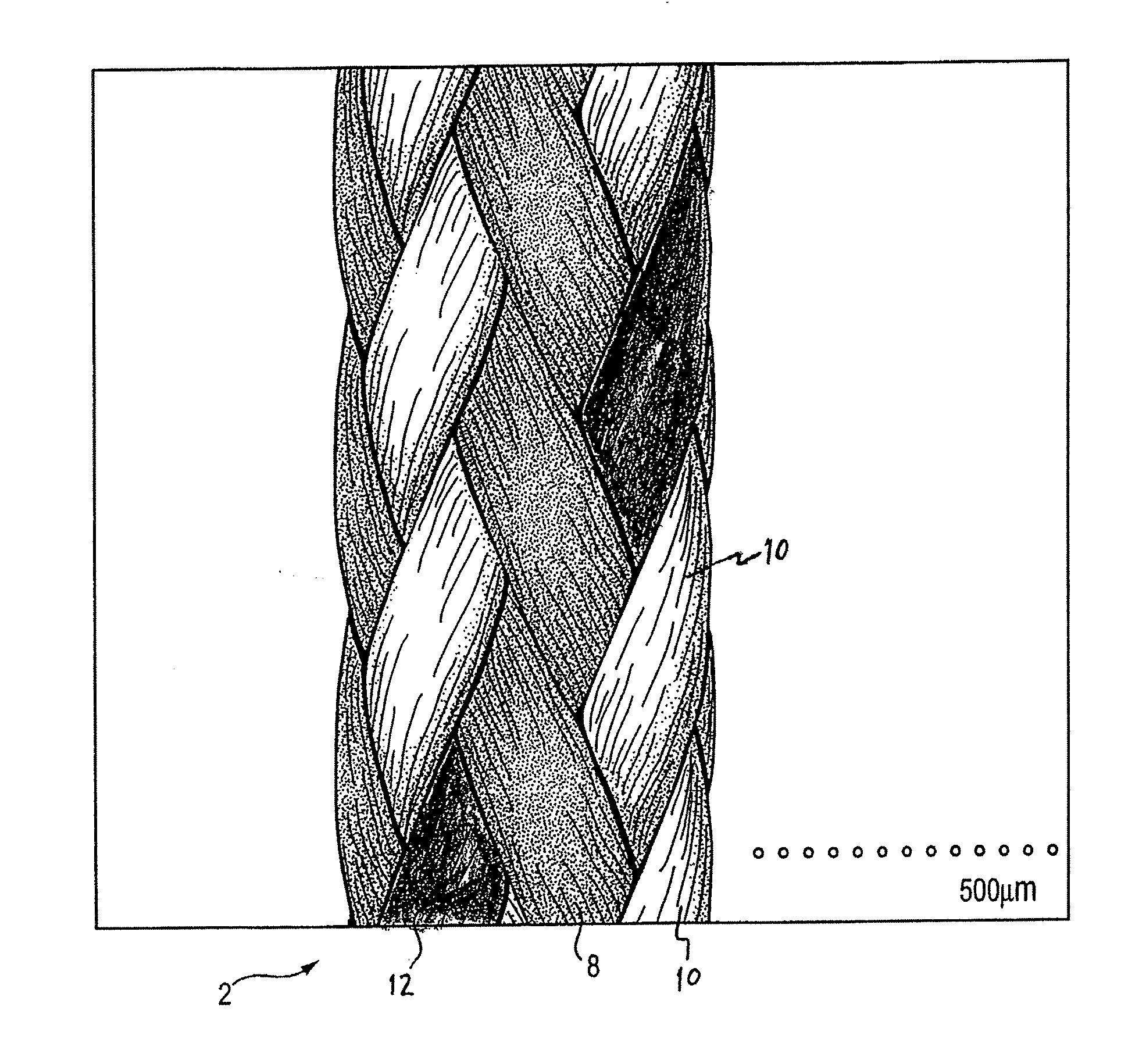
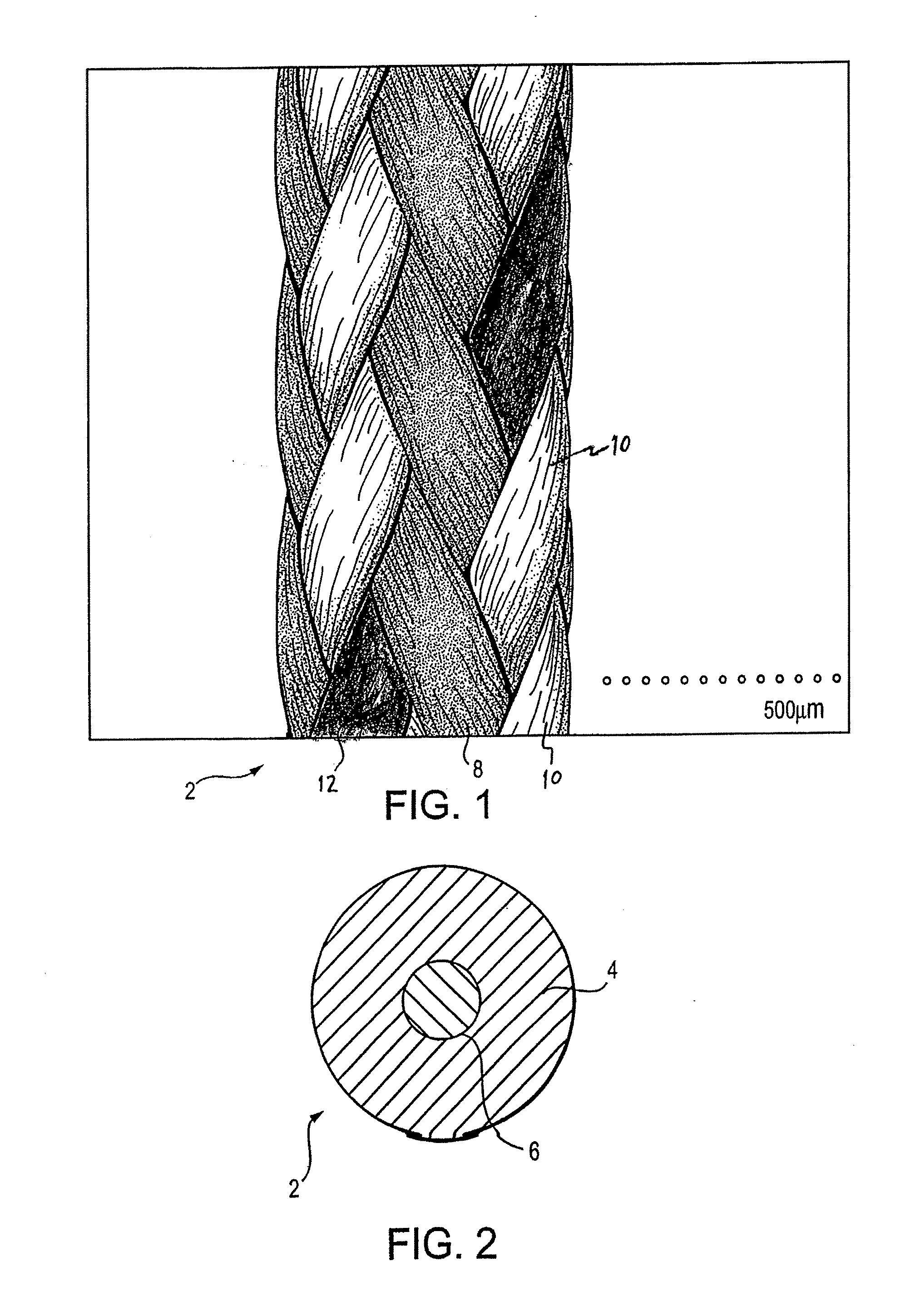
UHMWPE's Impact on the Durability of Surgical Sutures
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
UHMWPE Suture Evolution
The evolution of UHMWPE (Ultra-High Molecular Weight Polyethylene) sutures represents a significant advancement in surgical technology, particularly in terms of enhancing the durability and performance of surgical sutures. This evolution can be traced through several key stages, each marked by notable improvements and innovations.
In the early stages of suture development, traditional materials such as silk, catgut, and nylon were predominantly used. These materials, while effective, had limitations in terms of strength, durability, and biocompatibility. The introduction of UHMWPE in the 1950s marked the beginning of a new era in suture technology.
The initial application of UHMWPE in sutures was limited due to challenges in processing the material into fine, strong fibers. However, advancements in polymer science and manufacturing techniques in the 1970s and 1980s led to the development of UHMWPE fibers with exceptional strength-to-weight ratios. This breakthrough paved the way for the creation of high-performance sutures.
The 1990s saw the first commercial applications of UHMWPE in surgical sutures. These early UHMWPE sutures demonstrated superior tensile strength and abrasion resistance compared to traditional materials. However, they faced challenges in knot security and tissue reaction, which became focal points for further research and development.
In the early 2000s, significant progress was made in improving the surface properties of UHMWPE sutures. Techniques such as plasma treatment and chemical modification were employed to enhance the material's biocompatibility and reduce tissue reaction. This period also saw the development of hybrid sutures, combining UHMWPE with other materials to optimize performance characteristics.
The late 2000s and early 2010s marked a period of rapid innovation in UHMWPE suture technology. Advanced manufacturing processes, such as gel spinning and ultra-drawing, enabled the production of UHMWPE fibers with even higher strength and durability. These improvements led to the creation of sutures capable of withstanding extreme tensile forces, making them ideal for orthopedic and high-stress applications.
Recent years have seen a focus on enhancing the biological properties of UHMWPE sutures. Research has been directed towards developing coatings and surface modifications that promote faster healing, reduce infection risks, and improve overall biocompatibility. Additionally, efforts have been made to address the historical challenges of knot security and handling characteristics.
The latest developments in UHMWPE suture technology include the exploration of nanocomposite materials and the integration of smart technologies. These innovations aim to create sutures with enhanced properties such as antimicrobial activity, controlled drug release, and even the ability to monitor wound healing processes.
In the early stages of suture development, traditional materials such as silk, catgut, and nylon were predominantly used. These materials, while effective, had limitations in terms of strength, durability, and biocompatibility. The introduction of UHMWPE in the 1950s marked the beginning of a new era in suture technology.
The initial application of UHMWPE in sutures was limited due to challenges in processing the material into fine, strong fibers. However, advancements in polymer science and manufacturing techniques in the 1970s and 1980s led to the development of UHMWPE fibers with exceptional strength-to-weight ratios. This breakthrough paved the way for the creation of high-performance sutures.
The 1990s saw the first commercial applications of UHMWPE in surgical sutures. These early UHMWPE sutures demonstrated superior tensile strength and abrasion resistance compared to traditional materials. However, they faced challenges in knot security and tissue reaction, which became focal points for further research and development.
In the early 2000s, significant progress was made in improving the surface properties of UHMWPE sutures. Techniques such as plasma treatment and chemical modification were employed to enhance the material's biocompatibility and reduce tissue reaction. This period also saw the development of hybrid sutures, combining UHMWPE with other materials to optimize performance characteristics.
The late 2000s and early 2010s marked a period of rapid innovation in UHMWPE suture technology. Advanced manufacturing processes, such as gel spinning and ultra-drawing, enabled the production of UHMWPE fibers with even higher strength and durability. These improvements led to the creation of sutures capable of withstanding extreme tensile forces, making them ideal for orthopedic and high-stress applications.
Recent years have seen a focus on enhancing the biological properties of UHMWPE sutures. Research has been directed towards developing coatings and surface modifications that promote faster healing, reduce infection risks, and improve overall biocompatibility. Additionally, efforts have been made to address the historical challenges of knot security and handling characteristics.
The latest developments in UHMWPE suture technology include the exploration of nanocomposite materials and the integration of smart technologies. These innovations aim to create sutures with enhanced properties such as antimicrobial activity, controlled drug release, and even the ability to monitor wound healing processes.
Surgical Suture Market
The surgical suture market has experienced significant growth in recent years, driven by the increasing number of surgical procedures worldwide and advancements in medical technology. This market encompasses various types of sutures, including absorbable and non-absorbable sutures, with materials ranging from traditional options like silk and catgut to modern synthetic polymers.
The global surgical suture market size was valued at approximately $3.5 billion in 2020 and is projected to reach $5.4 billion by 2027, growing at a compound annual growth rate (CAGR) of around 6.5% during the forecast period. This growth is attributed to factors such as the rising prevalence of chronic diseases, an aging population, and the increasing number of surgical procedures across various medical specialties.
Geographically, North America holds the largest share of the surgical suture market, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare facilities and increasing healthcare awareness.
The market is characterized by the presence of several key players, including Johnson & Johnson, Medtronic, B. Braun Melsungen AG, and Ethicon, among others. These companies are focusing on product innovations and strategic collaborations to maintain their market positions and expand their product portfolios.
One of the key trends in the surgical suture market is the growing demand for advanced suture materials that offer improved performance and patient outcomes. This has led to the development of innovative suture technologies, such as antimicrobial sutures and barbed sutures, which aim to reduce the risk of surgical site infections and improve wound closure efficiency.
The introduction of Ultra-High Molecular Weight Polyethylene (UHMWPE) in surgical sutures represents a significant advancement in this field. UHMWPE sutures offer superior strength, durability, and biocompatibility compared to traditional suture materials. These properties make UHMWPE sutures particularly suitable for orthopedic and cardiovascular surgeries, where high tensile strength and minimal tissue reaction are crucial.
The market demand for UHMWPE sutures is expected to grow substantially in the coming years, driven by their ability to enhance surgical outcomes and reduce the risk of suture-related complications. This trend aligns with the overall market shift towards more advanced and specialized suture materials that cater to specific surgical needs and improve patient care.
The global surgical suture market size was valued at approximately $3.5 billion in 2020 and is projected to reach $5.4 billion by 2027, growing at a compound annual growth rate (CAGR) of around 6.5% during the forecast period. This growth is attributed to factors such as the rising prevalence of chronic diseases, an aging population, and the increasing number of surgical procedures across various medical specialties.
Geographically, North America holds the largest share of the surgical suture market, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare facilities and increasing healthcare awareness.
The market is characterized by the presence of several key players, including Johnson & Johnson, Medtronic, B. Braun Melsungen AG, and Ethicon, among others. These companies are focusing on product innovations and strategic collaborations to maintain their market positions and expand their product portfolios.
One of the key trends in the surgical suture market is the growing demand for advanced suture materials that offer improved performance and patient outcomes. This has led to the development of innovative suture technologies, such as antimicrobial sutures and barbed sutures, which aim to reduce the risk of surgical site infections and improve wound closure efficiency.
The introduction of Ultra-High Molecular Weight Polyethylene (UHMWPE) in surgical sutures represents a significant advancement in this field. UHMWPE sutures offer superior strength, durability, and biocompatibility compared to traditional suture materials. These properties make UHMWPE sutures particularly suitable for orthopedic and cardiovascular surgeries, where high tensile strength and minimal tissue reaction are crucial.
The market demand for UHMWPE sutures is expected to grow substantially in the coming years, driven by their ability to enhance surgical outcomes and reduce the risk of suture-related complications. This trend aligns with the overall market shift towards more advanced and specialized suture materials that cater to specific surgical needs and improve patient care.
UHMWPE Challenges
Despite the numerous advantages of Ultra-High Molecular Weight Polyethylene (UHMWPE) in surgical sutures, several challenges persist in its application and development. One of the primary concerns is the material's susceptibility to oxidative degradation. When exposed to oxygen and other reactive species, UHMWPE can undergo chemical changes that compromise its mechanical properties, potentially leading to premature failure of the sutures.
Another significant challenge lies in the processing of UHMWPE for suture production. The material's high molecular weight and long polymer chains, while contributing to its strength, make it difficult to melt and form into fine suture fibers. This necessitates specialized manufacturing techniques, which can increase production costs and limit scalability.
The biocompatibility of UHMWPE sutures also presents ongoing challenges. While generally considered inert, there are concerns about potential inflammatory responses in some patients. Long-term studies are still needed to fully understand the body's response to UHMWPE sutures over extended periods, particularly in diverse surgical applications.
Surface modification of UHMWPE sutures remains a complex issue. Enhancing the material's surface properties to improve tissue integration and reduce bacterial adhesion without compromising its core mechanical strengths is an area of active research. Current modification techniques often struggle to achieve a balance between improved surface characteristics and maintained bulk properties.
The sterilization of UHMWPE sutures poses another challenge. Traditional sterilization methods, such as gamma irradiation, can induce cross-linking and oxidation in the polymer, potentially altering its mechanical properties. Developing sterilization techniques that effectively sanitize without degrading the material's performance is crucial.
Lastly, the environmental impact of UHMWPE sutures is a growing concern. As a non-biodegradable polymer, UHMWPE contributes to medical waste accumulation. Developing eco-friendly alternatives or effective recycling methods for these sutures without compromising their medical efficacy remains a significant challenge for the industry.
Another significant challenge lies in the processing of UHMWPE for suture production. The material's high molecular weight and long polymer chains, while contributing to its strength, make it difficult to melt and form into fine suture fibers. This necessitates specialized manufacturing techniques, which can increase production costs and limit scalability.
The biocompatibility of UHMWPE sutures also presents ongoing challenges. While generally considered inert, there are concerns about potential inflammatory responses in some patients. Long-term studies are still needed to fully understand the body's response to UHMWPE sutures over extended periods, particularly in diverse surgical applications.
Surface modification of UHMWPE sutures remains a complex issue. Enhancing the material's surface properties to improve tissue integration and reduce bacterial adhesion without compromising its core mechanical strengths is an area of active research. Current modification techniques often struggle to achieve a balance between improved surface characteristics and maintained bulk properties.
The sterilization of UHMWPE sutures poses another challenge. Traditional sterilization methods, such as gamma irradiation, can induce cross-linking and oxidation in the polymer, potentially altering its mechanical properties. Developing sterilization techniques that effectively sanitize without degrading the material's performance is crucial.
Lastly, the environmental impact of UHMWPE sutures is a growing concern. As a non-biodegradable polymer, UHMWPE contributes to medical waste accumulation. Developing eco-friendly alternatives or effective recycling methods for these sutures without compromising their medical efficacy remains a significant challenge for the industry.
Current UHMWPE Solutions
01 Improving UHMWPE durability through crosslinking
Crosslinking techniques are employed to enhance the durability of UHMWPE. This process involves creating chemical bonds between polymer chains, resulting in improved wear resistance and mechanical properties. Various methods, such as radiation or chemical crosslinking, can be used to achieve this effect, leading to increased longevity of UHMWPE products.- Improving UHMWPE durability through crosslinking: Crosslinking techniques are employed to enhance the durability of UHMWPE. This process involves creating chemical bonds between polymer chains, resulting in improved wear resistance and mechanical properties. Various methods, such as radiation or chemical crosslinking, can be used to achieve this effect, leading to increased longevity of UHMWPE-based products.
- Enhancing UHMWPE durability with additives and fillers: The incorporation of additives and fillers into UHMWPE can significantly improve its durability. These additives may include antioxidants, stabilizers, or reinforcing materials such as carbon fibers or nanoparticles. The resulting composite materials exhibit enhanced mechanical properties, wear resistance, and overall durability compared to unmodified UHMWPE.
- UHMWPE processing techniques for improved durability: Advanced processing techniques are utilized to enhance the durability of UHMWPE. These may include specialized molding processes, compression molding, or ram extrusion. By optimizing processing parameters such as temperature, pressure, and cooling rates, the resulting UHMWPE products can achieve improved mechanical properties and durability.
- Surface modification of UHMWPE for enhanced durability: Surface modification techniques are applied to UHMWPE to improve its durability and performance in specific applications. These methods may include plasma treatment, chemical etching, or the application of protective coatings. Such modifications can enhance wear resistance, reduce friction, and improve the overall durability of UHMWPE surfaces.
- Testing and characterization of UHMWPE durability: Various testing and characterization methods are employed to assess and improve the durability of UHMWPE. These may include accelerated wear testing, fatigue testing, and advanced microscopy techniques. By understanding the mechanisms of wear and degradation, researchers can develop strategies to enhance the long-term durability of UHMWPE-based products.
02 Enhancing UHMWPE durability with additives and fillers
The incorporation of additives and fillers into UHMWPE can significantly improve its durability. These additives may include antioxidants, stabilizers, or reinforcing materials such as carbon fibers or nanoparticles. The resulting composite materials exhibit enhanced mechanical properties, wear resistance, and overall durability compared to unmodified UHMWPE.Expand Specific Solutions03 Surface modification techniques for improved UHMWPE durability
Various surface modification techniques can be applied to UHMWPE to enhance its durability. These methods may include plasma treatment, chemical etching, or the application of protective coatings. Such modifications can improve the material's resistance to wear, oxidation, and environmental degradation, thereby extending its service life in demanding applications.Expand Specific Solutions04 Optimizing processing conditions for enhanced UHMWPE durability
The durability of UHMWPE can be significantly influenced by its processing conditions. Factors such as molding temperature, pressure, and cooling rate can affect the material's crystallinity, molecular orientation, and overall mechanical properties. Optimizing these parameters during manufacturing can lead to UHMWPE products with improved durability and performance characteristics.Expand Specific Solutions05 Testing and characterization methods for UHMWPE durability
Various testing and characterization methods are employed to assess and predict the durability of UHMWPE. These may include accelerated aging tests, wear simulations, mechanical property evaluations, and microstructural analyses. Such methods help in understanding the material's behavior under different conditions and guide the development of more durable UHMWPE formulations and products.Expand Specific Solutions
Key UHMWPE Manufacturers
The UHMWPE surgical suture market is in a growth phase, driven by increasing demand for high-performance medical devices. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, UHMWPE sutures are advancing rapidly, with companies like DSM IP Assets BV, Arthrex GmbH, and Howmedica Osteonics Corp. leading innovation. These firms are developing enhanced UHMWPE fibers with improved strength and durability for surgical applications. Research institutions such as the University of Southern California and Massachusetts Institute of Technology are contributing to technological advancements, while hospitals like The General Hospital Corp. are conducting clinical trials to validate the efficacy of UHMWPE sutures in various surgical procedures.
DSM IP Assets BV
Technical Solution: DSM IP Assets BV has developed a proprietary UHMWPE fiber technology called Dyneema Purity®, specifically designed for medical applications including surgical sutures. Their process involves gel-spinning UHMWPE to create ultra-strong, ultra-lightweight fibers with excellent abrasion resistance and fatigue performance[1]. The company has further enhanced the material's properties through cross-linking techniques, improving its resistance to creep and wear[2]. DSM's UHMWPE sutures demonstrate superior tensile strength and knot security compared to traditional materials, potentially reducing the risk of suture failure during long-term implantation[3].
Strengths: Exceptional strength-to-weight ratio, high abrasion resistance, and improved long-term stability. Weaknesses: Higher cost compared to traditional suture materials, and potential challenges in sterilization due to the material's high melting point.
Teleflex Medical Inc
Technical Solution: Teleflex Medical Inc has developed a range of UHMWPE-based sutures under the brand name Force Fiber®. Their technology involves a unique braiding process that combines UHMWPE fibers with other materials to create hybrid sutures with optimized properties[4]. The company's approach focuses on tailoring the suture characteristics to specific surgical applications, such as orthopedics and sports medicine. Teleflex's UHMWPE sutures feature a proprietary surface treatment that enhances handling properties and knot security while maintaining the material's inherent strength[5]. Recent innovations include the development of colored UHMWPE sutures for improved visibility during minimally invasive procedures[6].
Strengths: Application-specific designs, improved handling characteristics, and enhanced visibility options. Weaknesses: Limited use in certain surgical fields due to the material's low coefficient of friction.
UHMWPE Suture Patents
High strength suture coated with collagen
PatentInactiveUS20080051834A1
Innovation
- A braided suture material combining ultrahigh molecular weight polyethylene fibers with polyester and a collagen coating, providing enhanced tie-down properties and aiding tendon-to-bone incorporation, with trace threads for identification during surgery.
High strength UHMWPE-based suture
PatentInactiveEP1293218B1
Innovation
- A surgical suture with a core of twisted ultrahigh molecular weight polyethylene fibers and a braided cover of ultrahigh molecular weight polyethylene fibers combined with long chain synthetic polymer or bioabsorbable fibers, optionally coated and featuring contrasting colored strands for enhanced visibility and handling.
Biocompatibility Analysis
Biocompatibility is a critical factor in the evaluation of UHMWPE's impact on surgical suture durability. The interaction between UHMWPE-based sutures and living tissues plays a crucial role in determining the overall performance and safety of these medical devices.
UHMWPE exhibits excellent biocompatibility properties, making it an ideal material for surgical sutures. Its inert nature minimizes the risk of adverse reactions when in contact with human tissues. The material's low coefficient of friction reduces tissue irritation and inflammation, contributing to improved wound healing and patient comfort.
Studies have shown that UHMWPE sutures elicit minimal foreign body response compared to traditional suture materials. This reduced inflammatory reaction is attributed to the material's smooth surface and low protein adsorption characteristics. As a result, UHMWPE sutures are less likely to trigger excessive scar tissue formation or promote bacterial adhesion, which can compromise suture integrity and wound healing.
The biocompatibility of UHMWPE sutures extends beyond the initial implantation period. Long-term studies have demonstrated that these sutures maintain their inert properties over time, with no significant degradation or release of harmful byproducts. This stability ensures consistent performance throughout the healing process and reduces the risk of delayed complications.
In terms of tissue integration, UHMWPE sutures have shown favorable results. While they do not actively promote tissue ingrowth, their surface properties allow for adequate cell adhesion and tissue attachment. This balance between integration and non-reactivity contributes to the sutures' ability to provide long-lasting support without compromising surrounding tissues.
The biocompatibility of UHMWPE sutures also extends to their resistance to microbial colonization. The material's low surface energy and hydrophobic nature make it less susceptible to bacterial adhesion compared to other suture materials. This property is particularly beneficial in reducing the risk of surgical site infections, a common complication in wound closure procedures.
Furthermore, UHMWPE's biocompatibility profile includes its non-allergenic nature. Unlike some synthetic or natural suture materials, UHMWPE does not contain proteins or other components that may trigger allergic reactions in sensitive individuals. This characteristic broadens its applicability across diverse patient populations and reduces the risk of hypersensitivity-related complications.
In conclusion, the biocompatibility analysis of UHMWPE in surgical sutures reveals a favorable profile that contributes significantly to their durability and overall performance. The material's inert nature, minimal tissue reactivity, long-term stability, and resistance to microbial colonization collectively enhance its suitability for various surgical applications, particularly in procedures requiring extended suture retention and optimal wound healing outcomes.
UHMWPE exhibits excellent biocompatibility properties, making it an ideal material for surgical sutures. Its inert nature minimizes the risk of adverse reactions when in contact with human tissues. The material's low coefficient of friction reduces tissue irritation and inflammation, contributing to improved wound healing and patient comfort.
Studies have shown that UHMWPE sutures elicit minimal foreign body response compared to traditional suture materials. This reduced inflammatory reaction is attributed to the material's smooth surface and low protein adsorption characteristics. As a result, UHMWPE sutures are less likely to trigger excessive scar tissue formation or promote bacterial adhesion, which can compromise suture integrity and wound healing.
The biocompatibility of UHMWPE sutures extends beyond the initial implantation period. Long-term studies have demonstrated that these sutures maintain their inert properties over time, with no significant degradation or release of harmful byproducts. This stability ensures consistent performance throughout the healing process and reduces the risk of delayed complications.
In terms of tissue integration, UHMWPE sutures have shown favorable results. While they do not actively promote tissue ingrowth, their surface properties allow for adequate cell adhesion and tissue attachment. This balance between integration and non-reactivity contributes to the sutures' ability to provide long-lasting support without compromising surrounding tissues.
The biocompatibility of UHMWPE sutures also extends to their resistance to microbial colonization. The material's low surface energy and hydrophobic nature make it less susceptible to bacterial adhesion compared to other suture materials. This property is particularly beneficial in reducing the risk of surgical site infections, a common complication in wound closure procedures.
Furthermore, UHMWPE's biocompatibility profile includes its non-allergenic nature. Unlike some synthetic or natural suture materials, UHMWPE does not contain proteins or other components that may trigger allergic reactions in sensitive individuals. This characteristic broadens its applicability across diverse patient populations and reduces the risk of hypersensitivity-related complications.
In conclusion, the biocompatibility analysis of UHMWPE in surgical sutures reveals a favorable profile that contributes significantly to their durability and overall performance. The material's inert nature, minimal tissue reactivity, long-term stability, and resistance to microbial colonization collectively enhance its suitability for various surgical applications, particularly in procedures requiring extended suture retention and optimal wound healing outcomes.
Regulatory Considerations
The regulatory landscape surrounding surgical sutures, particularly those incorporating Ultra-High Molecular Weight Polyethylene (UHMWPE), is complex and multifaceted. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) play crucial roles in ensuring the safety and efficacy of these medical devices.
In the United States, surgical sutures are classified as Class II medical devices, requiring a 510(k) premarket notification submission to the FDA. Manufacturers must demonstrate that their UHMWPE-enhanced sutures are substantially equivalent to predicate devices already on the market. This process involves rigorous testing to evaluate the suture's strength, durability, and biocompatibility.
The FDA's guidance document on surgical sutures outlines specific requirements for physical and mechanical properties, including tensile strength, knot pull strength, and diameter uniformity. For UHMWPE sutures, additional considerations may include evaluating the material's resistance to abrasion and its long-term stability in physiological conditions.
In the European Union, surgical sutures fall under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the Essential Requirements outlined in the MDR. This includes conducting a thorough risk assessment and providing clinical evidence of the device's performance and safety.
International standards, such as ISO 10993 for biocompatibility testing and USP <871> for suture-specific requirements, are widely recognized and often incorporated into regulatory frameworks. These standards provide a basis for evaluating the safety and performance of UHMWPE sutures across different jurisdictions.
Regulatory bodies are increasingly focusing on the long-term performance of implantable medical devices. For UHMWPE sutures, this may translate into requirements for extended durability testing and post-market surveillance to monitor their performance over time. Manufacturers may need to conduct long-term animal studies or provide clinical data demonstrating the sutures' ability to maintain strength and integrity in vivo.
As the use of UHMWPE in surgical sutures represents a relatively new application, regulatory agencies may require additional data to support its safety and efficacy. This could include studies on the material's degradation profile, potential for particle shedding, and its interaction with surrounding tissues over extended periods.
Regulatory considerations also extend to the manufacturing process of UHMWPE sutures. Good Manufacturing Practices (GMP) must be followed, with particular attention to maintaining the material's purity and consistency. Quality control measures, such as batch testing and process validation, are essential to ensure regulatory compliance and product reliability.
In the United States, surgical sutures are classified as Class II medical devices, requiring a 510(k) premarket notification submission to the FDA. Manufacturers must demonstrate that their UHMWPE-enhanced sutures are substantially equivalent to predicate devices already on the market. This process involves rigorous testing to evaluate the suture's strength, durability, and biocompatibility.
The FDA's guidance document on surgical sutures outlines specific requirements for physical and mechanical properties, including tensile strength, knot pull strength, and diameter uniformity. For UHMWPE sutures, additional considerations may include evaluating the material's resistance to abrasion and its long-term stability in physiological conditions.
In the European Union, surgical sutures fall under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the Essential Requirements outlined in the MDR. This includes conducting a thorough risk assessment and providing clinical evidence of the device's performance and safety.
International standards, such as ISO 10993 for biocompatibility testing and USP <871> for suture-specific requirements, are widely recognized and often incorporated into regulatory frameworks. These standards provide a basis for evaluating the safety and performance of UHMWPE sutures across different jurisdictions.
Regulatory bodies are increasingly focusing on the long-term performance of implantable medical devices. For UHMWPE sutures, this may translate into requirements for extended durability testing and post-market surveillance to monitor their performance over time. Manufacturers may need to conduct long-term animal studies or provide clinical data demonstrating the sutures' ability to maintain strength and integrity in vivo.
As the use of UHMWPE in surgical sutures represents a relatively new application, regulatory agencies may require additional data to support its safety and efficacy. This could include studies on the material's degradation profile, potential for particle shedding, and its interaction with surrounding tissues over extended periods.
Regulatory considerations also extend to the manufacturing process of UHMWPE sutures. Good Manufacturing Practices (GMP) must be followed, with particular attention to maintaining the material's purity and consistency. Quality control measures, such as batch testing and process validation, are essential to ensure regulatory compliance and product reliability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!