How to Monitor Lithium Nitrate Decomposition Using Infrared Spectroscopy
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate IR Spectroscopy Background and Objectives
Infrared spectroscopy has emerged as a powerful analytical technique for monitoring chemical reactions and decomposition processes since its development in the mid-20th century. The application of this technology to lithium nitrate decomposition represents a significant advancement in energy storage research, particularly for thermal energy storage systems and lithium-ion battery safety studies. The evolution of IR spectroscopy from simple dispersive instruments to sophisticated Fourier Transform Infrared (FTIR) systems has enabled researchers to capture detailed molecular information with unprecedented precision and temporal resolution.
The thermal decomposition of lithium nitrate (LiNO₃) is of particular interest due to its applications in molten salt technologies, thermal energy storage, and as an electrolyte additive in lithium-ion batteries. Understanding the decomposition pathways and kinetics of LiNO₃ is crucial for optimizing these applications and ensuring safety in energy storage systems.
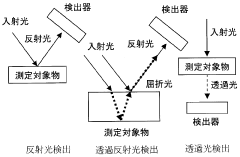
Recent technological developments in IR spectroscopy, including attenuated total reflection (ATR), diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), and in-situ/operando techniques, have opened new possibilities for real-time monitoring of decomposition processes. These advancements allow for the observation of transient species and intermediate compounds that form during the multi-step decomposition of lithium nitrate.
The primary objective of this technical research is to establish robust methodologies for monitoring lithium nitrate decomposition using infrared spectroscopy. This includes identifying characteristic IR absorption bands associated with LiNO₃ and its decomposition products, developing calibration methods for quantitative analysis, and optimizing experimental parameters for in-situ measurements under various temperature and atmospheric conditions.
Secondary objectives include correlating spectroscopic data with decomposition kinetics, elucidating the reaction mechanisms involved in LiNO₃ decomposition, and developing predictive models that can be applied to real-world energy storage systems. The research also aims to compare the effectiveness of different IR spectroscopic techniques for this specific application.
The technological trajectory suggests that combining IR spectroscopy with complementary analytical methods, such as mass spectrometry or thermal analysis, represents the next frontier in comprehensively characterizing lithium nitrate decomposition. Additionally, the miniaturization of IR spectroscopic equipment and the development of specialized sampling accessories are expected to facilitate field applications and industrial process monitoring.
This research addresses the growing need for advanced monitoring techniques in energy storage technologies, particularly as the demand for safer, more efficient lithium-based energy systems continues to increase globally. The findings will contribute to the broader understanding of molten salt chemistry and provide valuable insights for the design of next-generation thermal energy storage systems.
The thermal decomposition of lithium nitrate (LiNO₃) is of particular interest due to its applications in molten salt technologies, thermal energy storage, and as an electrolyte additive in lithium-ion batteries. Understanding the decomposition pathways and kinetics of LiNO₃ is crucial for optimizing these applications and ensuring safety in energy storage systems.
Recent technological developments in IR spectroscopy, including attenuated total reflection (ATR), diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), and in-situ/operando techniques, have opened new possibilities for real-time monitoring of decomposition processes. These advancements allow for the observation of transient species and intermediate compounds that form during the multi-step decomposition of lithium nitrate.
The primary objective of this technical research is to establish robust methodologies for monitoring lithium nitrate decomposition using infrared spectroscopy. This includes identifying characteristic IR absorption bands associated with LiNO₃ and its decomposition products, developing calibration methods for quantitative analysis, and optimizing experimental parameters for in-situ measurements under various temperature and atmospheric conditions.
Secondary objectives include correlating spectroscopic data with decomposition kinetics, elucidating the reaction mechanisms involved in LiNO₃ decomposition, and developing predictive models that can be applied to real-world energy storage systems. The research also aims to compare the effectiveness of different IR spectroscopic techniques for this specific application.
The technological trajectory suggests that combining IR spectroscopy with complementary analytical methods, such as mass spectrometry or thermal analysis, represents the next frontier in comprehensively characterizing lithium nitrate decomposition. Additionally, the miniaturization of IR spectroscopic equipment and the development of specialized sampling accessories are expected to facilitate field applications and industrial process monitoring.
This research addresses the growing need for advanced monitoring techniques in energy storage technologies, particularly as the demand for safer, more efficient lithium-based energy systems continues to increase globally. The findings will contribute to the broader understanding of molten salt chemistry and provide valuable insights for the design of next-generation thermal energy storage systems.
Market Applications for Lithium Nitrate Decomposition Monitoring
The monitoring of lithium nitrate decomposition using infrared spectroscopy presents significant market opportunities across multiple industries. In the energy storage sector, lithium-based batteries represent a rapidly growing market expected to reach $116 billion by 2030. Precise monitoring of lithium nitrate decomposition is critical for battery manufacturers seeking to enhance safety protocols and extend battery lifespan, particularly in electric vehicles and grid storage applications.
Thermal energy storage systems utilizing molten salts, including lithium nitrate mixtures, constitute another substantial market application. These systems are increasingly deployed in concentrated solar power plants, where real-time decomposition monitoring via infrared spectroscopy can prevent catastrophic system failures and optimize operational efficiency. The global thermal energy storage market is projected to grow at a compound annual growth rate of 12.8% through 2028.
The pharmaceutical industry represents a third significant application area. Lithium compounds are used in various medications, and monitoring their decomposition ensures product quality and safety. Infrared spectroscopy-based monitoring systems enable pharmaceutical manufacturers to comply with stringent regulatory requirements while minimizing production waste.
In the aerospace and defense sectors, lithium nitrate is utilized in specialized propellants and pyrotechnic compositions. Infrared monitoring technology provides crucial safety data during manufacturing, storage, and deployment phases. The market for advanced monitoring systems in this sector is estimated at $3.2 billion annually.
Environmental monitoring constitutes an emerging application area. As lithium mining and processing activities increase globally, regulatory bodies require more sophisticated tools to monitor potential environmental impacts. Infrared spectroscopy offers a non-invasive method to detect lithium nitrate decomposition products in soil and water samples.
Research institutions and academic laboratories form a smaller but technically sophisticated market segment. These organizations require high-precision analytical tools for fundamental research on lithium compounds and their decomposition mechanisms. The academic research equipment market specifically for spectroscopic analysis tools exceeds $1.5 billion globally.
Industrial process control systems represent perhaps the broadest application area. Manufacturing processes involving lithium compounds benefit from real-time monitoring capabilities, reducing waste and improving product consistency. The industrial process control market specifically for spectroscopic monitoring systems is growing at approximately 9% annually, driven by Industry 4.0 initiatives and increasing automation requirements.
Thermal energy storage systems utilizing molten salts, including lithium nitrate mixtures, constitute another substantial market application. These systems are increasingly deployed in concentrated solar power plants, where real-time decomposition monitoring via infrared spectroscopy can prevent catastrophic system failures and optimize operational efficiency. The global thermal energy storage market is projected to grow at a compound annual growth rate of 12.8% through 2028.
The pharmaceutical industry represents a third significant application area. Lithium compounds are used in various medications, and monitoring their decomposition ensures product quality and safety. Infrared spectroscopy-based monitoring systems enable pharmaceutical manufacturers to comply with stringent regulatory requirements while minimizing production waste.
In the aerospace and defense sectors, lithium nitrate is utilized in specialized propellants and pyrotechnic compositions. Infrared monitoring technology provides crucial safety data during manufacturing, storage, and deployment phases. The market for advanced monitoring systems in this sector is estimated at $3.2 billion annually.
Environmental monitoring constitutes an emerging application area. As lithium mining and processing activities increase globally, regulatory bodies require more sophisticated tools to monitor potential environmental impacts. Infrared spectroscopy offers a non-invasive method to detect lithium nitrate decomposition products in soil and water samples.
Research institutions and academic laboratories form a smaller but technically sophisticated market segment. These organizations require high-precision analytical tools for fundamental research on lithium compounds and their decomposition mechanisms. The academic research equipment market specifically for spectroscopic analysis tools exceeds $1.5 billion globally.
Industrial process control systems represent perhaps the broadest application area. Manufacturing processes involving lithium compounds benefit from real-time monitoring capabilities, reducing waste and improving product consistency. The industrial process control market specifically for spectroscopic monitoring systems is growing at approximately 9% annually, driven by Industry 4.0 initiatives and increasing automation requirements.
Current Challenges in IR Spectroscopic Analysis of Lithium Salts
Infrared (IR) spectroscopy has emerged as a powerful analytical technique for monitoring chemical reactions and material transformations. However, when applied to lithium salts, particularly lithium nitrate decomposition monitoring, several significant technical challenges persist that limit its effectiveness and reliability.
The primary challenge lies in the spectral interpretation of lithium-containing compounds. Lithium, being the lightest metal, forms bonds that vibrate at frequencies often overlapping with other functional groups. This spectral congestion makes it difficult to isolate and track specific lithium nitrate decomposition markers, especially in complex reaction environments where multiple species coexist.
Signal-to-noise ratio presents another substantial hurdle. The IR absorption bands of lithium salts are often weak compared to other components in the sample matrix. This is particularly problematic when monitoring decomposition processes where intermediate species may be present in low concentrations, requiring enhanced detection sensitivity that current standard IR instrumentation struggles to provide.
Temperature control during in-situ measurements creates additional complications. Lithium nitrate decomposition typically occurs at elevated temperatures (above 500°C), which introduces thermal noise and can damage conventional IR sampling accessories. The development of specialized high-temperature sampling cells with appropriate window materials that maintain transparency in the IR region while withstanding these extreme conditions remains technically challenging.
Sample preparation issues further compound these difficulties. Lithium salts are hygroscopic, readily absorbing atmospheric moisture that can interfere with spectral analysis. Additionally, the physical state changes during decomposition—from solid to molten to gaseous products—require versatile sampling techniques that can accommodate these transitions while maintaining spectral quality.
Resolution limitations of conventional FTIR systems (typically 4-8 cm⁻¹) may be insufficient to distinguish subtle spectral changes during the early stages of decomposition or to differentiate between closely related lithium-containing species. This becomes particularly problematic when attempting to identify reaction intermediates with similar structural features.
Data processing challenges also exist, as the complex spectral changes during decomposition require sophisticated chemometric approaches to extract meaningful kinetic and mechanistic information. Current algorithms often struggle with the multivariate nature of the decomposition process, especially when baseline drift occurs due to temperature fluctuations.
Finally, reference spectra for lithium nitrate decomposition products and intermediates are notably scarce in standard spectral libraries, complicating peak assignment and reaction pathway elucidation. This knowledge gap significantly hinders the development of robust monitoring protocols and automated analysis systems for lithium nitrate decomposition processes.
The primary challenge lies in the spectral interpretation of lithium-containing compounds. Lithium, being the lightest metal, forms bonds that vibrate at frequencies often overlapping with other functional groups. This spectral congestion makes it difficult to isolate and track specific lithium nitrate decomposition markers, especially in complex reaction environments where multiple species coexist.
Signal-to-noise ratio presents another substantial hurdle. The IR absorption bands of lithium salts are often weak compared to other components in the sample matrix. This is particularly problematic when monitoring decomposition processes where intermediate species may be present in low concentrations, requiring enhanced detection sensitivity that current standard IR instrumentation struggles to provide.
Temperature control during in-situ measurements creates additional complications. Lithium nitrate decomposition typically occurs at elevated temperatures (above 500°C), which introduces thermal noise and can damage conventional IR sampling accessories. The development of specialized high-temperature sampling cells with appropriate window materials that maintain transparency in the IR region while withstanding these extreme conditions remains technically challenging.
Sample preparation issues further compound these difficulties. Lithium salts are hygroscopic, readily absorbing atmospheric moisture that can interfere with spectral analysis. Additionally, the physical state changes during decomposition—from solid to molten to gaseous products—require versatile sampling techniques that can accommodate these transitions while maintaining spectral quality.
Resolution limitations of conventional FTIR systems (typically 4-8 cm⁻¹) may be insufficient to distinguish subtle spectral changes during the early stages of decomposition or to differentiate between closely related lithium-containing species. This becomes particularly problematic when attempting to identify reaction intermediates with similar structural features.
Data processing challenges also exist, as the complex spectral changes during decomposition require sophisticated chemometric approaches to extract meaningful kinetic and mechanistic information. Current algorithms often struggle with the multivariate nature of the decomposition process, especially when baseline drift occurs due to temperature fluctuations.
Finally, reference spectra for lithium nitrate decomposition products and intermediates are notably scarce in standard spectral libraries, complicating peak assignment and reaction pathway elucidation. This knowledge gap significantly hinders the development of robust monitoring protocols and automated analysis systems for lithium nitrate decomposition processes.
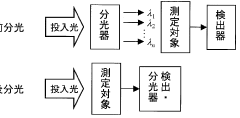
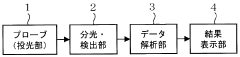
Established IR Methodologies for Lithium Compound Analysis
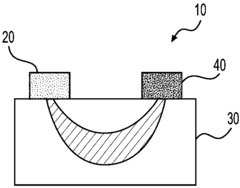
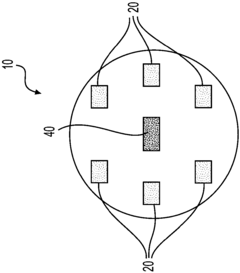
01 In-situ monitoring of lithium nitrate decomposition using infrared spectroscopy
Infrared spectroscopy techniques can be used for real-time monitoring of lithium nitrate decomposition processes. These methods allow for the detection of characteristic absorption bands associated with lithium nitrate and its decomposition products. In-situ monitoring enables researchers to track the decomposition kinetics and identify intermediate compounds formed during the thermal breakdown of lithium nitrate, providing valuable insights into the reaction mechanisms.- Infrared spectroscopy techniques for monitoring lithium compound decomposition: Various infrared spectroscopy techniques can be employed to monitor the decomposition of lithium compounds, including lithium nitrate. These techniques provide real-time data on the chemical changes occurring during the decomposition process, allowing researchers to track reaction progress and identify intermediate compounds. Advanced infrared spectroscopy methods offer high sensitivity for detecting subtle changes in molecular structure during thermal decomposition events.
- In-situ monitoring systems for lithium battery materials: Specialized monitoring systems have been developed for in-situ observation of lithium-containing materials during thermal processes. These systems integrate infrared spectroscopy with temperature control mechanisms to provide comprehensive data on decomposition kinetics. The monitoring setups allow for precise control of experimental conditions while simultaneously collecting spectral data, enabling researchers to establish correlations between temperature profiles and decomposition patterns of lithium nitrate and related compounds.
- Data analysis methods for decomposition spectral patterns: Advanced algorithms and data processing techniques have been developed specifically for analyzing infrared spectral data from lithium compound decomposition. These methods include pattern recognition approaches that can identify characteristic spectral signatures associated with different stages of the decomposition process. Machine learning techniques are increasingly being applied to interpret complex spectral changes and extract meaningful kinetic parameters from the decomposition monitoring data.
- Portable and miniaturized infrared monitoring devices: Compact and portable infrared spectroscopy systems have been designed for field monitoring of lithium compound decomposition. These devices integrate miniaturized infrared sensors with specialized sample holders suitable for lithium-containing materials. The portable nature of these systems allows for on-site monitoring of decomposition processes in various industrial and research settings, providing immediate feedback on reaction progress and potential safety concerns.
- Combined spectroscopic approaches for comprehensive decomposition analysis: Hybrid analytical systems that combine infrared spectroscopy with other spectroscopic techniques provide more comprehensive insights into lithium nitrate decomposition mechanisms. These multi-modal approaches may integrate infrared with Raman spectroscopy, mass spectrometry, or thermal analysis techniques to capture complementary data sets. The combined data allows researchers to develop more accurate models of the decomposition pathways and better understand the relationship between structural changes and thermal behavior during the decomposition process.
02 FTIR analysis for lithium-based battery materials
Fourier Transform Infrared (FTIR) spectroscopy is applied to analyze lithium-based battery materials, including those containing lithium nitrate. This technique helps in understanding the decomposition behavior of lithium compounds during battery operation and thermal events. FTIR analysis can identify chemical changes in the electrolyte and electrode materials, which is crucial for improving battery safety and performance by monitoring the decomposition products of lithium nitrate additives.Expand Specific Solutions03 Thermal analysis coupled with infrared spectroscopy for lithium compounds
Combining thermal analysis techniques with infrared spectroscopy provides comprehensive information about the decomposition behavior of lithium nitrate. Methods such as thermogravimetric analysis (TGA) coupled with infrared spectroscopy allow researchers to correlate mass loss events with specific chemical changes detected in the infrared spectrum. This approach enables precise identification of decomposition stages and the evolution of gaseous products during lithium nitrate thermal breakdown.Expand Specific Solutions04 Portable and miniaturized infrared systems for lithium compound analysis
Portable and miniaturized infrared spectroscopy systems have been developed for on-site monitoring of lithium compounds including lithium nitrate. These systems enable field analysis of decomposition processes without requiring laboratory equipment. The miniaturized technology incorporates specialized sensors and data processing algorithms to detect specific infrared signatures associated with lithium nitrate decomposition, making it suitable for industrial applications and quality control in lithium processing.Expand Specific Solutions05 Advanced data processing for infrared spectral analysis of lithium compounds
Advanced data processing techniques enhance the analysis of infrared spectral data collected during lithium nitrate decomposition. These methods include machine learning algorithms, multivariate analysis, and chemometric approaches that can extract subtle spectral changes and correlate them with specific decomposition events. The improved data processing enables more accurate quantification of decomposition products and reaction rates, leading to better understanding of the thermal behavior of lithium nitrate in various applications.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The infrared spectroscopy monitoring of lithium nitrate decomposition market is in its growth phase, with increasing applications in energy storage, materials science, and environmental monitoring. The global market size is expanding due to rising demand for advanced analytical techniques in lithium-based technologies. Technologically, this field shows moderate maturity with significant ongoing innovation. Leading players include Shimadzu Corp. and Air Liquide SA, who offer sophisticated spectroscopic equipment, while research institutions like Fraunhofer-Gesellschaft and China Institute of Atomic Energy drive fundamental advancements. Academic-industrial collaborations between entities like Chongqing University and Jinchuan Group are accelerating practical applications. Companies like Edwards Lifesciences and Verily Life Sciences are exploring specialized applications in biomedical fields, indicating cross-sector expansion potential.
China Institute of Atomic Energy
Technical Solution: The China Institute of Atomic Energy has pioneered a specialized infrared spectroscopy methodology for monitoring lithium nitrate decomposition under radiation conditions, addressing unique challenges in nuclear applications. Their approach combines time-resolved FTIR spectroscopy with radiation-resistant optical components to enable in-situ monitoring during both thermal and radiation-induced decomposition processes. The system features custom-designed sample holders that can withstand both high temperatures (up to 550°C) and radiation environments, with remote operation capabilities for hazardous conditions. Their methodology incorporates isotope-specific spectral analysis, allowing researchers to track decomposition pathways using isotopically labeled compounds (⁶Li vs. ⁷Li). The institute has developed specialized algorithms for deconvoluting overlapping spectral features characteristic of complex lithium-containing mixtures, enabling quantitative analysis of decomposition products even in the presence of interfering species. This technology has proven particularly valuable for studying lithium nitrate behavior in molten salt reactors and nuclear waste processing applications.
Strengths: Unique capability for monitoring decomposition under radiation conditions; specialized expertise in isotope-specific spectral analysis; robust design suitable for extreme environments. Weaknesses: Highly specialized equipment with limited commercial availability; requires significant expertise in both spectroscopy and nuclear materials; higher operational complexity compared to conventional FTIR systems.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed advanced FTIR (Fourier Transform Infrared) spectroscopy systems specifically optimized for monitoring lithium nitrate decomposition processes. Their technology employs high-resolution mid-infrared spectroscopy (4000-400 cm⁻¹) with specialized sampling accessories designed for both in-situ and ex-situ analysis of lithium compounds. The system features temperature-controlled sample chambers capable of operating up to 600°C, allowing real-time monitoring of decomposition kinetics. Shimadzu's proprietary software algorithms can identify and quantify multiple decomposition products simultaneously, including NO₂, NO, O₂, and various lithium oxide species. Their latest models incorporate chemometric analysis tools that enable automated peak identification and reaction progress tracking, making them particularly valuable for battery research and thermal energy storage applications.
Strengths: Superior spectral resolution (up to 0.25 cm⁻¹) allowing detection of subtle chemical changes; robust calibration models for quantitative analysis of lithium compounds; integrated data processing software specifically designed for decomposition studies. Weaknesses: Higher cost compared to general-purpose FTIR systems; requires specialized training for optimal operation; sampling accessories may need customization for specific research applications.
Key Spectral Features and Interpretation Techniques
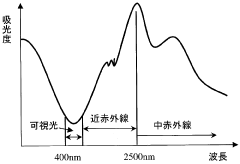
Method for nondestructively examining component of vegetable or the like by near-infrared spectroscopy and its device
PatentWO2005111583A1
Innovation
- A non-destructive near-infrared spectroscopy method that uses wavelengths between 400nm and 2500nm to analyze nitrate ion concentration in vegetables, employing singular value decomposition and multiple regression analysis to create a measurement model for quantitative and qualitative analysis, allowing for real-time measurement without sample preparation.
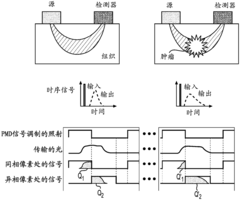
Continuous monitoring of tumor hypoxia using near-infrared spectroscopy and tomography with a photonic mixer device
PatentInactiveCN109069076A
Innovation
- Using a combination of PMD camera chip and amplitude-modulated near-infrared light source, by measuring the amplitude and phase shift of reflected light, calculating the absorption coefficient and reduced scattering coefficient, and combining data of multiple light wavelengths, the concentration of oxyhemoglobin and deoxygenated hemoglobin can be accurately measured to achieve Miniaturized, low-cost frequency domain spectroscopy and tomography equipment.
Safety Protocols for Lithium Compound Handling
When handling lithium nitrate for infrared spectroscopy monitoring of its decomposition, strict safety protocols must be implemented due to the compound's oxidizing properties and potential health hazards. Laboratory personnel must wear appropriate personal protective equipment including chemical-resistant gloves, safety goggles, lab coats, and in some cases, respiratory protection when dealing with powdered forms that may become airborne. Nitrile gloves are generally recommended, but compatibility should be verified for the specific experimental conditions.
Storage considerations are critical for lithium compounds, which must be kept in tightly sealed containers in cool, dry areas away from incompatible materials such as reducing agents, organic materials, and acids. Dedicated storage cabinets for oxidizers should be utilized, with clear labeling indicating the hazardous nature of the contents.
Workspace preparation requires adequate ventilation systems, preferably including fume hoods with proper face velocity for handling lithium nitrate, especially during sample preparation for spectroscopic analysis. Emergency equipment including eyewash stations, safety showers, and appropriate fire extinguishers (Class D for lithium metal fires) must be readily accessible in the laboratory.
Waste management protocols must address the proper disposal of lithium-containing waste, which should never be disposed of down drains or in regular trash. Segregation of waste streams and compliance with local regulations for hazardous waste disposal are essential components of the laboratory safety program.
Emergency response procedures must be established for potential incidents including spills, fires, or exposure. For spills, non-combustible absorbent materials should be used, avoiding materials that might react with the oxidizing compound. Personnel should be trained in these procedures before working with lithium compounds.
Special considerations apply when monitoring decomposition reactions using infrared spectroscopy. Temperature control systems must be properly designed to prevent runaway reactions, particularly when heating lithium nitrate to study its decomposition. Gas evolution during decomposition must be safely vented and, if necessary, scrubbed or trapped to prevent the release of nitrogen oxides into the laboratory environment.
Documentation and training requirements include maintaining current Safety Data Sheets (SDS), conducting regular safety briefings, and ensuring all personnel are familiar with the specific hazards of lithium compounds and the safety equipment available in the laboratory. Periodic reviews of safety protocols should be conducted to incorporate new information or address changing experimental conditions.
Storage considerations are critical for lithium compounds, which must be kept in tightly sealed containers in cool, dry areas away from incompatible materials such as reducing agents, organic materials, and acids. Dedicated storage cabinets for oxidizers should be utilized, with clear labeling indicating the hazardous nature of the contents.
Workspace preparation requires adequate ventilation systems, preferably including fume hoods with proper face velocity for handling lithium nitrate, especially during sample preparation for spectroscopic analysis. Emergency equipment including eyewash stations, safety showers, and appropriate fire extinguishers (Class D for lithium metal fires) must be readily accessible in the laboratory.
Waste management protocols must address the proper disposal of lithium-containing waste, which should never be disposed of down drains or in regular trash. Segregation of waste streams and compliance with local regulations for hazardous waste disposal are essential components of the laboratory safety program.
Emergency response procedures must be established for potential incidents including spills, fires, or exposure. For spills, non-combustible absorbent materials should be used, avoiding materials that might react with the oxidizing compound. Personnel should be trained in these procedures before working with lithium compounds.
Special considerations apply when monitoring decomposition reactions using infrared spectroscopy. Temperature control systems must be properly designed to prevent runaway reactions, particularly when heating lithium nitrate to study its decomposition. Gas evolution during decomposition must be safely vented and, if necessary, scrubbed or trapped to prevent the release of nitrogen oxides into the laboratory environment.
Documentation and training requirements include maintaining current Safety Data Sheets (SDS), conducting regular safety briefings, and ensuring all personnel are familiar with the specific hazards of lithium compounds and the safety equipment available in the laboratory. Periodic reviews of safety protocols should be conducted to incorporate new information or address changing experimental conditions.
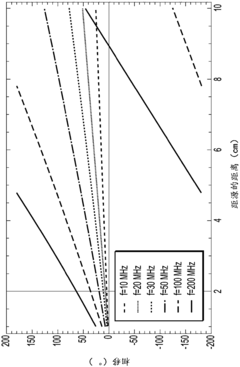
Data Processing Algorithms for Spectral Analysis
The spectral data collected during lithium nitrate decomposition monitoring contains complex information that requires sophisticated processing algorithms to extract meaningful insights. Principal Component Analysis (PCA) stands as a fundamental technique for dimensionality reduction in spectral datasets, allowing researchers to identify the most significant variations in the infrared spectra during the decomposition process. This statistical approach transforms potentially correlated variables into linearly uncorrelated components, effectively highlighting spectral changes that correspond to chemical transformations in lithium nitrate.
Partial Least Squares (PLS) regression algorithms offer another powerful approach, particularly valuable when correlating spectral data with quantitative measurements of decomposition progress. By establishing mathematical relationships between spectral features and decomposition parameters, PLS enables predictive modeling that can estimate decomposition rates and product formation without additional analytical techniques.
Multivariate Curve Resolution (MCR) algorithms have proven especially effective for decomposing overlapping spectral bands, a common challenge in monitoring lithium nitrate decomposition where multiple chemical species may contribute to the observed infrared spectrum simultaneously. MCR techniques can separate these contributions, providing concentration profiles of reactants, intermediates, and products throughout the decomposition process.
Machine learning approaches, particularly artificial neural networks (ANNs) and support vector machines (SVMs), represent the cutting edge in spectral analysis. These algorithms can be trained to recognize subtle spectral patterns associated with different stages of lithium nitrate decomposition, offering superior performance in complex reaction environments where traditional analytical methods struggle to provide clear results.
Savitzky-Golay filtering algorithms remain essential for preprocessing infrared spectral data, effectively reducing noise while preserving important spectral features. This mathematical procedure performs a local polynomial regression on a series of values, providing smoothed data that enhances subsequent analysis steps without distorting the underlying chemical information.
Derivative spectroscopy algorithms transform absorption spectra into their first or second derivatives, significantly improving the resolution of overlapping bands in the infrared spectrum of decomposing lithium nitrate. This mathematical transformation accentuates subtle spectral changes that might otherwise be overlooked in the original absorption data, enabling more precise monitoring of decomposition kinetics.
Partial Least Squares (PLS) regression algorithms offer another powerful approach, particularly valuable when correlating spectral data with quantitative measurements of decomposition progress. By establishing mathematical relationships between spectral features and decomposition parameters, PLS enables predictive modeling that can estimate decomposition rates and product formation without additional analytical techniques.
Multivariate Curve Resolution (MCR) algorithms have proven especially effective for decomposing overlapping spectral bands, a common challenge in monitoring lithium nitrate decomposition where multiple chemical species may contribute to the observed infrared spectrum simultaneously. MCR techniques can separate these contributions, providing concentration profiles of reactants, intermediates, and products throughout the decomposition process.
Machine learning approaches, particularly artificial neural networks (ANNs) and support vector machines (SVMs), represent the cutting edge in spectral analysis. These algorithms can be trained to recognize subtle spectral patterns associated with different stages of lithium nitrate decomposition, offering superior performance in complex reaction environments where traditional analytical methods struggle to provide clear results.
Savitzky-Golay filtering algorithms remain essential for preprocessing infrared spectral data, effectively reducing noise while preserving important spectral features. This mathematical procedure performs a local polynomial regression on a series of values, providing smoothed data that enhances subsequent analysis steps without distorting the underlying chemical information.
Derivative spectroscopy algorithms transform absorption spectra into their first or second derivatives, significantly improving the resolution of overlapping bands in the infrared spectrum of decomposing lithium nitrate. This mathematical transformation accentuates subtle spectral changes that might otherwise be overlooked in the original absorption data, enabling more precise monitoring of decomposition kinetics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!