCl is governed by strict regulations that vary depending on the mode of transport and the quantity being shipped. These regulations cover aspects such as container specifications, labeling requirements, and documentation. Drivers and handlers must receive specialized training in the safe transport of hazardous materials and emergency response procedures.
Environmental protection is a key focus of HCl industry regulations. Manufacturers must implement emission control technologies to minimize the release of HCl vapors into the atmosphere. Wastewater treatment systems are required to neutralize any acidic effluents before discharge. Regular monitoring and reporting of emissions and effluents are typically mandated by environmental agencies.
Occupational exposure limits for HCl are set by regulatory bodies to protect worker health. These limits specify the maximum allowable concentration of HCl in the air that workers can be exposed to over various time periods. Employers are required to conduct regular air quality monitoring and implement engineering controls or work practices to maintain exposure levels below these limits.
In recent years, there has been an increased focus on process safety information management within the HCl industry. This includes the development and maintenance of detailed process flow diagrams, piping and instrumentation diagrams, and material safety data sheets. These documents must be readily accessible to workers and emergency responders.
As research into cutting-edge HCl manufacturing techniques progresses, safety regulations are likely to evolve. Regulatory bodies will need to stay abreast of new technologies and potential hazards, updating their guidelines accordingly. This may include the development of new safety standards for novel production methods or the implementation of advanced monitoring technologies to enhance safety in HCl facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!