How to Validate FTIR Results With Reference Samples
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
FTIR Validation Background and Objectives
Fourier Transform Infrared Spectroscopy (FTIR) has evolved significantly since its inception in the mid-20th century, becoming an indispensable analytical technique across various industries including pharmaceuticals, materials science, environmental monitoring, and forensic analysis. The technology's development trajectory has been characterized by continuous improvements in resolution, sensitivity, and data processing capabilities, transforming it from a specialized laboratory tool to a widely accessible analytical method.
The validation of FTIR results represents a critical aspect of analytical quality assurance, ensuring that spectroscopic data accurately reflects the chemical composition and structure of analyzed samples. This validation process has become increasingly important as regulatory requirements have tightened across industries, particularly in pharmaceutical manufacturing, food safety, and environmental compliance monitoring.
Recent technological advancements have expanded FTIR applications beyond traditional laboratory settings into portable field devices and inline process monitoring systems. This evolution has created new validation challenges that must be addressed through systematic reference sample protocols. The integration of FTIR with other analytical techniques and artificial intelligence has further complicated validation methodologies, necessitating more sophisticated reference standards and validation procedures.
The primary objective of FTIR validation using reference samples is to establish and maintain analytical reliability through systematic verification of instrument performance, method accuracy, and result reproducibility. This involves developing comprehensive validation protocols that incorporate appropriate reference materials with well-characterized spectral properties that can serve as benchmarks for evaluating analytical results.
Secondary objectives include quantifying detection limits, establishing measurement uncertainty parameters, and determining the specificity and selectivity of FTIR methods for particular applications. These parameters are essential for ensuring that analytical results can be interpreted with appropriate confidence levels and that meaningful conclusions can be drawn from spectroscopic data.
Long-term validation goals focus on establishing standardized protocols that can be universally applied across different instrument platforms and laboratory environments. This standardization effort aims to improve inter-laboratory comparability of results and facilitate regulatory compliance across international boundaries, particularly important in global supply chains and collaborative research initiatives.
The technological trajectory suggests continued refinement of validation methodologies, with increasing emphasis on automated validation procedures, real-time quality control, and integration with digital data management systems. These developments align with broader industry trends toward process analytical technology (PAT) and quality by design (QbD) approaches that emphasize built-in quality assurance rather than end-product testing.
The validation of FTIR results represents a critical aspect of analytical quality assurance, ensuring that spectroscopic data accurately reflects the chemical composition and structure of analyzed samples. This validation process has become increasingly important as regulatory requirements have tightened across industries, particularly in pharmaceutical manufacturing, food safety, and environmental compliance monitoring.
Recent technological advancements have expanded FTIR applications beyond traditional laboratory settings into portable field devices and inline process monitoring systems. This evolution has created new validation challenges that must be addressed through systematic reference sample protocols. The integration of FTIR with other analytical techniques and artificial intelligence has further complicated validation methodologies, necessitating more sophisticated reference standards and validation procedures.
The primary objective of FTIR validation using reference samples is to establish and maintain analytical reliability through systematic verification of instrument performance, method accuracy, and result reproducibility. This involves developing comprehensive validation protocols that incorporate appropriate reference materials with well-characterized spectral properties that can serve as benchmarks for evaluating analytical results.
Secondary objectives include quantifying detection limits, establishing measurement uncertainty parameters, and determining the specificity and selectivity of FTIR methods for particular applications. These parameters are essential for ensuring that analytical results can be interpreted with appropriate confidence levels and that meaningful conclusions can be drawn from spectroscopic data.
Long-term validation goals focus on establishing standardized protocols that can be universally applied across different instrument platforms and laboratory environments. This standardization effort aims to improve inter-laboratory comparability of results and facilitate regulatory compliance across international boundaries, particularly important in global supply chains and collaborative research initiatives.
The technological trajectory suggests continued refinement of validation methodologies, with increasing emphasis on automated validation procedures, real-time quality control, and integration with digital data management systems. These developments align with broader industry trends toward process analytical technology (PAT) and quality by design (QbD) approaches that emphasize built-in quality assurance rather than end-product testing.
Market Demand for Accurate FTIR Analysis
The global market for Fourier Transform Infrared (FTIR) spectroscopy has witnessed substantial growth, driven by increasing demand for accurate analytical techniques across multiple industries. The market size for FTIR spectroscopy equipment reached approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate of 6.8% through 2028, highlighting the expanding need for precise molecular analysis capabilities.
Pharmaceutical and biotechnology sectors represent the largest market segments, collectively accounting for over 40% of FTIR applications. These industries require highly accurate analytical methods for drug development, quality control, and regulatory compliance. The FDA and other regulatory bodies have strengthened requirements for analytical method validation, creating significant demand for reference sample-validated FTIR techniques that can withstand regulatory scrutiny.
Environmental monitoring applications have emerged as the fastest-growing segment, expanding at nearly 8.5% annually. Government agencies and private organizations increasingly rely on FTIR for detecting and quantifying environmental contaminants, where validation against certified reference materials is essential for legal and compliance purposes.
The food and beverage industry has also embraced FTIR technology for quality control and authenticity verification, representing approximately 15% of the market. As food fraud cases increase globally, validated FTIR methods using appropriate reference standards have become critical tools for ensuring product integrity and consumer safety.
Academic and research institutions contribute significantly to market demand, particularly for advanced FTIR systems with comprehensive validation capabilities. Research funding directed toward spectroscopic method development has increased by 12% over the past three years, reflecting growing interest in improving analytical accuracy through proper validation protocols.
Geographically, North America leads the market with a 35% share, followed closely by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region demonstrates the highest growth rate, driven by expanding pharmaceutical manufacturing and increasing adoption of advanced analytical technologies in China and India.
Market research indicates that over 78% of end-users consider validation capabilities and reference sample compatibility as "very important" or "critical" factors when purchasing FTIR systems. This represents a significant shift from a decade ago when only 45% of users prioritized these features, underscoring the growing emphasis on analytical accuracy and reliability across industries.
Pharmaceutical and biotechnology sectors represent the largest market segments, collectively accounting for over 40% of FTIR applications. These industries require highly accurate analytical methods for drug development, quality control, and regulatory compliance. The FDA and other regulatory bodies have strengthened requirements for analytical method validation, creating significant demand for reference sample-validated FTIR techniques that can withstand regulatory scrutiny.
Environmental monitoring applications have emerged as the fastest-growing segment, expanding at nearly 8.5% annually. Government agencies and private organizations increasingly rely on FTIR for detecting and quantifying environmental contaminants, where validation against certified reference materials is essential for legal and compliance purposes.
The food and beverage industry has also embraced FTIR technology for quality control and authenticity verification, representing approximately 15% of the market. As food fraud cases increase globally, validated FTIR methods using appropriate reference standards have become critical tools for ensuring product integrity and consumer safety.
Academic and research institutions contribute significantly to market demand, particularly for advanced FTIR systems with comprehensive validation capabilities. Research funding directed toward spectroscopic method development has increased by 12% over the past three years, reflecting growing interest in improving analytical accuracy through proper validation protocols.
Geographically, North America leads the market with a 35% share, followed closely by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region demonstrates the highest growth rate, driven by expanding pharmaceutical manufacturing and increasing adoption of advanced analytical technologies in China and India.
Market research indicates that over 78% of end-users consider validation capabilities and reference sample compatibility as "very important" or "critical" factors when purchasing FTIR systems. This represents a significant shift from a decade ago when only 45% of users prioritized these features, underscoring the growing emphasis on analytical accuracy and reliability across industries.
Current Challenges in FTIR Reference Validation
Despite significant advancements in Fourier Transform Infrared (FTIR) spectroscopy, several persistent challenges impede the effective validation of FTIR results using reference samples. The primary challenge lies in establishing and maintaining appropriate reference standards that accurately represent the materials being analyzed. Many industries lack standardized reference materials specifically designed for FTIR validation, forcing researchers to develop in-house standards that may lack traceability to recognized metrological authorities.
Sample preparation inconsistencies present another significant obstacle. Even minor variations in sample thickness, homogeneity, or surface characteristics can dramatically alter spectral outputs, making direct comparisons between test and reference samples problematic. This challenge is particularly acute when dealing with complex matrices or heterogeneous materials where representative sampling becomes exceedingly difficult.
Environmental factors further complicate validation efforts. FTIR measurements are notably sensitive to temperature fluctuations, humidity levels, and atmospheric conditions. These variables can introduce spectral artifacts or baseline shifts that obscure true chemical information, making it difficult to distinguish between actual sample differences and environmental interference when comparing against reference standards.
The inherent limitations of the FTIR technique itself pose additional validation challenges. Issues such as spectral overlap in complex mixtures, varying absorption coefficients between compounds, and the non-linear relationship between concentration and absorbance at high concentrations can all compromise validation accuracy. These technical constraints often necessitate complementary analytical techniques to confirm FTIR findings.
Data processing and interpretation present further complications. The lack of standardized protocols for spectral preprocessing (baseline correction, normalization, etc.) introduces subjectivity that can significantly impact validation outcomes. Different mathematical treatments of the same raw data can yield divergent results when compared against reference spectra.
Instrument-to-instrument variability remains a persistent issue in the field. Differences in optical configurations, detector sensitivities, and resolution capabilities between instruments mean that reference spectra generated on one system may not be directly comparable to measurements taken on another, even within the same laboratory.
Lastly, the challenge of quantifying uncertainty in FTIR validation cannot be overlooked. Establishing confidence intervals and determining acceptable tolerance limits for spectral matching is often approached inconsistently across different applications and industries, making it difficult to establish universal validation protocols that can be widely adopted.
Sample preparation inconsistencies present another significant obstacle. Even minor variations in sample thickness, homogeneity, or surface characteristics can dramatically alter spectral outputs, making direct comparisons between test and reference samples problematic. This challenge is particularly acute when dealing with complex matrices or heterogeneous materials where representative sampling becomes exceedingly difficult.
Environmental factors further complicate validation efforts. FTIR measurements are notably sensitive to temperature fluctuations, humidity levels, and atmospheric conditions. These variables can introduce spectral artifacts or baseline shifts that obscure true chemical information, making it difficult to distinguish between actual sample differences and environmental interference when comparing against reference standards.
The inherent limitations of the FTIR technique itself pose additional validation challenges. Issues such as spectral overlap in complex mixtures, varying absorption coefficients between compounds, and the non-linear relationship between concentration and absorbance at high concentrations can all compromise validation accuracy. These technical constraints often necessitate complementary analytical techniques to confirm FTIR findings.
Data processing and interpretation present further complications. The lack of standardized protocols for spectral preprocessing (baseline correction, normalization, etc.) introduces subjectivity that can significantly impact validation outcomes. Different mathematical treatments of the same raw data can yield divergent results when compared against reference spectra.
Instrument-to-instrument variability remains a persistent issue in the field. Differences in optical configurations, detector sensitivities, and resolution capabilities between instruments mean that reference spectra generated on one system may not be directly comparable to measurements taken on another, even within the same laboratory.
Lastly, the challenge of quantifying uncertainty in FTIR validation cannot be overlooked. Establishing confidence intervals and determining acceptable tolerance limits for spectral matching is often approached inconsistently across different applications and industries, making it difficult to establish universal validation protocols that can be widely adopted.
Current Reference Sample Validation Protocols
01 FTIR validation methods and protocols
Various methods and protocols are used for validating FTIR spectroscopy systems to ensure accuracy and reliability of results. These include standardized procedures for calibration, performance verification, and quality control. Validation protocols typically involve measuring reference materials with known spectral characteristics, assessing spectral resolution, wavelength accuracy, and signal-to-noise ratio. These methods help establish the reliability of FTIR systems for analytical applications in research and industry.- FTIR validation methods and protocols: Various methods and protocols are used for validating FTIR spectroscopy systems to ensure accuracy and reliability of results. These include standardized procedures for calibration, verification of instrument performance, and quality control measures. Validation protocols typically involve testing against known reference standards, assessing spectral resolution, wavelength accuracy, and signal-to-noise ratio to confirm that the FTIR system meets required specifications for analytical applications.
- FTIR system components and hardware validation: Validation of FTIR hardware components is essential for ensuring system integrity. This includes verification of optical components, detectors, interferometers, and sampling accessories. Hardware validation procedures assess the physical performance of these components through tests that measure beam alignment, mirror positioning accuracy, detector response, and overall system stability. Regular hardware validation helps identify potential mechanical or electronic issues that could affect spectral data quality.
- Software and data processing validation for FTIR analysis: Validation of software and data processing algorithms is crucial for ensuring accurate interpretation of FTIR spectral data. This includes verification of mathematical transformations, baseline correction algorithms, peak identification methods, and quantitative analysis tools. Software validation procedures test the accuracy of spectral processing functions, database matching capabilities, and reporting features to confirm that the analytical results are reliable and reproducible across different samples and conditions.
- FTIR validation for specific applications and industries: FTIR validation requirements vary across different applications and industries, with specialized protocols developed for pharmaceutical, environmental, food safety, and materials science applications. These application-specific validation approaches focus on parameters most relevant to the particular use case, such as detection limits for contaminants, identification accuracy for specific compounds, or precision in quantitative measurements. Industry standards and regulatory guidelines often dictate the validation requirements for FTIR systems in regulated environments.
- Automated and continuous FTIR validation systems: Advanced automated systems for continuous validation of FTIR instruments help maintain ongoing quality assurance. These systems incorporate real-time monitoring of instrument performance, automated calibration checks, and intelligent diagnostics to detect drift or malfunction. Continuous validation approaches use reference standards, internal calibration sources, or comparative analysis techniques to verify system performance without interrupting routine operation, enabling more efficient quality control and reducing downtime for validation procedures.
02 FTIR instrumentation and hardware validation
Validation of FTIR hardware components and instrumentation is essential for ensuring system performance. This includes validation of optical components, detectors, interferometers, and sample handling systems. Hardware validation procedures verify that the physical components of the FTIR system are functioning correctly and meeting specifications. Regular maintenance and validation of these components help prevent measurement errors and ensure consistent analytical results across different samples and over time.Expand Specific Solutions03 FTIR software validation and data processing
Validation of FTIR software and data processing algorithms ensures accurate interpretation of spectral data. This includes validation of spectral analysis software, chemometric models, and data transformation algorithms. Software validation procedures verify that data processing tools correctly interpret raw spectral data and produce reliable analytical results. These procedures may include testing with standard data sets, algorithm verification, and comparison with established reference methods to ensure consistency and accuracy in spectral analysis.Expand Specific Solutions04 FTIR application-specific validation techniques
Different applications of FTIR spectroscopy require specific validation approaches tailored to the particular analytical needs. These include validation methods for pharmaceutical analysis, material identification, environmental monitoring, and biological sample analysis. Application-specific validation techniques ensure that FTIR methods are suitable for their intended use and can reliably detect and quantify the compounds of interest. These techniques often involve testing with industry-specific reference materials and following regulatory guidelines relevant to the application domain.Expand Specific Solutions05 Automated FTIR validation systems
Automated systems for FTIR validation streamline the validation process and reduce human error. These systems include automated calibration, performance verification, and quality control monitoring. Automated validation systems can perform routine checks on FTIR instruments, track performance metrics over time, and alert users to potential issues before they affect analytical results. Integration of automated validation with laboratory information management systems enhances documentation and compliance with regulatory requirements while improving efficiency in analytical laboratories.Expand Specific Solutions
Key Industry Players in FTIR Instrumentation
FTIR validation with reference samples is currently in a mature development stage within the analytical instrumentation market, which is estimated at $8-10 billion globally. The competitive landscape features established analytical instrument manufacturers like Shimadzu Corp. and Horiba Ltd. dominating with comprehensive validation solutions, while oil industry giants such as ExxonMobil Technology & Engineering Co. and Saudi Arabian Oil Co. have developed specialized FTIR validation protocols for petroleum applications. Academic institutions including Peking University and Heriot-Watt University contribute significant research advancements. The technology has reached high maturity with standardized validation methods, though innovation continues in automated reference sample preparation and AI-enhanced spectral analysis, particularly from Thermo Electron Scientific Instruments LLC and Bruker Nano, Inc., who are advancing reference material certification and multi-component analysis capabilities.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed a proprietary FTIR validation methodology specifically optimized for petroleum and petrochemical applications. Their approach utilizes a custom-designed reference sample library that includes synthetic blends mimicking complex hydrocarbon matrices encountered in real-world samples. ExxonMobil's validation protocol employs a multi-component reference standard system where each standard contains precisely quantified concentrations of key chemical markers relevant to fuel and lubricant analysis. Their technique incorporates chemometric modeling to validate both qualitative identification and quantitative measurement accuracy across different sample types. The validation process includes regular verification using certified reference materials to ensure consistent performance across different instruments and laboratories within their global network. ExxonMobil's system also features adaptive algorithms that can compensate for matrix effects when validating FTIR results in complex hydrocarbon samples, improving accuracy in challenging analytical scenarios. Their validation approach emphasizes reproducibility across different operating conditions, ensuring reliable results in both laboratory and field environments[7][9].
Strengths: Highly specialized for petroleum industry applications; robust performance with complex hydrocarbon matrices; validated across global laboratory network ensuring consistency. Weaknesses: Limited applicability outside petroleum/petrochemical sector; proprietary nature restricts broader adoption; may require specialized training specific to ExxonMobil's protocols.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has pioneered an integrated FTIR validation approach specifically designed for oilfield and geological sample analysis. Their methodology employs a dual-reference validation system that combines both synthetic standards and natural reference materials collected from well-characterized geological formations. Schlumberger's validation protocol utilizes a library of reference spectra obtained from samples with compositions verified through multiple analytical techniques (XRD, XRF, NMR) to provide cross-validation of FTIR results. Their system incorporates advanced chemometric algorithms that can identify and quantify multiple components in complex mixtures by comparing spectral patterns against reference databases. For field applications, Schlumberger has developed portable FTIR systems with built-in validation routines that automatically verify instrument performance using integrated reference cells before analyzing unknown samples. Their validation approach also includes environmental correction factors that account for temperature, pressure, and humidity variations when comparing field measurements to laboratory reference standards, ensuring consistent results across diverse operational environments[8][10].
Strengths: Specifically optimized for geological and oilfield applications; robust field validation capabilities; integration with complementary analytical techniques. Weaknesses: Highly specialized for energy sector applications; complex validation algorithms require significant computational resources; reference database may have gaps for unusual or novel sample types.
Critical Spectral Analysis Techniques
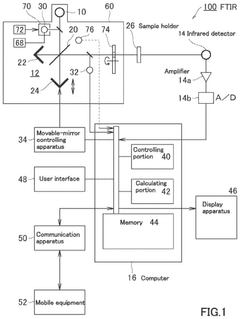
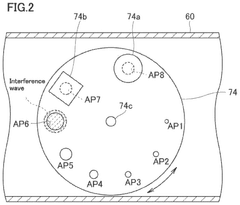
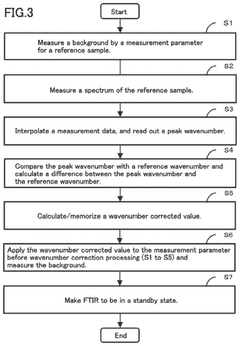
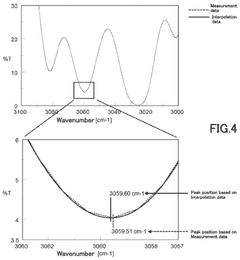
Fourier transform infrared spectrometer
PatentActiveUS12372460B2
Innovation
- A Fourier transform infrared spectrometer utilizing a solid reference sample, such as a polystyrene film, with a computer program for interpolating and correcting the wavenumber by adjusting the semiconductor laser's wavelength through temperature control or applied current, ensuring precise wavenumber correction even in nitrogen-purged or vacuumed states.
Measuring low levels of contaminants in fuels using Fourier Transform Infra red spectroscopy with dynamic reference analysis
PatentActiveGB2466802A
Innovation
- A sampling apparatus and process using a Fourier Transform InfraRed (FTIR) spectrometer with a dual through-flow cell and solid phase sorbent to create dynamic reference spectra, eliminating the need for solvents and enabling real-time comparison of sample and reference spectra for accurate measurement of contaminants and additives.
Regulatory Standards for Analytical Validation
Regulatory standards for analytical validation in FTIR spectroscopy are essential frameworks that ensure the reliability, reproducibility, and accuracy of analytical methods across laboratories and industries. These standards are established by various international and regional regulatory bodies, including the International Conference on Harmonisation (ICH), the United States Pharmacopeia (USP), the European Medicines Agency (EMA), and the Food and Drug Administration (FDA).
The ICH Q2(R1) guideline, "Validation of Analytical Procedures: Text and Methodology," provides comprehensive requirements for analytical method validation, applicable to FTIR spectroscopy. This guideline outlines key validation parameters including specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. For FTIR validation specifically, these parameters must be adapted to address the unique characteristics of vibrational spectroscopy.
USP <1225> "Validation of Compendial Procedures" complements the ICH guidelines by providing specific requirements for pharmaceutical analysis. When validating FTIR methods, this standard requires demonstration that the analytical procedure is suitable for its intended purpose through systematic evaluation of method performance characteristics.
The FDA's Guidance for Industry on Analytical Procedures and Methods Validation emphasizes the importance of reference standards in validation processes. For FTIR validation, certified reference materials (CRMs) with known spectral properties must be used to establish traceability to recognized standards. These materials should be characterized using multiple analytical techniques to confirm their composition and purity.
ISO/IEC 17025, which specifies general requirements for the competence of testing and calibration laboratories, mandates rigorous validation protocols for all analytical methods, including FTIR spectroscopy. This standard emphasizes the need for documented evidence that demonstrates the method's fitness for purpose.
For environmental applications, the EPA has established Method 320 for FTIR analysis, which includes specific validation requirements using reference samples with known concentrations of target analytes. This method requires demonstration of acceptable accuracy and precision through analysis of these reference materials.
Industry-specific standards also exist, such as ASTM E1655 for multivariate calibration of FTIR spectrometers and ASTM E2310 for standard practice for use of reference materials in FTIR analysis. These standards provide detailed protocols for establishing calibration models and validating analytical results against reference samples.
Compliance with these regulatory standards not only ensures the scientific validity of FTIR results but also facilitates regulatory approval processes and international recognition of analytical data. Organizations must maintain comprehensive documentation of validation procedures, including reference sample selection criteria, validation protocols, and statistical analysis of validation data.
The ICH Q2(R1) guideline, "Validation of Analytical Procedures: Text and Methodology," provides comprehensive requirements for analytical method validation, applicable to FTIR spectroscopy. This guideline outlines key validation parameters including specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. For FTIR validation specifically, these parameters must be adapted to address the unique characteristics of vibrational spectroscopy.
USP <1225> "Validation of Compendial Procedures" complements the ICH guidelines by providing specific requirements for pharmaceutical analysis. When validating FTIR methods, this standard requires demonstration that the analytical procedure is suitable for its intended purpose through systematic evaluation of method performance characteristics.
The FDA's Guidance for Industry on Analytical Procedures and Methods Validation emphasizes the importance of reference standards in validation processes. For FTIR validation, certified reference materials (CRMs) with known spectral properties must be used to establish traceability to recognized standards. These materials should be characterized using multiple analytical techniques to confirm their composition and purity.
ISO/IEC 17025, which specifies general requirements for the competence of testing and calibration laboratories, mandates rigorous validation protocols for all analytical methods, including FTIR spectroscopy. This standard emphasizes the need for documented evidence that demonstrates the method's fitness for purpose.
For environmental applications, the EPA has established Method 320 for FTIR analysis, which includes specific validation requirements using reference samples with known concentrations of target analytes. This method requires demonstration of acceptable accuracy and precision through analysis of these reference materials.
Industry-specific standards also exist, such as ASTM E1655 for multivariate calibration of FTIR spectrometers and ASTM E2310 for standard practice for use of reference materials in FTIR analysis. These standards provide detailed protocols for establishing calibration models and validating analytical results against reference samples.
Compliance with these regulatory standards not only ensures the scientific validity of FTIR results but also facilitates regulatory approval processes and international recognition of analytical data. Organizations must maintain comprehensive documentation of validation procedures, including reference sample selection criteria, validation protocols, and statistical analysis of validation data.
Data Management in Spectroscopic Validation
Effective data management is crucial for ensuring the reliability and reproducibility of FTIR validation processes using reference samples. Organizations must establish robust systems for collecting, storing, and processing spectroscopic data throughout the validation workflow. This begins with implementing standardized data acquisition protocols that specify parameters such as spectral resolution, number of scans, and environmental conditions during measurement to ensure consistency across all validation activities.
Database architecture specifically designed for spectroscopic validation should incorporate both raw spectral data and processed results, maintaining clear relationships between reference samples and their corresponding FTIR spectra. Metadata management becomes particularly important, requiring comprehensive documentation of sample characteristics, preparation methods, measurement conditions, and instrument calibration status for each validation dataset.
Version control systems must be implemented to track changes in spectral processing algorithms and validation methodologies over time. This creates an audit trail that allows analysts to reproduce validation results and understand how processing parameters may have evolved, which is essential for long-term data integrity and regulatory compliance in industries such as pharmaceuticals and materials science.
Data integrity safeguards should include automated validation checks that flag anomalous spectral features, unexpected deviations from reference standards, or instrument performance issues. These systems can employ statistical process control methods to monitor validation trends and detect subtle shifts in instrument performance before they compromise validation accuracy.
Cloud-based solutions are increasingly being adopted for spectroscopic data management, offering advantages in terms of accessibility, scalability, and collaborative capabilities. These platforms can integrate with laboratory information management systems (LIMS) to streamline the validation workflow and ensure seamless data transfer between different stages of the process.
Machine learning approaches are emerging as powerful tools for enhancing spectroscopic data management. Algorithms can be trained to recognize patterns in reference sample spectra, automatically classify unknown samples, and even predict potential validation issues before they occur. This predictive capability can significantly reduce the time and resources required for routine validation activities.
Standardized data exchange formats such as JCAMP-DX and AnIML facilitate interoperability between different spectroscopic systems and software platforms, enabling more efficient collaboration and data sharing across organizations. These standards are particularly valuable when external reference libraries are used for validation purposes, ensuring that spectral data can be accurately compared regardless of the original acquisition system.
Database architecture specifically designed for spectroscopic validation should incorporate both raw spectral data and processed results, maintaining clear relationships between reference samples and their corresponding FTIR spectra. Metadata management becomes particularly important, requiring comprehensive documentation of sample characteristics, preparation methods, measurement conditions, and instrument calibration status for each validation dataset.
Version control systems must be implemented to track changes in spectral processing algorithms and validation methodologies over time. This creates an audit trail that allows analysts to reproduce validation results and understand how processing parameters may have evolved, which is essential for long-term data integrity and regulatory compliance in industries such as pharmaceuticals and materials science.
Data integrity safeguards should include automated validation checks that flag anomalous spectral features, unexpected deviations from reference standards, or instrument performance issues. These systems can employ statistical process control methods to monitor validation trends and detect subtle shifts in instrument performance before they compromise validation accuracy.
Cloud-based solutions are increasingly being adopted for spectroscopic data management, offering advantages in terms of accessibility, scalability, and collaborative capabilities. These platforms can integrate with laboratory information management systems (LIMS) to streamline the validation workflow and ensure seamless data transfer between different stages of the process.
Machine learning approaches are emerging as powerful tools for enhancing spectroscopic data management. Algorithms can be trained to recognize patterns in reference sample spectra, automatically classify unknown samples, and even predict potential validation issues before they occur. This predictive capability can significantly reduce the time and resources required for routine validation activities.
Standardized data exchange formats such as JCAMP-DX and AnIML facilitate interoperability between different spectroscopic systems and software platforms, enabling more efficient collaboration and data sharing across organizations. These standards are particularly valuable when external reference libraries are used for validation purposes, ensuring that spectral data can be accurately compared regardless of the original acquisition system.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!