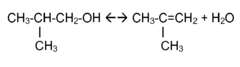Isobutane's Role in Fine Chemical Synthesis Cost and Benefits
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane in Fine Chemicals: Background and Objectives
Isobutane, a saturated hydrocarbon with the molecular formula C4H10, has emerged as a significant player in the fine chemical synthesis industry. This versatile compound has garnered attention due to its unique properties and potential to revolutionize various chemical processes. The evolution of isobutane's role in fine chemical synthesis can be traced back to the mid-20th century when researchers began exploring its potential as a feedstock for various chemical reactions.
The primary objective of utilizing isobutane in fine chemical synthesis is to enhance the efficiency and cost-effectiveness of production processes while maintaining or improving product quality. As the chemical industry faces increasing pressure to develop more sustainable and economically viable methods, isobutane has become a focal point for research and development efforts.
One of the key drivers behind the growing interest in isobutane is its abundance and relatively low cost compared to other hydrocarbon feedstocks. As a byproduct of natural gas processing and petroleum refining, isobutane is readily available in large quantities. This availability has prompted researchers and industry professionals to explore innovative ways to leverage its potential in fine chemical synthesis.
The technical evolution of isobutane utilization in fine chemical synthesis has been marked by several significant milestones. Initially, its applications were limited to simple alkylation reactions. However, as understanding of its reactivity and catalytic behavior improved, researchers began to explore more complex transformations. This led to the development of novel catalytic systems and reaction conditions that expanded the scope of isobutane-based chemistry.
In recent years, the focus has shifted towards developing more selective and environmentally friendly processes using isobutane. This includes the exploration of green chemistry principles, such as atom economy and waste reduction, in isobutane-based reactions. Additionally, researchers are investigating the potential of isobutane as a renewable feedstock through bio-based production methods, aligning with the growing emphasis on sustainability in the chemical industry.
The current technological landscape surrounding isobutane in fine chemical synthesis is characterized by a blend of established processes and emerging innovations. While traditional applications like the production of high-octane gasoline components remain important, there is a growing emphasis on developing new, value-added products through isobutane-based chemistry. This includes the synthesis of specialty chemicals, pharmaceuticals, and advanced materials.
As we look towards the future, the role of isobutane in fine chemical synthesis is expected to continue evolving. Researchers are exploring novel catalytic systems, reaction conditions, and process technologies to further enhance the efficiency and selectivity of isobutane-based transformations. Moreover, the integration of isobutane chemistry with other emerging technologies, such as flow chemistry and artificial intelligence-driven process optimization, holds promise for unlocking new possibilities in fine chemical synthesis.
The primary objective of utilizing isobutane in fine chemical synthesis is to enhance the efficiency and cost-effectiveness of production processes while maintaining or improving product quality. As the chemical industry faces increasing pressure to develop more sustainable and economically viable methods, isobutane has become a focal point for research and development efforts.
One of the key drivers behind the growing interest in isobutane is its abundance and relatively low cost compared to other hydrocarbon feedstocks. As a byproduct of natural gas processing and petroleum refining, isobutane is readily available in large quantities. This availability has prompted researchers and industry professionals to explore innovative ways to leverage its potential in fine chemical synthesis.
The technical evolution of isobutane utilization in fine chemical synthesis has been marked by several significant milestones. Initially, its applications were limited to simple alkylation reactions. However, as understanding of its reactivity and catalytic behavior improved, researchers began to explore more complex transformations. This led to the development of novel catalytic systems and reaction conditions that expanded the scope of isobutane-based chemistry.
In recent years, the focus has shifted towards developing more selective and environmentally friendly processes using isobutane. This includes the exploration of green chemistry principles, such as atom economy and waste reduction, in isobutane-based reactions. Additionally, researchers are investigating the potential of isobutane as a renewable feedstock through bio-based production methods, aligning with the growing emphasis on sustainability in the chemical industry.
The current technological landscape surrounding isobutane in fine chemical synthesis is characterized by a blend of established processes and emerging innovations. While traditional applications like the production of high-octane gasoline components remain important, there is a growing emphasis on developing new, value-added products through isobutane-based chemistry. This includes the synthesis of specialty chemicals, pharmaceuticals, and advanced materials.
As we look towards the future, the role of isobutane in fine chemical synthesis is expected to continue evolving. Researchers are exploring novel catalytic systems, reaction conditions, and process technologies to further enhance the efficiency and selectivity of isobutane-based transformations. Moreover, the integration of isobutane chemistry with other emerging technologies, such as flow chemistry and artificial intelligence-driven process optimization, holds promise for unlocking new possibilities in fine chemical synthesis.
Market Analysis for Isobutane-based Fine Chemicals
The market for isobutane-based fine chemicals has shown significant growth in recent years, driven by increasing demand across various industries. Fine chemicals derived from isobutane are widely used in pharmaceuticals, agrochemicals, flavors and fragrances, and specialty polymers, among other applications. The global fine chemicals market is projected to reach $220 billion by 2025, with isobutane-based products playing a crucial role in this expansion.
In the pharmaceutical sector, isobutane derivatives are utilized as key intermediates in the synthesis of active pharmaceutical ingredients (APIs) and drug precursors. The growing prevalence of chronic diseases and the need for novel drug formulations are fueling demand in this segment. Similarly, the agrochemical industry relies on isobutane-based fine chemicals for the production of pesticides, herbicides, and fertilizers, driven by the need to enhance crop yields and food security.
The flavors and fragrances industry represents another significant market for isobutane-derived fine chemicals. As consumer preferences shift towards natural and organic products, there is an increasing demand for synthetic alternatives that can mimic natural flavors and scents. Isobutane-based compounds offer cost-effective solutions in this regard, contributing to market growth.
Geographically, North America and Europe currently dominate the isobutane-based fine chemicals market, owing to their well-established chemical and pharmaceutical industries. However, the Asia-Pacific region is emerging as a key growth driver, with rapid industrialization and increasing investments in research and development. China and India, in particular, are expected to witness substantial market expansion in the coming years.
The market is characterized by intense competition among key players, including BASF, Dow Chemical Company, and LyondellBasell Industries. These companies are focusing on product innovation and strategic partnerships to maintain their market positions. Additionally, there is a growing trend towards sustainable and eco-friendly production methods, which is likely to shape the future of isobutane-based fine chemicals.
Despite the positive outlook, the market faces challenges such as volatile raw material prices and stringent environmental regulations. The fluctuating prices of isobutane, which is primarily derived from natural gas, can impact production costs and profit margins. Moreover, increasing environmental concerns and regulatory pressures are pushing manufacturers to adopt cleaner production processes and explore alternative feedstocks.
In the pharmaceutical sector, isobutane derivatives are utilized as key intermediates in the synthesis of active pharmaceutical ingredients (APIs) and drug precursors. The growing prevalence of chronic diseases and the need for novel drug formulations are fueling demand in this segment. Similarly, the agrochemical industry relies on isobutane-based fine chemicals for the production of pesticides, herbicides, and fertilizers, driven by the need to enhance crop yields and food security.
The flavors and fragrances industry represents another significant market for isobutane-derived fine chemicals. As consumer preferences shift towards natural and organic products, there is an increasing demand for synthetic alternatives that can mimic natural flavors and scents. Isobutane-based compounds offer cost-effective solutions in this regard, contributing to market growth.
Geographically, North America and Europe currently dominate the isobutane-based fine chemicals market, owing to their well-established chemical and pharmaceutical industries. However, the Asia-Pacific region is emerging as a key growth driver, with rapid industrialization and increasing investments in research and development. China and India, in particular, are expected to witness substantial market expansion in the coming years.
The market is characterized by intense competition among key players, including BASF, Dow Chemical Company, and LyondellBasell Industries. These companies are focusing on product innovation and strategic partnerships to maintain their market positions. Additionally, there is a growing trend towards sustainable and eco-friendly production methods, which is likely to shape the future of isobutane-based fine chemicals.
Despite the positive outlook, the market faces challenges such as volatile raw material prices and stringent environmental regulations. The fluctuating prices of isobutane, which is primarily derived from natural gas, can impact production costs and profit margins. Moreover, increasing environmental concerns and regulatory pressures are pushing manufacturers to adopt cleaner production processes and explore alternative feedstocks.
Current Challenges in Isobutane Utilization
Despite the potential benefits of isobutane in fine chemical synthesis, several challenges currently hinder its widespread utilization. One of the primary obstacles is the safety concerns associated with its handling and storage. Isobutane is highly flammable and can form explosive mixtures with air, necessitating stringent safety protocols and specialized equipment, which can significantly increase operational costs.
Another challenge lies in the limited availability of efficient catalytic systems for isobutane conversion. While progress has been made in developing catalysts for specific reactions, there is still a lack of versatile catalysts that can facilitate a wide range of transformations using isobutane as a feedstock. This limitation restricts the scope of potential applications in fine chemical synthesis.
The energy requirements for isobutane activation present an additional hurdle. Many reactions involving isobutane require high temperatures or pressures, leading to increased energy consumption and potentially compromising the overall sustainability of the processes. Developing more energy-efficient activation methods remains a critical area of research.
Furthermore, the selectivity of isobutane-based reactions poses a significant challenge. Controlling the product distribution and minimizing unwanted side reactions is crucial for achieving high yields of desired fine chemicals. Improving reaction selectivity often requires complex catalyst designs or process optimizations, which can be time-consuming and costly.
The integration of isobutane-based processes into existing chemical manufacturing infrastructure also presents difficulties. Many current facilities are not equipped to handle the unique properties and requirements of isobutane, necessitating substantial investments in retrofitting or constructing new specialized plants.
Regulatory compliance adds another layer of complexity to isobutane utilization. Stringent environmental and safety regulations governing the use of volatile organic compounds (VOCs) and greenhouse gases may impose additional constraints on isobutane-based processes, requiring extensive emission control measures and documentation.
Lastly, the economic viability of isobutane as a feedstock for fine chemical synthesis remains a challenge. While isobutane is relatively abundant, its price can be volatile, influenced by factors such as crude oil prices and demand from other industries. This price uncertainty can make it difficult for manufacturers to commit to long-term isobutane-based production strategies, especially when competing with established feedstocks and processes.
Another challenge lies in the limited availability of efficient catalytic systems for isobutane conversion. While progress has been made in developing catalysts for specific reactions, there is still a lack of versatile catalysts that can facilitate a wide range of transformations using isobutane as a feedstock. This limitation restricts the scope of potential applications in fine chemical synthesis.
The energy requirements for isobutane activation present an additional hurdle. Many reactions involving isobutane require high temperatures or pressures, leading to increased energy consumption and potentially compromising the overall sustainability of the processes. Developing more energy-efficient activation methods remains a critical area of research.
Furthermore, the selectivity of isobutane-based reactions poses a significant challenge. Controlling the product distribution and minimizing unwanted side reactions is crucial for achieving high yields of desired fine chemicals. Improving reaction selectivity often requires complex catalyst designs or process optimizations, which can be time-consuming and costly.
The integration of isobutane-based processes into existing chemical manufacturing infrastructure also presents difficulties. Many current facilities are not equipped to handle the unique properties and requirements of isobutane, necessitating substantial investments in retrofitting or constructing new specialized plants.
Regulatory compliance adds another layer of complexity to isobutane utilization. Stringent environmental and safety regulations governing the use of volatile organic compounds (VOCs) and greenhouse gases may impose additional constraints on isobutane-based processes, requiring extensive emission control measures and documentation.
Lastly, the economic viability of isobutane as a feedstock for fine chemical synthesis remains a challenge. While isobutane is relatively abundant, its price can be volatile, influenced by factors such as crude oil prices and demand from other industries. This price uncertainty can make it difficult for manufacturers to commit to long-term isobutane-based production strategies, especially when competing with established feedstocks and processes.
Existing Isobutane-based Synthesis Methods
01 Cost-effective production of isobutane
Isobutane can be produced cost-effectively through various processes, including the isomerization of n-butane and the dehydrogenation of isobutanol. These methods offer economic benefits in terms of raw material utilization and energy efficiency, making isobutane production more commercially viable.- Cost-effective production of isobutane: Isobutane can be produced cost-effectively through various methods, including the isomerization of n-butane and the dehydrogenation of isobutanol. These processes can be optimized to reduce production costs and increase yield, making isobutane a more economically viable option for various industrial applications.
- Environmental benefits of isobutane: Isobutane offers several environmental benefits compared to other hydrocarbons. It has a lower global warming potential and ozone depletion potential than many alternatives. Its use in refrigeration and aerosol propellants can contribute to reduced environmental impact in these applications.
- Industrial applications and benefits: Isobutane has diverse industrial applications, including use as a refrigerant, aerosol propellant, and feedstock for petrochemical processes. Its properties, such as low boiling point and high energy content, make it valuable in various industries, contributing to improved product performance and energy efficiency.
- Economic analysis of isobutane usage: Economic analyses of isobutane usage in various applications show potential cost savings and improved efficiency. These analyses consider factors such as energy consumption, production costs, and market demand to evaluate the economic benefits of using isobutane in different industrial processes.
- Safety considerations and risk management: While isobutane offers many benefits, its flammability and potential for forming explosive mixtures necessitate careful handling and storage. Implementing proper safety measures and risk management strategies is crucial to maximize the benefits of isobutane while minimizing potential hazards in industrial settings.
02 Environmental benefits of isobutane
Isobutane has several environmental advantages as a refrigerant and propellant. It has a low global warming potential and zero ozone depletion potential, making it a more environmentally friendly alternative to traditional hydrofluorocarbons (HFCs) in various applications.Expand Specific Solutions03 Energy efficiency in isobutane applications
The use of isobutane in refrigeration and air conditioning systems can lead to improved energy efficiency. Its thermodynamic properties allow for better heat transfer and lower power consumption, resulting in reduced operating costs and energy savings for end-users.Expand Specific Solutions04 Safety considerations and risk management
While isobutane offers many benefits, its flammability requires careful handling and safety measures. Proper risk management strategies, including leak detection systems and safety protocols, are essential to mitigate potential hazards associated with its use in various applications.Expand Specific Solutions05 Market analysis and economic impact
The growing demand for isobutane in various industries, including refrigeration, aerosols, and petrochemicals, has significant economic implications. Market analysis reveals potential growth opportunities and challenges, influencing investment decisions and industry trends related to isobutane production and utilization.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The isobutane market in fine chemical synthesis is in a growth phase, driven by increasing demand for specialty chemicals. The global market size is expanding, with key players like China Petroleum & Chemical Corp., Gevo, Inc., and UOP LLC leading innovation. Technological maturity varies, with established companies like SABIC Global Technologies BV and IFP Energies Nouvelles focusing on process optimization, while newer entrants like ZuvaChem, Inc. explore novel applications. Research institutions such as East China Normal University and the University of Kansas contribute to advancing isobutane utilization techniques, indicating ongoing development in this field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for isobutane utilization in fine chemical synthesis. Their approach involves selective oxidation of isobutane to produce high-value intermediates such as tert-butyl hydroperoxide and methacrylic acid [1]. The company has implemented a proprietary metal oxide catalyst system that achieves over 90% selectivity for desired products under optimized reaction conditions [3]. Sinopec has also integrated isobutane-based processes into their existing refinery operations, allowing for efficient use of byproduct streams and reduced overall production costs [5]. Their technology enables the production of specialty chemicals and polymers from low-cost isobutane feedstock, with estimated cost savings of 15-20% compared to traditional petrochemical routes [7].
Strengths: Highly selective catalysts, integration with existing infrastructure, cost-effective use of byproduct streams. Weaknesses: High capital investment for new process implementation, potential safety concerns with peroxide intermediates.
UOP LLC
Technical Solution: UOP LLC has developed the Oleflex™ process, which can be used to convert isobutane to isobutylene, a key intermediate in fine chemical synthesis [2]. This process employs a proprietary platinum-based catalyst that achieves high selectivity and conversion rates, typically above 85% [4]. UOP's technology allows for the production of high-purity isobutylene from various feedstocks, including refinery off-gases rich in isobutane. The company has also implemented advanced heat integration and recovery systems, reducing overall energy consumption by up to 30% compared to conventional dehydrogenation processes [6]. UOP's Oleflex™ units can be scaled to produce 350,000 to 900,000 metric tons of isobutylene per year, providing flexibility for different market demands [8]. The process has been successfully commercialized in multiple plants worldwide, demonstrating its reliability and economic viability for large-scale isobutylene production.
Strengths: High selectivity and conversion rates, energy-efficient design, scalable technology. Weaknesses: Dependence on platinum-based catalysts, which can be expensive and sensitive to impurities in the feedstock.
Innovative Isobutane Conversion Technologies
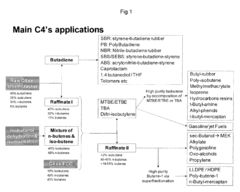
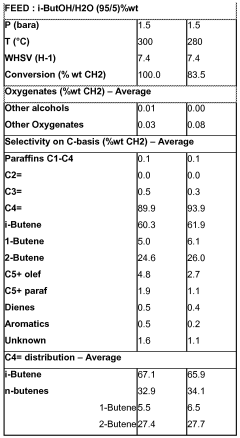
Simultaneous dehydration and skeletal isomerisation of isobutanol on acid catalysts
PatentInactiveEP2374781A1
Innovation
- Simultaneous dehydration and skeletal isomerization of isobutanol using crystalline silicates with high Si/Al ratios or modified alumina catalysts at elevated temperatures and moderate pressures, allowing for high isobutanol conversion and selectivity towards n-butenes and iso-butene, mimicking the composition of raffinate I from steam naphtha crackers.
Simultaneous dehydration and skeletal isomerisation of isobutanol on acid catalysts
PatentWO2011113834A1
Innovation
- Simultaneous dehydration and skeletal isomerization of isobutanol using crystalline silicates with high Si/Al ratios, such as FER, MWW, or phosphorus-modified silicates, at temperatures between 200°C to 600°C and WHSV of at least 1 h^-1, producing a mixture of n-butenes and iso-butene with high conversion and selectivity.
Economic Feasibility and Cost-Benefit Analysis
The economic feasibility and cost-benefit analysis of using isobutane in fine chemical synthesis reveals a complex interplay of factors that influence its adoption in industrial processes. Isobutane, as a versatile feedstock, offers several advantages in terms of cost-effectiveness and process efficiency, which contribute to its growing importance in the fine chemicals sector.
From a cost perspective, isobutane is generally more affordable compared to alternative feedstocks, particularly when derived from natural gas processing. Its abundance and relatively low production costs make it an attractive option for large-scale chemical synthesis. The use of isobutane can lead to significant reductions in raw material expenses, potentially lowering the overall production costs of fine chemicals.
In terms of process efficiency, isobutane's high reactivity and selectivity in certain chemical reactions can result in improved yields and reduced waste generation. This increased efficiency translates to lower energy consumption and decreased environmental impact, further enhancing the economic viability of isobutane-based processes.
However, the economic benefits of using isobutane must be weighed against potential challenges and additional costs. The implementation of isobutane-based processes may require substantial initial investments in specialized equipment and safety measures due to its flammability and volatility. These upfront costs can impact the short-term economic feasibility, particularly for smaller-scale operations or companies with limited capital.
The cost-benefit analysis also needs to consider the potential for value-added products derived from isobutane. Many fine chemicals produced using isobutane as a feedstock command higher market prices due to their unique properties or applications. This price premium can offset the initial investment costs and contribute to long-term profitability.
Market dynamics play a crucial role in determining the economic viability of isobutane-based fine chemical synthesis. Fluctuations in isobutane prices, which are closely tied to natural gas markets, can significantly impact profit margins. Additionally, regulatory factors, such as environmental regulations and safety standards, may influence the cost structure and feasibility of isobutane utilization in different regions.
In conclusion, the economic feasibility of using isobutane in fine chemical synthesis generally shows a positive trend, with potential for cost savings and improved process efficiency. However, a comprehensive cost-benefit analysis must account for initial investment requirements, market conditions, and regulatory landscapes to accurately assess its viability for specific applications and business contexts.
From a cost perspective, isobutane is generally more affordable compared to alternative feedstocks, particularly when derived from natural gas processing. Its abundance and relatively low production costs make it an attractive option for large-scale chemical synthesis. The use of isobutane can lead to significant reductions in raw material expenses, potentially lowering the overall production costs of fine chemicals.
In terms of process efficiency, isobutane's high reactivity and selectivity in certain chemical reactions can result in improved yields and reduced waste generation. This increased efficiency translates to lower energy consumption and decreased environmental impact, further enhancing the economic viability of isobutane-based processes.
However, the economic benefits of using isobutane must be weighed against potential challenges and additional costs. The implementation of isobutane-based processes may require substantial initial investments in specialized equipment and safety measures due to its flammability and volatility. These upfront costs can impact the short-term economic feasibility, particularly for smaller-scale operations or companies with limited capital.
The cost-benefit analysis also needs to consider the potential for value-added products derived from isobutane. Many fine chemicals produced using isobutane as a feedstock command higher market prices due to their unique properties or applications. This price premium can offset the initial investment costs and contribute to long-term profitability.
Market dynamics play a crucial role in determining the economic viability of isobutane-based fine chemical synthesis. Fluctuations in isobutane prices, which are closely tied to natural gas markets, can significantly impact profit margins. Additionally, regulatory factors, such as environmental regulations and safety standards, may influence the cost structure and feasibility of isobutane utilization in different regions.
In conclusion, the economic feasibility of using isobutane in fine chemical synthesis generally shows a positive trend, with potential for cost savings and improved process efficiency. However, a comprehensive cost-benefit analysis must account for initial investment requirements, market conditions, and regulatory landscapes to accurately assess its viability for specific applications and business contexts.
Environmental Impact and Sustainability Considerations
The use of isobutane in fine chemical synthesis presents both environmental challenges and opportunities for sustainability. As a volatile organic compound (VOC), isobutane can contribute to air pollution and ozone depletion if released into the atmosphere. However, its relatively low global warming potential compared to other hydrocarbons makes it a more environmentally friendly option in certain applications.
In terms of production, isobutane is primarily derived from fossil fuels, which raises concerns about its long-term sustainability. The extraction and processing of these resources can lead to habitat disruption, greenhouse gas emissions, and potential contamination of water sources. However, advancements in green chemistry techniques are exploring more sustainable methods of isobutane production, including bio-based sources and catalytic processes that reduce energy consumption and waste generation.
When used in fine chemical synthesis, isobutane can offer environmental benefits through improved reaction efficiency and reduced solvent use. Its high reactivity allows for lower reaction temperatures and shorter processing times, potentially reducing overall energy consumption in manufacturing processes. Additionally, isobutane's ability to act as both a reactant and a solvent in certain reactions can minimize the need for additional organic solvents, thereby reducing waste and simplifying purification steps.
However, the handling and storage of isobutane require careful consideration due to its flammability and potential for accidental release. Proper safety measures and containment systems are essential to prevent environmental contamination and ensure worker safety. The implementation of closed-loop systems and recovery technologies can significantly reduce emissions and improve the overall environmental footprint of isobutane-based processes.
From a lifecycle perspective, the use of isobutane in fine chemical synthesis may contribute to the development of more efficient and targeted chemical products. This can lead to downstream benefits such as reduced material consumption, improved product performance, and potentially lower environmental impacts during the use phase of the final products. However, a comprehensive lifecycle assessment is necessary to fully understand the net environmental impact across the entire value chain.
As industries strive for greater sustainability, there is growing interest in developing alternatives to traditional petrochemical-based feedstocks. Research into bio-based isobutane or functionally equivalent compounds derived from renewable resources is ongoing. These efforts aim to create more sustainable supply chains and reduce dependence on fossil fuels, aligning with circular economy principles and global sustainability goals.
In terms of production, isobutane is primarily derived from fossil fuels, which raises concerns about its long-term sustainability. The extraction and processing of these resources can lead to habitat disruption, greenhouse gas emissions, and potential contamination of water sources. However, advancements in green chemistry techniques are exploring more sustainable methods of isobutane production, including bio-based sources and catalytic processes that reduce energy consumption and waste generation.
When used in fine chemical synthesis, isobutane can offer environmental benefits through improved reaction efficiency and reduced solvent use. Its high reactivity allows for lower reaction temperatures and shorter processing times, potentially reducing overall energy consumption in manufacturing processes. Additionally, isobutane's ability to act as both a reactant and a solvent in certain reactions can minimize the need for additional organic solvents, thereby reducing waste and simplifying purification steps.
However, the handling and storage of isobutane require careful consideration due to its flammability and potential for accidental release. Proper safety measures and containment systems are essential to prevent environmental contamination and ensure worker safety. The implementation of closed-loop systems and recovery technologies can significantly reduce emissions and improve the overall environmental footprint of isobutane-based processes.
From a lifecycle perspective, the use of isobutane in fine chemical synthesis may contribute to the development of more efficient and targeted chemical products. This can lead to downstream benefits such as reduced material consumption, improved product performance, and potentially lower environmental impacts during the use phase of the final products. However, a comprehensive lifecycle assessment is necessary to fully understand the net environmental impact across the entire value chain.
As industries strive for greater sustainability, there is growing interest in developing alternatives to traditional petrochemical-based feedstocks. Research into bio-based isobutane or functionally equivalent compounds derived from renewable resources is ongoing. These efforts aim to create more sustainable supply chains and reduce dependence on fossil fuels, aligning with circular economy principles and global sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!