Investigation of UHMWPE's Role in Joint Replacement Technologies
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
UHMWPE in Orthopedics: Background and Objectives
Ultra-high-molecular-weight polyethylene (UHMWPE) has emerged as a pivotal material in orthopedic joint replacement technologies, revolutionizing the field since its introduction in the 1960s. This high-performance polymer has become the gold standard for articulating surfaces in total joint arthroplasty, particularly in hip and knee replacements, due to its exceptional wear resistance, low friction coefficient, and biocompatibility.
The development of UHMWPE for orthopedic applications stems from the need to address the limitations of earlier materials used in joint replacements, such as metal-on-metal or ceramic-on-ceramic bearings. These previous solutions often led to issues like excessive wear, implant loosening, and adverse tissue reactions. UHMWPE's unique molecular structure, characterized by extremely long chains of polyethylene, provides it with superior mechanical properties that make it ideal for withstanding the high stresses and repetitive motions experienced in artificial joints.
Over the past several decades, continuous research and development efforts have focused on enhancing the performance of UHMWPE in orthopedic applications. These advancements have led to the creation of highly crosslinked UHMWPE and vitamin E-doped UHMWPE, which offer improved wear resistance and oxidation stability. The evolution of UHMWPE technology has significantly contributed to increasing the longevity of joint implants, reducing the need for revision surgeries and improving patient outcomes.
The primary objective of investigating UHMWPE's role in joint replacement technologies is to further optimize its properties and performance. This includes exploring new manufacturing techniques, surface modifications, and composite formulations to enhance wear resistance, reduce particle generation, and improve long-term stability in the biological environment. Additionally, researchers aim to develop UHMWPE variants that can better mimic the natural joint's biomechanics and load distribution characteristics.
Another crucial aspect of UHMWPE research is understanding and mitigating the biological response to wear particles. While UHMWPE has shown excellent biocompatibility, the accumulation of wear debris over time can lead to osteolysis and implant loosening. Therefore, a key objective is to develop strategies to minimize wear particle generation and to modulate the body's response to these particles.
As the global population ages and the demand for joint replacements continues to rise, the importance of advancing UHMWPE technology becomes increasingly critical. The ongoing investigation into UHMWPE's role in joint replacement technologies aims not only to improve the quality of life for millions of patients but also to reduce the economic burden associated with revision surgeries and long-term complications.
The development of UHMWPE for orthopedic applications stems from the need to address the limitations of earlier materials used in joint replacements, such as metal-on-metal or ceramic-on-ceramic bearings. These previous solutions often led to issues like excessive wear, implant loosening, and adverse tissue reactions. UHMWPE's unique molecular structure, characterized by extremely long chains of polyethylene, provides it with superior mechanical properties that make it ideal for withstanding the high stresses and repetitive motions experienced in artificial joints.
Over the past several decades, continuous research and development efforts have focused on enhancing the performance of UHMWPE in orthopedic applications. These advancements have led to the creation of highly crosslinked UHMWPE and vitamin E-doped UHMWPE, which offer improved wear resistance and oxidation stability. The evolution of UHMWPE technology has significantly contributed to increasing the longevity of joint implants, reducing the need for revision surgeries and improving patient outcomes.
The primary objective of investigating UHMWPE's role in joint replacement technologies is to further optimize its properties and performance. This includes exploring new manufacturing techniques, surface modifications, and composite formulations to enhance wear resistance, reduce particle generation, and improve long-term stability in the biological environment. Additionally, researchers aim to develop UHMWPE variants that can better mimic the natural joint's biomechanics and load distribution characteristics.
Another crucial aspect of UHMWPE research is understanding and mitigating the biological response to wear particles. While UHMWPE has shown excellent biocompatibility, the accumulation of wear debris over time can lead to osteolysis and implant loosening. Therefore, a key objective is to develop strategies to minimize wear particle generation and to modulate the body's response to these particles.
As the global population ages and the demand for joint replacements continues to rise, the importance of advancing UHMWPE technology becomes increasingly critical. The ongoing investigation into UHMWPE's role in joint replacement technologies aims not only to improve the quality of life for millions of patients but also to reduce the economic burden associated with revision surgeries and long-term complications.
Market Analysis for Joint Replacement Materials
The global market for joint replacement materials has been experiencing steady growth, driven by an aging population, increasing prevalence of osteoarthritis, and advancements in medical technology. Ultra-high molecular weight polyethylene (UHMWPE) has emerged as a critical component in joint replacement technologies, particularly in hip and knee arthroplasty.
The demand for joint replacement materials is primarily fueled by the rising incidence of degenerative joint diseases and sports-related injuries. According to recent market research, the global joint replacement market is projected to reach significant value in the coming years, with UHMWPE playing a substantial role in this growth.
UHMWPE's popularity in joint replacement stems from its excellent wear resistance, low friction coefficient, and biocompatibility. These properties make it an ideal material for articulating surfaces in artificial joints, contributing to improved implant longevity and patient outcomes. The market for UHMWPE in joint replacement is expected to expand further as manufacturers continue to innovate and enhance its performance characteristics.
Geographically, North America and Europe dominate the joint replacement materials market, owing to their advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth potential, driven by improving healthcare access and rising disposable incomes.
The competitive landscape of the joint replacement materials market is characterized by the presence of several key players, including major medical device manufacturers and specialized biomaterials companies. These companies are investing heavily in research and development to improve UHMWPE's properties and explore new applications in joint replacement technologies.
Recent trends in the market include the development of highly cross-linked UHMWPE, which offers superior wear resistance compared to conventional UHMWPE. Additionally, there is growing interest in vitamin E-infused UHMWPE, which has shown promise in reducing oxidation and improving long-term implant performance.
Despite the positive outlook, the market faces challenges such as the high cost of joint replacement procedures and stringent regulatory requirements. However, ongoing technological advancements and increasing healthcare expenditure are expected to mitigate these challenges and drive market growth.
In conclusion, the market analysis for joint replacement materials, particularly UHMWPE, indicates a robust growth trajectory. The material's unique properties, coupled with ongoing research and development efforts, position it as a key player in the future of joint replacement technologies. As the global population continues to age and the demand for improved quality of life increases, UHMWPE is likely to maintain its critical role in the joint replacement materials market.
The demand for joint replacement materials is primarily fueled by the rising incidence of degenerative joint diseases and sports-related injuries. According to recent market research, the global joint replacement market is projected to reach significant value in the coming years, with UHMWPE playing a substantial role in this growth.
UHMWPE's popularity in joint replacement stems from its excellent wear resistance, low friction coefficient, and biocompatibility. These properties make it an ideal material for articulating surfaces in artificial joints, contributing to improved implant longevity and patient outcomes. The market for UHMWPE in joint replacement is expected to expand further as manufacturers continue to innovate and enhance its performance characteristics.
Geographically, North America and Europe dominate the joint replacement materials market, owing to their advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth potential, driven by improving healthcare access and rising disposable incomes.
The competitive landscape of the joint replacement materials market is characterized by the presence of several key players, including major medical device manufacturers and specialized biomaterials companies. These companies are investing heavily in research and development to improve UHMWPE's properties and explore new applications in joint replacement technologies.
Recent trends in the market include the development of highly cross-linked UHMWPE, which offers superior wear resistance compared to conventional UHMWPE. Additionally, there is growing interest in vitamin E-infused UHMWPE, which has shown promise in reducing oxidation and improving long-term implant performance.
Despite the positive outlook, the market faces challenges such as the high cost of joint replacement procedures and stringent regulatory requirements. However, ongoing technological advancements and increasing healthcare expenditure are expected to mitigate these challenges and drive market growth.
In conclusion, the market analysis for joint replacement materials, particularly UHMWPE, indicates a robust growth trajectory. The material's unique properties, coupled with ongoing research and development efforts, position it as a key player in the future of joint replacement technologies. As the global population continues to age and the demand for improved quality of life increases, UHMWPE is likely to maintain its critical role in the joint replacement materials market.
Current Challenges in UHMWPE Technology
Despite the widespread use of Ultra-High Molecular Weight Polyethylene (UHMWPE) in joint replacement technologies, several challenges persist in its application. One of the primary concerns is wear resistance. While UHMWPE has shown remarkable durability, the constant friction in joint replacements can lead to the generation of wear particles. These particles may trigger an inflammatory response in the surrounding tissues, potentially causing osteolysis and implant loosening over time.
Another significant challenge is the balance between mechanical properties and oxidation resistance. Crosslinking techniques have been employed to enhance wear resistance, but this process can compromise the material's mechanical strength and fatigue resistance. Additionally, the free radicals produced during crosslinking can lead to oxidative degradation, affecting the long-term stability of the implant.
The biocompatibility of UHMWPE also presents ongoing challenges. Although generally considered biocompatible, the interaction between UHMWPE and the biological environment over extended periods is not fully understood. There are concerns about potential long-term effects of UHMWPE particles on the immune system and surrounding tissues.
Manufacturing consistency and quality control pose additional challenges. Ensuring uniform molecular weight distribution and crystallinity across batches is crucial for maintaining consistent performance in implants. Variations in these properties can lead to unpredictable wear characteristics and mechanical behavior.
The sterilization process for UHMWPE components remains a challenge. Traditional methods like gamma irradiation in air can lead to oxidation and degradation of the material. While alternative methods have been developed, such as ethylene oxide sterilization or gamma irradiation in an inert atmosphere, each comes with its own set of limitations and potential effects on material properties.
Lastly, the development of UHMWPE for specific patient populations presents a unique challenge. Different age groups, activity levels, and medical conditions may require tailored UHMWPE formulations. Creating customized solutions while maintaining manufacturing efficiency and regulatory compliance is an ongoing area of research and development in the field of joint replacement technologies.
Another significant challenge is the balance between mechanical properties and oxidation resistance. Crosslinking techniques have been employed to enhance wear resistance, but this process can compromise the material's mechanical strength and fatigue resistance. Additionally, the free radicals produced during crosslinking can lead to oxidative degradation, affecting the long-term stability of the implant.
The biocompatibility of UHMWPE also presents ongoing challenges. Although generally considered biocompatible, the interaction between UHMWPE and the biological environment over extended periods is not fully understood. There are concerns about potential long-term effects of UHMWPE particles on the immune system and surrounding tissues.
Manufacturing consistency and quality control pose additional challenges. Ensuring uniform molecular weight distribution and crystallinity across batches is crucial for maintaining consistent performance in implants. Variations in these properties can lead to unpredictable wear characteristics and mechanical behavior.
The sterilization process for UHMWPE components remains a challenge. Traditional methods like gamma irradiation in air can lead to oxidation and degradation of the material. While alternative methods have been developed, such as ethylene oxide sterilization or gamma irradiation in an inert atmosphere, each comes with its own set of limitations and potential effects on material properties.
Lastly, the development of UHMWPE for specific patient populations presents a unique challenge. Different age groups, activity levels, and medical conditions may require tailored UHMWPE formulations. Creating customized solutions while maintaining manufacturing efficiency and regulatory compliance is an ongoing area of research and development in the field of joint replacement technologies.
Existing UHMWPE Formulations and Processing
01 Synthesis and processing of UHMWPE
Various methods for synthesizing and processing Ultra-High Molecular Weight Polyethylene (UHMWPE) are explored. These include polymerization techniques, extrusion processes, and molding methods to produce UHMWPE with desired properties. The focus is on improving the molecular weight, crystallinity, and overall performance of the material.- Synthesis and processing of UHMWPE: Various methods for synthesizing and processing Ultra-High Molecular Weight Polyethylene (UHMWPE) are explored. These include polymerization techniques, extrusion processes, and molding methods to produce UHMWPE with desired properties. The focus is on improving the molecular weight, crystallinity, and overall performance of the material.
- UHMWPE composites and blends: Development of UHMWPE-based composites and blends to enhance specific properties. This includes incorporating additives, reinforcing materials, or blending with other polymers to improve mechanical strength, wear resistance, or other desired characteristics. The aim is to create tailored materials for specific applications.
- Surface modification of UHMWPE: Techniques for modifying the surface of UHMWPE to enhance its properties or compatibility with other materials. This may involve chemical treatments, plasma processing, or coating applications to improve adhesion, wettability, or biocompatibility of UHMWPE surfaces for various applications.
- UHMWPE in medical applications: Utilization of UHMWPE in medical devices and implants due to its biocompatibility and wear resistance. This includes the development of specialized grades of UHMWPE for orthopedic implants, prosthetics, and other medical applications, focusing on improving longevity and performance in the human body.
- UHMWPE fibers and films: Production and application of UHMWPE in fiber and film forms. This involves specialized processing techniques to create high-strength fibers or thin films with exceptional properties. Applications include ballistic protection, high-performance textiles, and industrial uses requiring high strength-to-weight ratios.
02 UHMWPE composites and blends
Development of UHMWPE-based composites and blends to enhance specific properties. This includes incorporating additives, fillers, or other polymers to improve mechanical strength, wear resistance, or thermal properties. The aim is to create tailored materials for specific applications while maintaining the inherent benefits of UHMWPE.Expand Specific Solutions03 Surface modification of UHMWPE
Techniques for modifying the surface of UHMWPE to enhance its properties or compatibility with other materials. This may include chemical treatments, plasma treatments, or grafting methods to improve adhesion, wettability, or biocompatibility. The goal is to expand the range of applications for UHMWPE in various industries.Expand Specific Solutions04 UHMWPE fibers and films
Production and application of UHMWPE in fiber and film forms. This includes spinning techniques, drawing processes, and film extrusion methods to create high-strength fibers and thin films. The focus is on developing materials for applications such as protective gear, high-performance textiles, and packaging.Expand Specific Solutions05 Medical and biomedical applications of UHMWPE
Utilization of UHMWPE in medical and biomedical applications, particularly in orthopedic implants and prosthetics. This includes developing wear-resistant UHMWPE components, improving longevity of implants, and enhancing biocompatibility. Research also focuses on sterilization methods and the incorporation of antimicrobial properties.Expand Specific Solutions
Key Players in UHMWPE Manufacturing
The investigation of UHMWPE's role in joint replacement technologies reveals a competitive landscape in a mature yet evolving market. With a global market size exceeding $19 billion, the industry is dominated by established players like Smith & Nephew Orthopaedics GmbH, Howmedica Osteonics Corp., and Zimmer, Inc. These companies leverage advanced materials technology, including UHMWPE, to develop innovative joint replacement solutions. The technology's maturity is evident in its widespread adoption, yet ongoing research by institutions such as Nanjing University of Science & Technology and Sichuan University indicates potential for further advancements. Emerging players like B One Medical Suzhou Co. Ltd. are also contributing to the field, focusing on specialized products for specific patient demographics.
Smith & Nephew Orthopaedics GmbH
Technical Solution: Smith & Nephew has developed advanced UHMWPE-based materials for joint replacement, including their OXINIUM™ Oxidized Zirconium technology. This technology combines a ceramic surface with a metal core, which is then paired with highly cross-linked UHMWPE. The company's VERILAST™ Technology for hip and knee replacements utilizes OXINIUM material articulating against XLPE (cross-linked polyethylene), showing significantly reduced wear rates compared to traditional materials[1][2]. Their research has demonstrated that this combination can potentially extend implant longevity, with simulator studies showing up to 80% reduction in wear compared to traditional cobalt chrome on standard polyethylene[3].
Strengths: Excellent wear resistance, potential for extended implant life, and reduced risk of osteolysis. Weaknesses: Higher cost compared to traditional materials, limited long-term clinical data for newer technologies.
Howmedica Osteonics Corp.
Technical Solution: Howmedica Osteonics, a subsidiary of Stryker Corporation, has made significant advancements in UHMWPE technology for joint replacements. Their X3™ Advanced Bearing Technology utilizes a proprietary sequential irradiation and annealing process to create highly cross-linked UHMWPE. This process enhances wear resistance while maintaining mechanical strength and oxidation resistance[4]. The company's research has shown that X3 polyethylene demonstrates up to 97% wear reduction compared to conventional polyethylene in hip simulator studies[5]. Additionally, they have developed the Tritanium™ technology, which combines a porous titanium coating with UHMWPE, promoting bone ingrowth and implant stability[6].
Strengths: Highly wear-resistant UHMWPE, maintained mechanical properties, and potential for improved implant fixation. Weaknesses: Complexity of manufacturing process may increase costs, and long-term clinical performance data is still accumulating.
Innovations in UHMWPE Material Science
Ultra-high molecular weight polyethylene and preparation method therefor
PatentPendingEP4286420A1
Innovation
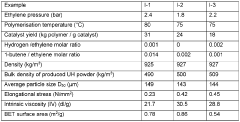
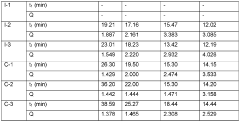
- The use of a supported non-metallocene catalyst system with an alkane or mixed alkane solvent in the ethylene slurry polymerization process, in the absence of hydrogen gas, to produce UHMWPE with low metal element content and high mechanical properties, allowing for stable polymerization and simplified post-treatment.
Ultra-high molecular weight polyethylene powder having improved swelling performance
PatentWO2021228735A1
Innovation
- Developing a UHMWPE powder with a BET specific surface area of at least 0.50 m2/g, prepared via slurry polymerization, allows for a gel solution with a desired swelling ratio to be achieved at moderate temperatures within a reduced swelling period, enhancing solvent absorption and reducing polymer degradation.
Biocompatibility and Safety Regulations
Ultra-high-molecular-weight polyethylene (UHMWPE) has become a crucial material in joint replacement technologies, particularly for its use in artificial joint components. The biocompatibility and safety of UHMWPE are paramount concerns in its application, necessitating stringent regulations and testing protocols to ensure patient safety and long-term implant success.
The biocompatibility of UHMWPE is primarily attributed to its inert nature and resistance to degradation within the human body. However, the material's interaction with surrounding tissues and potential long-term effects require thorough evaluation. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established comprehensive guidelines for assessing the biocompatibility of implant materials, including UHMWPE.
These regulations typically mandate a series of in vitro and in vivo tests to evaluate the material's cytotoxicity, sensitization potential, irritation or intracutaneous reactivity, systemic toxicity, and genotoxicity. For UHMWPE specifically, additional tests focus on its wear properties and the biological response to wear particles, as these can potentially lead to osteolysis and implant loosening over time.
Safety regulations also extend to the manufacturing processes of UHMWPE components. Strict quality control measures are required to ensure the purity of the material and prevent contamination that could compromise its biocompatibility. This includes regulations on sterilization methods, as improper sterilization can alter the material properties of UHMWPE and potentially introduce harmful substances.
The FDA's guidance document on the use of UHMWPE in orthopedic devices outlines specific requirements for mechanical testing, wear testing, and chemical characterization. These tests aim to predict the long-term performance of the material in vivo and assess potential risks associated with its use.
International standards, such as ISO 10993 for biological evaluation of medical devices, provide a framework for assessing the biocompatibility of UHMWPE and other implant materials. These standards are continually updated to reflect advances in materials science and our understanding of the body's response to implanted materials.
Recent developments in UHMWPE technology, such as highly cross-linked and vitamin E-doped variants, have necessitated additional regulatory considerations. These modifications aim to improve wear resistance and oxidative stability but require thorough evaluation to ensure they do not compromise the material's biocompatibility or introduce new safety concerns.
As research continues to advance our understanding of the long-term effects of implanted materials, regulatory bodies are adapting their guidelines to address emerging concerns. This includes a growing focus on the potential systemic effects of wear particles and the need for more sophisticated in vitro models to predict in vivo performance accurately.
The biocompatibility of UHMWPE is primarily attributed to its inert nature and resistance to degradation within the human body. However, the material's interaction with surrounding tissues and potential long-term effects require thorough evaluation. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established comprehensive guidelines for assessing the biocompatibility of implant materials, including UHMWPE.
These regulations typically mandate a series of in vitro and in vivo tests to evaluate the material's cytotoxicity, sensitization potential, irritation or intracutaneous reactivity, systemic toxicity, and genotoxicity. For UHMWPE specifically, additional tests focus on its wear properties and the biological response to wear particles, as these can potentially lead to osteolysis and implant loosening over time.
Safety regulations also extend to the manufacturing processes of UHMWPE components. Strict quality control measures are required to ensure the purity of the material and prevent contamination that could compromise its biocompatibility. This includes regulations on sterilization methods, as improper sterilization can alter the material properties of UHMWPE and potentially introduce harmful substances.
The FDA's guidance document on the use of UHMWPE in orthopedic devices outlines specific requirements for mechanical testing, wear testing, and chemical characterization. These tests aim to predict the long-term performance of the material in vivo and assess potential risks associated with its use.
International standards, such as ISO 10993 for biological evaluation of medical devices, provide a framework for assessing the biocompatibility of UHMWPE and other implant materials. These standards are continually updated to reflect advances in materials science and our understanding of the body's response to implanted materials.
Recent developments in UHMWPE technology, such as highly cross-linked and vitamin E-doped variants, have necessitated additional regulatory considerations. These modifications aim to improve wear resistance and oxidative stability but require thorough evaluation to ensure they do not compromise the material's biocompatibility or introduce new safety concerns.
As research continues to advance our understanding of the long-term effects of implanted materials, regulatory bodies are adapting their guidelines to address emerging concerns. This includes a growing focus on the potential systemic effects of wear particles and the need for more sophisticated in vitro models to predict in vivo performance accurately.
Long-term Clinical Outcomes of UHMWPE Implants
Long-term clinical outcomes of Ultra-High Molecular Weight Polyethylene (UHMWPE) implants have been extensively studied over the past few decades, providing valuable insights into the performance and durability of these materials in joint replacement technologies. The clinical data collected from numerous studies and patient follow-ups have demonstrated the overall effectiveness of UHMWPE in improving patient quality of life and reducing pain associated with joint disorders.
One of the key findings from long-term studies is the significant improvement in wear resistance of UHMWPE implants. Early generations of UHMWPE components showed wear rates that led to osteolysis and implant loosening. However, advancements in manufacturing processes, such as cross-linking and the addition of antioxidants, have substantially reduced wear rates. Studies comparing conventional UHMWPE with highly cross-linked polyethylene (HXLPE) have consistently shown lower wear rates and reduced incidence of osteolysis in HXLPE implants.
The longevity of UHMWPE implants has also been a focus of long-term clinical studies. Research has shown that modern UHMWPE implants can maintain their structural integrity and functionality for 15-20 years or more in many patients. This extended lifespan has significantly reduced the need for revision surgeries, particularly in older patient populations. However, it's important to note that implant longevity can vary depending on factors such as patient activity level, body weight, and implant design.
Clinical outcomes have also highlighted the importance of proper implant positioning and surgical technique in maximizing the performance of UHMWPE components. Studies have shown that mal-alignment or improper sizing can lead to increased wear and reduced implant lifespan. This has led to improvements in surgical techniques and the development of more precise implant positioning tools.
Long-term follow-up studies have also provided insights into the biological response to UHMWPE wear particles. While early concerns about the potential for these particles to cause adverse tissue reactions have been largely addressed through improved manufacturing processes, ongoing research continues to monitor the long-term effects of UHMWPE wear debris on surrounding tissues.
Patient-reported outcomes have been a crucial aspect of long-term clinical studies. These have consistently shown high levels of patient satisfaction, improved mobility, and reduced pain levels following joint replacement with UHMWPE implants. Quality of life measures have demonstrated sustained improvements over extended periods, further supporting the efficacy of these implants.
In conclusion, long-term clinical outcomes of UHMWPE implants have been largely positive, showcasing the material's effectiveness in joint replacement technologies. However, ongoing research continues to focus on further improving implant performance, reducing wear rates, and extending implant longevity to meet the needs of an increasingly active and aging population.
One of the key findings from long-term studies is the significant improvement in wear resistance of UHMWPE implants. Early generations of UHMWPE components showed wear rates that led to osteolysis and implant loosening. However, advancements in manufacturing processes, such as cross-linking and the addition of antioxidants, have substantially reduced wear rates. Studies comparing conventional UHMWPE with highly cross-linked polyethylene (HXLPE) have consistently shown lower wear rates and reduced incidence of osteolysis in HXLPE implants.
The longevity of UHMWPE implants has also been a focus of long-term clinical studies. Research has shown that modern UHMWPE implants can maintain their structural integrity and functionality for 15-20 years or more in many patients. This extended lifespan has significantly reduced the need for revision surgeries, particularly in older patient populations. However, it's important to note that implant longevity can vary depending on factors such as patient activity level, body weight, and implant design.
Clinical outcomes have also highlighted the importance of proper implant positioning and surgical technique in maximizing the performance of UHMWPE components. Studies have shown that mal-alignment or improper sizing can lead to increased wear and reduced implant lifespan. This has led to improvements in surgical techniques and the development of more precise implant positioning tools.
Long-term follow-up studies have also provided insights into the biological response to UHMWPE wear particles. While early concerns about the potential for these particles to cause adverse tissue reactions have been largely addressed through improved manufacturing processes, ongoing research continues to monitor the long-term effects of UHMWPE wear debris on surrounding tissues.
Patient-reported outcomes have been a crucial aspect of long-term clinical studies. These have consistently shown high levels of patient satisfaction, improved mobility, and reduced pain levels following joint replacement with UHMWPE implants. Quality of life measures have demonstrated sustained improvements over extended periods, further supporting the efficacy of these implants.
In conclusion, long-term clinical outcomes of UHMWPE implants have been largely positive, showcasing the material's effectiveness in joint replacement technologies. However, ongoing research continues to focus on further improving implant performance, reducing wear rates, and extending implant longevity to meet the needs of an increasingly active and aging population.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!