Hydrofluoric Acid vs Chlorofluorocarbons: Environmental Impact
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF and CFC Environmental Impact Background and Objectives
Hydrofluoric acid (HF) and chlorofluorocarbons (CFCs) represent two significant chemical compounds that have played pivotal roles in industrial development while simultaneously posing substantial environmental challenges. The historical trajectory of these substances traces back to the early 20th century, with HF's industrial production beginning in the 1920s for applications in aluminum processing and uranium enrichment, while CFCs were first synthesized in 1928 as safe refrigerants to replace toxic alternatives.
The evolution of these chemicals has been marked by increasing recognition of their environmental impacts. CFCs gained widespread industrial adoption throughout the mid-20th century, valued for their stability and non-toxicity, before scientific evidence in the 1970s and 1980s linked them to stratospheric ozone depletion. This discovery led to the landmark Montreal Protocol in 1987, initiating the global phaseout of these compounds. HF, meanwhile, has maintained its industrial importance despite growing awareness of its acute toxicity and potential for environmental contamination.
Current technological trends indicate a shift toward finding environmentally sustainable alternatives to both substances. For CFCs, this has manifested in the development of hydrofluorocarbons (HFCs) and more recently hydrofluoroolefins (HFOs), though concerns about their global warming potential persist. For HF, research focuses on developing safer handling protocols and alternative processes that reduce or eliminate its use in industrial applications.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of the environmental impacts associated with hydrofluoric acid and chlorofluorocarbons throughout their lifecycle. This includes examining their production processes, industrial applications, release mechanisms, environmental persistence, and ecological effects.
Secondary objectives include quantifying the relative environmental footprints of these substances across different metrics including ozone depletion potential, global warming contribution, water system contamination, and ecosystem toxicity. Additionally, this research aims to evaluate current regulatory frameworks governing these chemicals globally and identify technological innovations that may mitigate their environmental impacts.
The ultimate goal is to provide a scientific foundation for informed decision-making regarding the continued use, potential replacement, or enhanced management of these substances across industrial sectors. This research recognizes the tension between industrial utility and environmental protection, seeking to illuminate pathways toward more sustainable chemical management practices that balance technological needs with ecological preservation.
The evolution of these chemicals has been marked by increasing recognition of their environmental impacts. CFCs gained widespread industrial adoption throughout the mid-20th century, valued for their stability and non-toxicity, before scientific evidence in the 1970s and 1980s linked them to stratospheric ozone depletion. This discovery led to the landmark Montreal Protocol in 1987, initiating the global phaseout of these compounds. HF, meanwhile, has maintained its industrial importance despite growing awareness of its acute toxicity and potential for environmental contamination.
Current technological trends indicate a shift toward finding environmentally sustainable alternatives to both substances. For CFCs, this has manifested in the development of hydrofluorocarbons (HFCs) and more recently hydrofluoroolefins (HFOs), though concerns about their global warming potential persist. For HF, research focuses on developing safer handling protocols and alternative processes that reduce or eliminate its use in industrial applications.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of the environmental impacts associated with hydrofluoric acid and chlorofluorocarbons throughout their lifecycle. This includes examining their production processes, industrial applications, release mechanisms, environmental persistence, and ecological effects.
Secondary objectives include quantifying the relative environmental footprints of these substances across different metrics including ozone depletion potential, global warming contribution, water system contamination, and ecosystem toxicity. Additionally, this research aims to evaluate current regulatory frameworks governing these chemicals globally and identify technological innovations that may mitigate their environmental impacts.
The ultimate goal is to provide a scientific foundation for informed decision-making regarding the continued use, potential replacement, or enhanced management of these substances across industrial sectors. This research recognizes the tension between industrial utility and environmental protection, seeking to illuminate pathways toward more sustainable chemical management practices that balance technological needs with ecological preservation.
Market Analysis of HF and CFC Alternatives
The global market for hydrofluoric acid (HF) and chlorofluorocarbons (CFCs) alternatives has undergone significant transformation driven by environmental regulations and sustainability concerns. Currently, the HF market is valued at approximately $2.7 billion with a compound annual growth rate of 3.8%, primarily fueled by applications in aluminum production, refrigerants, and electronics manufacturing.
Since the Montreal Protocol implementation, traditional CFCs have been largely phased out, creating a robust market for alternatives worth over $22 billion. Hydrofluoroolefins (HFOs) and hydrochlorofluoroolefins (HCFOs) have emerged as leading replacements, with market adoption accelerating at 6.2% annually as industries transition to more environmentally compatible solutions.
The electronics sector represents the fastest-growing segment for HF alternatives, expanding at 5.7% annually due to semiconductor manufacturing demands. Meanwhile, refrigeration and air conditioning industries account for 42% of the total market for CFC alternatives, with automotive air conditioning systems driving significant innovation in low Global Warming Potential (GWP) refrigerants.
Regional analysis reveals Asia-Pacific dominates the market with 38% share, followed by North America (27%) and Europe (24%). China leads global HF production capacity, while the United States and European Union are pioneering in next-generation CFC alternatives development, particularly in HFO technology.
Consumer preferences are increasingly favoring products with lower environmental impact, creating premium market segments for appliances and systems using natural refrigerants. This trend has expanded the market for propane, ammonia, and CO2-based systems by 8.3% annually, particularly in commercial refrigeration applications.
Regulatory frameworks continue to reshape market dynamics, with the Kigali Amendment to the Montreal Protocol accelerating the phase-down of hydrofluorocarbons (HFCs). This has created immediate market opportunities for HFO-1234yf and HFO-1234ze, which have seen production capacity increases of over 200% in the past five years.
Investment in research and development for safer HF handling technologies and completely halogen-free alternatives has reached $1.2 billion annually. Major chemical manufacturers have allocated significant resources toward developing drop-in replacements that minimize conversion costs for end-users while meeting increasingly stringent environmental standards.
The market forecast indicates continued growth for environmentally superior alternatives, with projected market expansion to $32 billion by 2028. Price premiums for environmentally friendly alternatives are gradually decreasing as production scales up, with current premiums averaging 15-30% above conventional options but expected to normalize within 3-5 years as manufacturing efficiencies improve.
Since the Montreal Protocol implementation, traditional CFCs have been largely phased out, creating a robust market for alternatives worth over $22 billion. Hydrofluoroolefins (HFOs) and hydrochlorofluoroolefins (HCFOs) have emerged as leading replacements, with market adoption accelerating at 6.2% annually as industries transition to more environmentally compatible solutions.
The electronics sector represents the fastest-growing segment for HF alternatives, expanding at 5.7% annually due to semiconductor manufacturing demands. Meanwhile, refrigeration and air conditioning industries account for 42% of the total market for CFC alternatives, with automotive air conditioning systems driving significant innovation in low Global Warming Potential (GWP) refrigerants.
Regional analysis reveals Asia-Pacific dominates the market with 38% share, followed by North America (27%) and Europe (24%). China leads global HF production capacity, while the United States and European Union are pioneering in next-generation CFC alternatives development, particularly in HFO technology.
Consumer preferences are increasingly favoring products with lower environmental impact, creating premium market segments for appliances and systems using natural refrigerants. This trend has expanded the market for propane, ammonia, and CO2-based systems by 8.3% annually, particularly in commercial refrigeration applications.
Regulatory frameworks continue to reshape market dynamics, with the Kigali Amendment to the Montreal Protocol accelerating the phase-down of hydrofluorocarbons (HFCs). This has created immediate market opportunities for HFO-1234yf and HFO-1234ze, which have seen production capacity increases of over 200% in the past five years.
Investment in research and development for safer HF handling technologies and completely halogen-free alternatives has reached $1.2 billion annually. Major chemical manufacturers have allocated significant resources toward developing drop-in replacements that minimize conversion costs for end-users while meeting increasingly stringent environmental standards.
The market forecast indicates continued growth for environmentally superior alternatives, with projected market expansion to $32 billion by 2028. Price premiums for environmentally friendly alternatives are gradually decreasing as production scales up, with current premiums averaging 15-30% above conventional options but expected to normalize within 3-5 years as manufacturing efficiencies improve.
Current Technical Challenges in HF and CFC Management
The management of hydrofluoric acid (HF) and chlorofluorocarbons (CFCs) presents significant technical challenges due to their environmental impact and hazardous properties. Despite regulatory frameworks like the Montreal Protocol that have successfully reduced CFC production, both substances continue to pose substantial environmental and safety concerns that require innovative technical solutions.
HF management faces critical containment challenges due to its highly corrosive nature and ability to penetrate materials that typically resist other acids. Current containment systems using specialized fluoropolymers like PTFE and PFA show limitations in long-term durability under industrial conditions. The development of more resilient materials capable of withstanding HF's aggressive properties remains an ongoing technical hurdle.
Monitoring technologies for HF emissions and workplace exposure represent another significant challenge. While electrochemical sensors offer real-time detection capabilities, they suffer from cross-sensitivity issues and limited operational lifespans in industrial environments. Optical sensing technologies show promise but require further refinement to achieve the necessary sensitivity and reliability for comprehensive monitoring solutions.
For CFCs, despite production phase-outs, legacy systems containing these compounds continue to operate worldwide. The technical challenge lies in developing cost-effective recovery and destruction methods for these remaining CFC stocks. Current destruction technologies like plasma arc systems and cement kiln incineration achieve destruction efficiencies of 99.99%, but their high energy requirements and operational costs limit widespread implementation.
The identification and remediation of CFC-contaminated sites presents additional technical difficulties. Current detection methods often lack the sensitivity required to identify low-concentration leaks from aging equipment. Advanced spectroscopic techniques show promise but require further development for field deployment.
Both substances face challenges in recycling and circular economy integration. For HF, recovery processes from spent catalysts and etching solutions remain energy-intensive and generate secondary waste streams. CFC recovery from insulation foams and refrigeration systems faces similar efficiency limitations, with current mechanical separation techniques capturing only 60-80% of embedded compounds.
Climate change considerations further complicate management strategies, as rising temperatures may accelerate the degradation of containment systems and increase volatilization rates. This necessitates the development of more robust management approaches that account for changing environmental conditions.
Cross-disciplinary technical solutions integrating materials science, sensor technology, and process engineering are urgently needed to address these challenges. The development of next-generation containment materials, more sensitive detection systems, and energy-efficient destruction technologies represents critical areas for technical innovation in the management of these environmentally impactful substances.
HF management faces critical containment challenges due to its highly corrosive nature and ability to penetrate materials that typically resist other acids. Current containment systems using specialized fluoropolymers like PTFE and PFA show limitations in long-term durability under industrial conditions. The development of more resilient materials capable of withstanding HF's aggressive properties remains an ongoing technical hurdle.
Monitoring technologies for HF emissions and workplace exposure represent another significant challenge. While electrochemical sensors offer real-time detection capabilities, they suffer from cross-sensitivity issues and limited operational lifespans in industrial environments. Optical sensing technologies show promise but require further refinement to achieve the necessary sensitivity and reliability for comprehensive monitoring solutions.
For CFCs, despite production phase-outs, legacy systems containing these compounds continue to operate worldwide. The technical challenge lies in developing cost-effective recovery and destruction methods for these remaining CFC stocks. Current destruction technologies like plasma arc systems and cement kiln incineration achieve destruction efficiencies of 99.99%, but their high energy requirements and operational costs limit widespread implementation.
The identification and remediation of CFC-contaminated sites presents additional technical difficulties. Current detection methods often lack the sensitivity required to identify low-concentration leaks from aging equipment. Advanced spectroscopic techniques show promise but require further development for field deployment.
Both substances face challenges in recycling and circular economy integration. For HF, recovery processes from spent catalysts and etching solutions remain energy-intensive and generate secondary waste streams. CFC recovery from insulation foams and refrigeration systems faces similar efficiency limitations, with current mechanical separation techniques capturing only 60-80% of embedded compounds.
Climate change considerations further complicate management strategies, as rising temperatures may accelerate the degradation of containment systems and increase volatilization rates. This necessitates the development of more robust management approaches that account for changing environmental conditions.
Cross-disciplinary technical solutions integrating materials science, sensor technology, and process engineering are urgently needed to address these challenges. The development of next-generation containment materials, more sensitive detection systems, and energy-efficient destruction technologies represents critical areas for technical innovation in the management of these environmentally impactful substances.
Existing Mitigation and Containment Solutions
01 Ozone depletion and atmospheric impact of chlorofluorocarbons
Chlorofluorocarbons (CFCs) have been identified as significant contributors to ozone layer depletion in the upper atmosphere. When released into the air, these compounds rise to the stratosphere where ultraviolet radiation breaks them down, releasing chlorine atoms that destroy ozone molecules. This depletion allows more harmful UV radiation to reach the Earth's surface, increasing risks of skin cancer, eye damage, and harm to marine ecosystems. The environmental persistence of these compounds exacerbates their long-term impact on global atmospheric conditions.- Ozone depletion by chlorofluorocarbons: Chlorofluorocarbons (CFCs) have been identified as major contributors to ozone layer depletion. When released into the atmosphere, these compounds break down and release chlorine atoms that catalytically destroy ozone molecules in the stratosphere. This depletion of the ozone layer increases the amount of harmful ultraviolet radiation reaching the Earth's surface, leading to increased risks of skin cancer, eye damage, and harm to marine ecosystems.
- Hydrofluoric acid pollution control methods: Various technologies have been developed to control and reduce hydrofluoric acid emissions and their environmental impact. These include scrubbing systems, neutralization processes, and containment strategies that prevent the release of hydrofluoric acid into the environment. Such methods are crucial in industrial settings where hydrofluoric acid is used, as uncontrolled releases can lead to air pollution, water contamination, and damage to vegetation and wildlife.
- Alternative compounds with reduced environmental impact: Research has focused on developing alternative compounds to replace chlorofluorocarbons and hydrofluoric acid in various applications. These alternatives include hydrofluoroolefins (HFOs), hydrochlorofluoroolefins (HCFOs), and other compounds with lower global warming potential and ozone depletion potential. The development of these environmentally friendly alternatives aims to maintain industrial functionality while reducing negative environmental impacts.
- Recycling and recovery technologies: Technologies for recycling and recovering chlorofluorocarbons and hydrofluoric acid from end-of-life products and industrial processes have been developed to prevent their release into the environment. These technologies include adsorption systems, membrane separation, and chemical conversion processes that allow for the reuse of these compounds or their transformation into less harmful substances, thereby reducing their environmental footprint.
- Monitoring and detection systems: Advanced monitoring and detection systems have been developed to identify and measure chlorofluorocarbon and hydrofluoric acid emissions in industrial settings and the environment. These systems employ various technologies including spectroscopy, electrochemical sensors, and remote sensing to provide early warning of leaks or releases, enabling prompt response to minimize environmental damage and protect human health.
02 Alternative technologies and replacement compounds for CFCs
Due to environmental concerns, significant research has focused on developing alternatives to chlorofluorocarbons and processes using hydrofluoric acid. These alternatives include hydrofluoroolefins (HFOs), hydrochlorofluorocarbons (HCFCs), and hydrofluorocarbons (HFCs) that have lower ozone depletion potential. Various manufacturing processes have been developed to produce these environmentally friendlier compounds while maintaining the functional properties required for industrial applications such as refrigeration, propellants, and foam blowing agents.Expand Specific Solutions03 Waste management and disposal techniques for hydrofluoric acid
Proper disposal and treatment of hydrofluoric acid waste presents significant environmental challenges due to its high toxicity and corrosive properties. Advanced treatment methods have been developed to neutralize and safely dispose of hydrofluoric acid waste, including chemical precipitation, ion exchange processes, and specialized containment systems. These techniques aim to prevent contamination of soil and water resources while reducing the environmental footprint of industrial processes that utilize hydrofluoric acid.Expand Specific Solutions04 Greenhouse effect and climate change implications
Beyond ozone depletion, chlorofluorocarbons and some of their replacements are potent greenhouse gases with global warming potentials hundreds to thousands of times greater than carbon dioxide. Their contribution to climate change has prompted international regulations and agreements to phase out their production and use. Research has focused on quantifying their atmospheric lifetimes, radiative forcing effects, and developing climate models to predict their long-term impact on global temperature patterns and weather systems.Expand Specific Solutions05 Industrial emission control and regulatory compliance
Industries using hydrofluoric acid and chlorofluorocarbons have developed various emission control technologies to comply with increasingly stringent environmental regulations. These include scrubber systems, catalytic converters, thermal oxidizers, and closed-loop production processes that minimize releases to the environment. Monitoring systems and protocols have been established to detect leaks and emissions, while regulatory frameworks like the Montreal Protocol and subsequent amendments have created timelines for the complete phase-out of environmentally harmful compounds.Expand Specific Solutions
Key Industry Stakeholders and Manufacturers
The environmental impact comparison between Hydrofluoric Acid and Chlorofluorocarbons reveals an industry in transition. While CFCs have been largely phased out under the Montreal Protocol due to ozone depletion concerns, hydrofluoric acid presents significant toxicity challenges despite lower ozone impacts. The market is evolving toward more environmentally sustainable alternatives, with major players like Honeywell, Chemours, and DuPont leading innovation in this space. Asian manufacturers including Zhejiang Quhua and AGC are rapidly gaining market share, particularly in fluorochemical alternatives. Companies such as 3M and Arkema are focusing on developing safer handling protocols and substitutes with reduced environmental footprints, reflecting the industry's movement toward greater sustainability while balancing industrial application needs.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed the Solstice® line of hydrofluoroolefin (HFO) products as environmentally preferable alternatives to both hydrofluoric acid applications and chlorofluorocarbons. Their technology platform focuses on molecules with ultra-low global warming potential (GWP) values typically below 10, compared to thousands for traditional CFCs. Honeywell's Solstice® yf refrigerant (HFO-1234yf) has a GWP of less than 1 and atmospheric lifetime of just 11 days versus 13 years for common HFCs[7]. Their innovation extends to manufacturing processes that reduce environmental impact during production. Honeywell has invested over $1 billion in R&D and new capacity for these fourth-generation fluorochemicals. Their solutions maintain or improve performance characteristics while addressing environmental concerns through molecular design that ensures rapid atmospheric breakdown without forming persistent byproducts[8].
Strengths: Comprehensive product portfolio covering refrigeration, foam blowing, and industrial applications; extensive regulatory approval across global markets; proven performance in commercial applications. Weaknesses: Implementation requires system modifications in some applications; higher production costs than previous generation chemicals; some applications still require performance optimization.
The Chemours Co.
Technical Solution: Chemours has developed the Opteon™ portfolio as a sustainable alternative to both hydrofluoric acid and chlorofluorocarbons. Their technology focuses on hydrofluoroolefins (HFOs) with significantly lower global warming potential (GWP) compared to traditional HFCs and CFCs. The Opteon™ YF (HFO-1234yf) refrigerant has a GWP of less than 1, which is 99.9% lower than previous refrigerants[1]. Chemours has invested over $300 million in production facilities for these next-generation solutions that address environmental concerns while maintaining performance requirements. Their approach includes comprehensive lifecycle assessments to ensure reduced environmental impact from manufacturing through disposal, with particular attention to minimizing atmospheric lifetime and ozone depletion potential[2].
Strengths: Industry-leading low GWP solutions with proven commercial viability; extensive manufacturing infrastructure; strong regulatory compliance positioning. Weaknesses: Higher production costs compared to traditional chemicals; requires system modifications for implementation in existing equipment; some HFO products still have trace environmental concerns requiring ongoing research.
Critical Patents and Innovations in Fluorochemical Safety
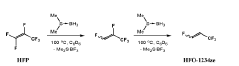
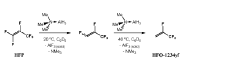
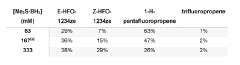
Method of HFO synthesis
PatentWO2020002932A1
Innovation
- A method involving the reaction of a fluoroolefin with a group III element-based reductant, such as boron or aluminum, which does not generate toxic intermediates and uses inexpensive starting materials, proceeding through a one-pot process to produce HFOs like HFO-1234ze and HFO-1234yf without the need for catalysts or specialized equipment.
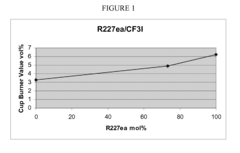
Azeotrope-like compositions of heptafluoropropane and trifluoroiodomethane
PatentInactiveUS8017030B2
Innovation
- The combination of 1,1,1,2,3,3,3-heptafluoropropane (HFC-227ea) and trifluoroiodomethane (CF3I) forms an azeotrope-like composition, which is used in various applications such as fire suppressants, refrigerants, and blowing agents, offering a safer alternative with a predictable, non-fractionating mixture.
Regulatory Framework and Global Compliance Standards
The regulatory landscape governing hydrofluoric acid (HF) and chlorofluorocarbons (CFCs) has evolved significantly over the past four decades, reflecting growing environmental concerns and scientific understanding. The Montreal Protocol, established in 1987, stands as the cornerstone of international efforts to phase out CFCs due to their ozone-depleting properties. This landmark agreement has undergone multiple amendments, including the Kigali Amendment of 2016, which expanded its scope to include hydrofluorocarbons (HFCs), demonstrating the dynamic nature of global environmental governance.
For hydrofluoric acid, regulatory frameworks are more fragmented but equally stringent. The U.S. Environmental Protection Agency regulates HF under multiple statutes including the Clean Air Act, the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), and the Emergency Planning and Community Right-to-Know Act (EPCRA). In the European Union, HF falls under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Classification, Labelling and Packaging (CLP) Regulation.
Compliance standards for both substances incorporate tiered approaches based on quantity, concentration, and application. For CFCs, complete phase-out schedules have been implemented with different timelines for developed versus developing nations. The remaining legitimate uses, primarily in laboratory and analytical applications, require special exemptions and strict reporting protocols. HF compliance standards focus on workplace safety measures, emissions controls, and emergency response planning due to its acute toxicity profile.
Global harmonization efforts have been more successful with CFCs than with HF. The United Nations Environment Programme (UNEP) maintains a comprehensive monitoring system for CFC production, consumption, and trade through its Ozone Secretariat. In contrast, HF monitoring remains primarily within national regulatory frameworks, with international coordination occurring through the Strategic Approach to International Chemicals Management (SAICM) and the Rotterdam Convention on Prior Informed Consent.
Enforcement mechanisms vary significantly across jurisdictions. Developed nations typically employ sophisticated monitoring technologies, regular facility inspections, and substantial penalties for non-compliance. Developing nations often face implementation challenges due to limited resources, technical capacity, and competing economic priorities. International assistance programs, including the Multilateral Fund for the Implementation of the Montreal Protocol, have been established to address these disparities.
Recent regulatory trends indicate increasing scrutiny of both substances. For CFCs, attention has shifted to preventing illegal trade and ensuring the destruction of legacy stockpiles. For HF, regulatory focus is expanding beyond occupational safety to include broader environmental impacts, particularly regarding fluoride emissions and potential groundwater contamination from industrial applications.
For hydrofluoric acid, regulatory frameworks are more fragmented but equally stringent. The U.S. Environmental Protection Agency regulates HF under multiple statutes including the Clean Air Act, the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), and the Emergency Planning and Community Right-to-Know Act (EPCRA). In the European Union, HF falls under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Classification, Labelling and Packaging (CLP) Regulation.
Compliance standards for both substances incorporate tiered approaches based on quantity, concentration, and application. For CFCs, complete phase-out schedules have been implemented with different timelines for developed versus developing nations. The remaining legitimate uses, primarily in laboratory and analytical applications, require special exemptions and strict reporting protocols. HF compliance standards focus on workplace safety measures, emissions controls, and emergency response planning due to its acute toxicity profile.
Global harmonization efforts have been more successful with CFCs than with HF. The United Nations Environment Programme (UNEP) maintains a comprehensive monitoring system for CFC production, consumption, and trade through its Ozone Secretariat. In contrast, HF monitoring remains primarily within national regulatory frameworks, with international coordination occurring through the Strategic Approach to International Chemicals Management (SAICM) and the Rotterdam Convention on Prior Informed Consent.
Enforcement mechanisms vary significantly across jurisdictions. Developed nations typically employ sophisticated monitoring technologies, regular facility inspections, and substantial penalties for non-compliance. Developing nations often face implementation challenges due to limited resources, technical capacity, and competing economic priorities. International assistance programs, including the Multilateral Fund for the Implementation of the Montreal Protocol, have been established to address these disparities.
Recent regulatory trends indicate increasing scrutiny of both substances. For CFCs, attention has shifted to preventing illegal trade and ensuring the destruction of legacy stockpiles. For HF, regulatory focus is expanding beyond occupational safety to include broader environmental impacts, particularly regarding fluoride emissions and potential groundwater contamination from industrial applications.
Life Cycle Assessment of Fluorochemical Compounds
Life Cycle Assessment (LCA) provides a comprehensive framework for evaluating the environmental impacts of fluorochemical compounds throughout their entire existence. When comparing hydrofluoric acid (HF) and chlorofluorocarbons (CFCs), LCA reveals significant differences in their environmental footprints across production, use, and disposal phases.
The production phase of hydrofluoric acid typically involves the reaction of calcium fluoride (fluorspar) with sulfuric acid, generating considerable energy consumption and sulfur dioxide emissions. Conversely, CFC production historically utilized chloroform and hydrogen fluoride as primary feedstocks, with different energy requirements and emission profiles. Both processes demand substantial energy inputs, though HF production generally exhibits higher direct toxicity concerns during manufacturing.
During the use phase, these compounds demonstrate markedly different environmental behaviors. Hydrofluoric acid, primarily utilized in industrial applications such as aluminum production, glass etching, and as a catalyst in petroleum refining, presents localized environmental risks through potential soil and water contamination. CFCs, once widely used as refrigerants, propellants, and solvents, exhibit minimal local environmental impact during use but contribute significantly to stratospheric ozone depletion upon release.
End-of-life considerations reveal further distinctions. HF can be neutralized through appropriate treatment processes, though improper disposal may lead to groundwater contamination and ecosystem damage. CFCs, with atmospheric lifetimes ranging from 50-100+ years, continue to impact global environmental systems long after disposal, contributing to both ozone depletion and climate change as potent greenhouse gases.
Global warming potential (GWP) metrics indicate that while HF itself has minimal direct climate impact, its production generates substantial CO2 emissions. CFCs possess GWP values thousands of times greater than CO2, with significant long-term climate implications despite their production phase having potentially lower carbon footprints than HF manufacturing.
Ozone depletion potential (ODP) represents the most striking difference between these compounds. Hydrofluoric acid shows negligible ODP, whereas CFCs demonstrate exceptionally high ODP values, leading to their phase-out under the Montreal Protocol. This fundamental distinction has driven the transition toward HFC alternatives despite other environmental considerations.
Water and soil impact assessments indicate that HF presents more immediate and severe localized environmental threats through potential acidification and toxicity to aquatic ecosystems, while CFCs primarily affect environmental systems through atmospheric pathways rather than direct contamination of water bodies or soil.
The production phase of hydrofluoric acid typically involves the reaction of calcium fluoride (fluorspar) with sulfuric acid, generating considerable energy consumption and sulfur dioxide emissions. Conversely, CFC production historically utilized chloroform and hydrogen fluoride as primary feedstocks, with different energy requirements and emission profiles. Both processes demand substantial energy inputs, though HF production generally exhibits higher direct toxicity concerns during manufacturing.
During the use phase, these compounds demonstrate markedly different environmental behaviors. Hydrofluoric acid, primarily utilized in industrial applications such as aluminum production, glass etching, and as a catalyst in petroleum refining, presents localized environmental risks through potential soil and water contamination. CFCs, once widely used as refrigerants, propellants, and solvents, exhibit minimal local environmental impact during use but contribute significantly to stratospheric ozone depletion upon release.
End-of-life considerations reveal further distinctions. HF can be neutralized through appropriate treatment processes, though improper disposal may lead to groundwater contamination and ecosystem damage. CFCs, with atmospheric lifetimes ranging from 50-100+ years, continue to impact global environmental systems long after disposal, contributing to both ozone depletion and climate change as potent greenhouse gases.
Global warming potential (GWP) metrics indicate that while HF itself has minimal direct climate impact, its production generates substantial CO2 emissions. CFCs possess GWP values thousands of times greater than CO2, with significant long-term climate implications despite their production phase having potentially lower carbon footprints than HF manufacturing.
Ozone depletion potential (ODP) represents the most striking difference between these compounds. Hydrofluoric acid shows negligible ODP, whereas CFCs demonstrate exceptionally high ODP values, leading to their phase-out under the Montreal Protocol. This fundamental distinction has driven the transition toward HFC alternatives despite other environmental considerations.
Water and soil impact assessments indicate that HF presents more immediate and severe localized environmental threats through potential acidification and toxicity to aquatic ecosystems, while CFCs primarily affect environmental systems through atmospheric pathways rather than direct contamination of water bodies or soil.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!