Isobutane Utilization in Synthesis of Alkylate for High-Octane Fuels
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane Alkylation Background and Objectives
Isobutane alkylation has been a cornerstone process in the production of high-octane fuels since its inception in the 1930s. This technology emerged from the growing demand for higher-performance gasoline, particularly during World War II when aviation fuel requirements became critical. The process involves the reaction of isobutane with light olefins, typically butenes, to produce a mixture of highly branched alkanes known as alkylate.
The primary objective of isobutane alkylation is to synthesize high-octane blending components for gasoline, which significantly improve the fuel's anti-knock properties. This process has become increasingly important as environmental regulations have tightened, leading to the phaseout of lead-based octane enhancers and the need for cleaner-burning, high-performance fuels.
Over the decades, the technology has evolved to address various challenges, including catalyst efficiency, process safety, and environmental concerns. Initially, sulfuric acid was the predominant catalyst, but hydrofluoric acid catalysts gained popularity due to their ability to operate at lower temperatures and pressures. More recently, solid acid catalysts have been developed to mitigate the environmental and safety risks associated with liquid acid catalysts.
The evolution of isobutane alkylation technology has been driven by several factors. These include the need for improved octane ratings to meet engine performance requirements, the push for more environmentally friendly processes, and the desire to optimize feedstock utilization in refineries. As a result, research has focused on enhancing catalyst selectivity, improving reactor designs, and developing more efficient separation techniques.
Current technological goals in isobutane alkylation include developing safer and more environmentally benign catalysts, improving process efficiency to reduce energy consumption, and enhancing the flexibility of feedstock utilization. There is also a growing interest in integrating alkylation units with other refinery processes to optimize overall plant efficiency and product quality.
Looking forward, the isobutane alkylation process faces both challenges and opportunities. The shift towards electric vehicles may impact long-term demand for high-octane fuels, but in the near to medium term, the technology remains crucial for meeting stringent fuel quality standards and supporting the transition to cleaner transportation fuels. Additionally, there is potential for applying alkylation technology in the production of specialty chemicals and petrochemicals, expanding its relevance beyond traditional fuel markets.
The primary objective of isobutane alkylation is to synthesize high-octane blending components for gasoline, which significantly improve the fuel's anti-knock properties. This process has become increasingly important as environmental regulations have tightened, leading to the phaseout of lead-based octane enhancers and the need for cleaner-burning, high-performance fuels.
Over the decades, the technology has evolved to address various challenges, including catalyst efficiency, process safety, and environmental concerns. Initially, sulfuric acid was the predominant catalyst, but hydrofluoric acid catalysts gained popularity due to their ability to operate at lower temperatures and pressures. More recently, solid acid catalysts have been developed to mitigate the environmental and safety risks associated with liquid acid catalysts.
The evolution of isobutane alkylation technology has been driven by several factors. These include the need for improved octane ratings to meet engine performance requirements, the push for more environmentally friendly processes, and the desire to optimize feedstock utilization in refineries. As a result, research has focused on enhancing catalyst selectivity, improving reactor designs, and developing more efficient separation techniques.
Current technological goals in isobutane alkylation include developing safer and more environmentally benign catalysts, improving process efficiency to reduce energy consumption, and enhancing the flexibility of feedstock utilization. There is also a growing interest in integrating alkylation units with other refinery processes to optimize overall plant efficiency and product quality.
Looking forward, the isobutane alkylation process faces both challenges and opportunities. The shift towards electric vehicles may impact long-term demand for high-octane fuels, but in the near to medium term, the technology remains crucial for meeting stringent fuel quality standards and supporting the transition to cleaner transportation fuels. Additionally, there is potential for applying alkylation technology in the production of specialty chemicals and petrochemicals, expanding its relevance beyond traditional fuel markets.
High-Octane Fuel Market Analysis
The high-octane fuel market has experienced significant growth in recent years, driven by increasing demand for improved engine performance and fuel efficiency. This trend is particularly evident in the automotive sector, where manufacturers are continuously seeking ways to enhance vehicle power output while meeting stringent emission standards. The global high-octane fuel market is characterized by a complex interplay of factors, including regulatory pressures, technological advancements, and shifting consumer preferences.
In the context of isobutane utilization for alkylate synthesis, the market demand is closely tied to the broader high-octane fuel landscape. Alkylate, a key component in high-octane gasoline blends, has gained prominence due to its superior combustion properties and low environmental impact. The market for alkylate-based high-octane fuels has shown robust growth, particularly in regions with strict fuel quality regulations.
The automotive industry remains the primary driver of high-octane fuel demand. As vehicle manufacturers continue to develop more advanced, high-compression engines, the need for fuels with higher octane ratings has intensified. This trend is especially pronounced in the premium and performance vehicle segments, where consumers are willing to pay a premium for enhanced engine performance and fuel efficiency.
Aviation represents another significant market for high-octane fuels, with a steady demand for high-performance aviation gasoline. The general aviation sector, in particular, relies heavily on high-octane fuels to power piston-engine aircraft. This niche market segment provides a stable demand base for alkylate-based fuels.
Geographically, North America and Europe lead in high-octane fuel consumption, driven by stringent environmental regulations and a well-established automotive industry. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth, fueled by increasing vehicle ownership and improving economic conditions.
The market is also influenced by fluctuating crude oil prices and the availability of alternative fuel technologies. While electric vehicles pose a long-term challenge to traditional fuel markets, the transition is gradual, ensuring a continued demand for high-octane fuels in the medium term. Additionally, the push for cleaner-burning fuels has created opportunities for alkylate-based products, which offer reduced emissions compared to conventional gasoline blends.
Looking ahead, the high-octane fuel market is expected to continue its growth trajectory, with a particular emphasis on cleaner, more efficient fuel formulations. The role of isobutane-derived alkylate in this evolving landscape remains crucial, as it offers a pathway to meeting both performance and environmental objectives in the fuel industry.
In the context of isobutane utilization for alkylate synthesis, the market demand is closely tied to the broader high-octane fuel landscape. Alkylate, a key component in high-octane gasoline blends, has gained prominence due to its superior combustion properties and low environmental impact. The market for alkylate-based high-octane fuels has shown robust growth, particularly in regions with strict fuel quality regulations.
The automotive industry remains the primary driver of high-octane fuel demand. As vehicle manufacturers continue to develop more advanced, high-compression engines, the need for fuels with higher octane ratings has intensified. This trend is especially pronounced in the premium and performance vehicle segments, where consumers are willing to pay a premium for enhanced engine performance and fuel efficiency.
Aviation represents another significant market for high-octane fuels, with a steady demand for high-performance aviation gasoline. The general aviation sector, in particular, relies heavily on high-octane fuels to power piston-engine aircraft. This niche market segment provides a stable demand base for alkylate-based fuels.
Geographically, North America and Europe lead in high-octane fuel consumption, driven by stringent environmental regulations and a well-established automotive industry. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth, fueled by increasing vehicle ownership and improving economic conditions.
The market is also influenced by fluctuating crude oil prices and the availability of alternative fuel technologies. While electric vehicles pose a long-term challenge to traditional fuel markets, the transition is gradual, ensuring a continued demand for high-octane fuels in the medium term. Additionally, the push for cleaner-burning fuels has created opportunities for alkylate-based products, which offer reduced emissions compared to conventional gasoline blends.
Looking ahead, the high-octane fuel market is expected to continue its growth trajectory, with a particular emphasis on cleaner, more efficient fuel formulations. The role of isobutane-derived alkylate in this evolving landscape remains crucial, as it offers a pathway to meeting both performance and environmental objectives in the fuel industry.
Alkylation Technology Status and Challenges
Alkylation technology for the synthesis of high-octane fuels has been a cornerstone in the petroleum refining industry for decades. However, the current status of this technology faces several significant challenges that require innovative solutions. The primary process, which involves the reaction of isobutane with light olefins to produce alkylate, is confronted with issues related to catalyst efficiency, environmental concerns, and economic viability.
One of the major challenges in alkylation technology is the continued use of liquid acid catalysts, particularly hydrofluoric acid (HF) and sulfuric acid (H2SO4). These catalysts, while effective, pose substantial safety and environmental risks. The potential for accidental releases and the corrosive nature of these acids have led to increased scrutiny and regulatory pressure. Consequently, there is a pressing need for the development of safer, more environmentally friendly catalytic systems.
Solid acid catalysts have emerged as a potential alternative, offering improved safety profiles and reduced environmental impact. However, their widespread adoption has been hindered by issues such as rapid deactivation and lower alkylate quality compared to liquid acid processes. The challenge lies in developing solid catalysts that can maintain high activity and selectivity over extended periods while producing alkylate of comparable quality to traditional methods.
Another significant hurdle is the optimization of isobutane utilization. Current processes often require a high isobutane to olefin ratio to achieve desired product quality, leading to increased operational costs and energy consumption. Improving isobutane efficiency without compromising alkylate quality remains a key area of focus for researchers and industry professionals.
The alkylation industry is also grappling with the need for process intensification to reduce capital and operational expenditures. Traditional alkylation units are complex and require substantial investment. Developing more compact, efficient reactor designs that can achieve the same or better performance is crucial for the economic viability of alkylation technology in an increasingly competitive refining landscape.
Furthermore, the variability in feedstock quality presents ongoing challenges. As refineries process crudes from diverse sources, including unconventional oils, the composition of the light olefin streams can vary significantly. This variability impacts alkylation process stability and product quality, necessitating more robust and flexible alkylation technologies capable of handling a wider range of feedstocks.
Lastly, the push towards cleaner fuels and stricter environmental regulations has placed additional pressure on alkylation technology. There is a growing demand for processes that can produce ultra-low sulfur alkylate while minimizing overall emissions and waste generation. This challenge intersects with the broader industry trend towards sustainability and carbon footprint reduction.
One of the major challenges in alkylation technology is the continued use of liquid acid catalysts, particularly hydrofluoric acid (HF) and sulfuric acid (H2SO4). These catalysts, while effective, pose substantial safety and environmental risks. The potential for accidental releases and the corrosive nature of these acids have led to increased scrutiny and regulatory pressure. Consequently, there is a pressing need for the development of safer, more environmentally friendly catalytic systems.
Solid acid catalysts have emerged as a potential alternative, offering improved safety profiles and reduced environmental impact. However, their widespread adoption has been hindered by issues such as rapid deactivation and lower alkylate quality compared to liquid acid processes. The challenge lies in developing solid catalysts that can maintain high activity and selectivity over extended periods while producing alkylate of comparable quality to traditional methods.
Another significant hurdle is the optimization of isobutane utilization. Current processes often require a high isobutane to olefin ratio to achieve desired product quality, leading to increased operational costs and energy consumption. Improving isobutane efficiency without compromising alkylate quality remains a key area of focus for researchers and industry professionals.
The alkylation industry is also grappling with the need for process intensification to reduce capital and operational expenditures. Traditional alkylation units are complex and require substantial investment. Developing more compact, efficient reactor designs that can achieve the same or better performance is crucial for the economic viability of alkylation technology in an increasingly competitive refining landscape.
Furthermore, the variability in feedstock quality presents ongoing challenges. As refineries process crudes from diverse sources, including unconventional oils, the composition of the light olefin streams can vary significantly. This variability impacts alkylation process stability and product quality, necessitating more robust and flexible alkylation technologies capable of handling a wider range of feedstocks.
Lastly, the push towards cleaner fuels and stricter environmental regulations has placed additional pressure on alkylation technology. There is a growing demand for processes that can produce ultra-low sulfur alkylate while minimizing overall emissions and waste generation. This challenge intersects with the broader industry trend towards sustainability and carbon footprint reduction.
Current Isobutane Alkylation Methods
01 Octane number measurement and improvement for isobutane
Methods and apparatus for measuring and improving the octane number of isobutane are discussed. This includes techniques for accurately determining the octane rating of isobutane-containing fuel mixtures and processes for enhancing the octane number through various refining and blending techniques.- Octane number determination for isobutane: Methods and apparatus for determining the octane number of isobutane, which is an important parameter in fuel quality assessment. This involves specialized testing equipment and procedures to measure the anti-knock properties of isobutane in comparison to reference fuels.
- Isobutane as a component in high-octane fuel blends: Utilization of isobutane as a key component in formulating high-octane fuel blends. Isobutane's branched structure contributes to improved octane ratings in gasoline and other fuel mixtures, enhancing engine performance and efficiency.
- Isobutane production processes for octane enhancement: Various processes for producing high-purity isobutane, focusing on methods that yield a product with optimal octane characteristics. These processes may involve isomerization, separation, or other refining techniques to maximize the octane-boosting potential of isobutane.
- Isobutane in fuel systems and engine performance: The role of isobutane in fuel systems and its impact on engine performance, particularly in relation to its octane properties. This includes studies on combustion characteristics, emissions, and overall engine efficiency when using isobutane-rich fuels.
- Analytical methods for isobutane octane number: Development and application of analytical techniques for accurately measuring and predicting the octane number of isobutane. This may involve spectroscopic methods, chromatography, or other advanced analytical approaches to assess fuel quality and performance.
02 Isobutane in fuel compositions
The use of isobutane in various fuel compositions is explored, particularly its role in enhancing octane ratings. This includes the formulation of high-octane fuel blends incorporating isobutane and the impact on engine performance and emissions.Expand Specific Solutions03 Isobutane production and purification
Processes for producing and purifying isobutane are discussed, with a focus on methods that yield high-octane isobutane. This includes catalytic conversion processes, isomerization techniques, and separation methods to obtain high-purity isobutane suitable for use in high-octane fuel applications.Expand Specific Solutions04 Isobutane in alternative fuel systems
The application of isobutane in alternative fuel systems is examined, including its use in LPG (Liquefied Petroleum Gas) blends and other non-traditional fuel compositions. The impact on octane ratings and overall fuel performance in these systems is discussed.Expand Specific Solutions05 Octane boosting additives for isobutane-containing fuels
Various additives and compounds that can be used to boost the octane number of isobutane-containing fuels are explored. This includes both chemical and organic additives, as well as their synergistic effects when combined with isobutane in fuel formulations.Expand Specific Solutions
Key Players in Alkylation Industry
The isobutane utilization for high-octane alkylate synthesis is in a mature stage of development, with a substantial global market size driven by increasing demand for cleaner-burning fuels. Major players like ExxonMobil, Shell, and Sinopec have well-established technologies, while UOP and DuPont offer licensed processes. The technology is commercially proven, with ongoing incremental improvements in catalysts and process efficiency. Research institutes from China Petroleum University and Sinopec are actively involved in advancing the technology further, indicating continued innovation potential in this field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced alkylation technology called S-Alkylation for high-octane fuel production. This process utilizes isobutane and light olefins to produce high-quality alkylate. The S-Alkylation technology employs a proprietary solid acid catalyst, which operates at lower temperatures and pressures compared to traditional sulfuric acid alkylation [1]. The process achieves high isobutane utilization efficiency, with conversion rates exceeding 99% [2]. Sinopec's technology also incorporates a unique reactor design that enhances mass transfer and reaction kinetics, resulting in improved alkylate yield and quality. The company has successfully implemented this technology in multiple refineries across China, demonstrating its commercial viability and scalability [3].
Strengths: Higher safety due to solid catalyst, reduced environmental impact, and improved product quality. Weaknesses: Potential higher initial capital costs and the need for specialized catalyst handling and regeneration.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed the AlkyClean® solid acid alkylation technology for high-octane fuel production. This process efficiently utilizes isobutane and light olefins to produce premium alkylate. The AlkyClean® technology employs a proprietary fixed-bed reactor system with a solid acid catalyst, eliminating the need for liquid acids [4]. The process operates at moderate temperatures and pressures, achieving high isobutane conversion rates of up to 99.5% [5]. ExxonMobil's technology features a unique regeneration system that maintains catalyst activity and extends catalyst life. The company has demonstrated the technology's effectiveness through successful commercial-scale operations, with multiple units in operation globally [6]. The AlkyClean® process also incorporates advanced control systems for optimized performance and product quality consistency.
Strengths: Improved safety profile, reduced environmental impact, and lower operating costs. Weaknesses: Potential higher initial investment and the need for specialized operator training.
Innovative Alkylation Catalyst Technologies
Isobutane alkylation
PatentInactiveUS7550644B2
Innovation
- The process operates a fixed-bed alkylation reactor in a mass-transfer controlled regime with olefin as the limiting reactant, controlling olefin diffusion to the catalyst surface to minimize polymerization by using laminar flow and introducing olefin downstream of channel entrances, and injecting it along the flow channel centerlines to maintain low surface concentrations, thus allowing lower isobutane-to-olefin ratios and reducing catalyst deactivation.
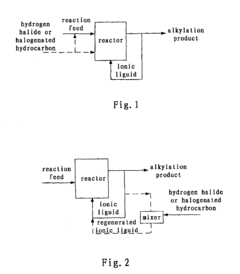
Methods for regenerating and maintaining activity of ionic liquid catalyst and producing alkylate
PatentInactiveUS20120283500A1
Innovation
- Supplying hydrogen halide or halogenated hydrocarbon to the acidic ionic liquid catalyst during the alkylation reaction to regenerate the Brönsted acid and maintain catalytic activity, allowing for a controlled amount within a 0.01-1 wt% range in the alkylate product to extend the catalyst's useful life.
Environmental Impact of Alkylation Processes
The alkylation process for producing high-octane fuels, particularly using isobutane, has significant environmental implications that warrant careful consideration. This process, while crucial for producing cleaner-burning fuels, also poses potential environmental risks that need to be addressed.
One of the primary environmental concerns associated with alkylation is the use and handling of hydrofluoric acid (HF) or sulfuric acid as catalysts. These strong acids can pose serious environmental hazards if not properly managed. Accidental releases of HF, in particular, can lead to severe air and water pollution, potentially causing harm to both human health and ecosystems in the vicinity of production facilities.
Water consumption and wastewater generation are also notable environmental aspects of the alkylation process. The process requires substantial amounts of water for cooling and washing, which can strain local water resources. Additionally, the wastewater produced often contains trace amounts of hydrocarbons and acids, necessitating thorough treatment before discharge to prevent water pollution.
Air emissions from alkylation units are another environmental concern. Volatile organic compounds (VOCs) and other hazardous air pollutants can be released during the process, contributing to air quality issues and potentially impacting local communities. Proper emission control technologies and monitoring systems are essential to mitigate these impacts.
Energy consumption in the alkylation process is significant, contributing to indirect environmental impacts through greenhouse gas emissions. The process requires substantial heating and cooling, often relying on fossil fuel-based energy sources. Improving energy efficiency in alkylation units is crucial for reducing the overall carbon footprint of fuel production.
Solid waste generation, although not as prominent as other environmental aspects, is still a consideration. Spent catalysts and other process residues require proper disposal or recycling to prevent soil and groundwater contamination.
On the positive side, the production of high-octane alkylate contributes to the manufacture of cleaner-burning fuels. These fuels can lead to reduced emissions from vehicles, potentially offsetting some of the environmental impacts of the production process itself. The higher efficiency of engines using high-octane fuels can also contribute to overall reductions in fuel consumption and associated emissions.
Efforts to improve the environmental performance of alkylation processes are ongoing. These include the development of solid acid catalysts to replace liquid acids, which could significantly reduce the environmental risks associated with acid handling. Additionally, process intensification techniques and advanced control systems are being explored to optimize resource use and minimize waste generation.
In conclusion, while the alkylation process for high-octane fuel production presents several environmental challenges, ongoing technological advancements and stringent regulatory frameworks are driving improvements in its environmental performance. Balancing the need for high-quality fuels with environmental protection remains a key focus for the industry.
One of the primary environmental concerns associated with alkylation is the use and handling of hydrofluoric acid (HF) or sulfuric acid as catalysts. These strong acids can pose serious environmental hazards if not properly managed. Accidental releases of HF, in particular, can lead to severe air and water pollution, potentially causing harm to both human health and ecosystems in the vicinity of production facilities.
Water consumption and wastewater generation are also notable environmental aspects of the alkylation process. The process requires substantial amounts of water for cooling and washing, which can strain local water resources. Additionally, the wastewater produced often contains trace amounts of hydrocarbons and acids, necessitating thorough treatment before discharge to prevent water pollution.
Air emissions from alkylation units are another environmental concern. Volatile organic compounds (VOCs) and other hazardous air pollutants can be released during the process, contributing to air quality issues and potentially impacting local communities. Proper emission control technologies and monitoring systems are essential to mitigate these impacts.
Energy consumption in the alkylation process is significant, contributing to indirect environmental impacts through greenhouse gas emissions. The process requires substantial heating and cooling, often relying on fossil fuel-based energy sources. Improving energy efficiency in alkylation units is crucial for reducing the overall carbon footprint of fuel production.
Solid waste generation, although not as prominent as other environmental aspects, is still a consideration. Spent catalysts and other process residues require proper disposal or recycling to prevent soil and groundwater contamination.
On the positive side, the production of high-octane alkylate contributes to the manufacture of cleaner-burning fuels. These fuels can lead to reduced emissions from vehicles, potentially offsetting some of the environmental impacts of the production process itself. The higher efficiency of engines using high-octane fuels can also contribute to overall reductions in fuel consumption and associated emissions.
Efforts to improve the environmental performance of alkylation processes are ongoing. These include the development of solid acid catalysts to replace liquid acids, which could significantly reduce the environmental risks associated with acid handling. Additionally, process intensification techniques and advanced control systems are being explored to optimize resource use and minimize waste generation.
In conclusion, while the alkylation process for high-octane fuel production presents several environmental challenges, ongoing technological advancements and stringent regulatory frameworks are driving improvements in its environmental performance. Balancing the need for high-quality fuels with environmental protection remains a key focus for the industry.
Regulatory Framework for Fuel Production
The regulatory framework for fuel production plays a crucial role in shaping the development and implementation of high-octane fuel technologies, including those involving isobutane utilization in alkylate synthesis. This framework encompasses a complex web of regulations, standards, and policies at various levels of governance.
At the international level, organizations such as the United Nations Framework Convention on Climate Change (UNFCCC) and the International Civil Aviation Organization (ICAO) set overarching goals for reducing greenhouse gas emissions from transportation fuels. These global initiatives influence national and regional policies, driving the demand for cleaner, more efficient fuels.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing fuel production and quality. The EPA's Renewable Fuel Standard (RFS) program mandates the blending of renewable fuels into transportation fuels, which indirectly affects the demand for high-octane alkylates. Additionally, the Corporate Average Fuel Economy (CAFE) standards incentivize automakers to produce more fuel-efficient vehicles, potentially increasing the market for high-octane fuels.
The European Union has implemented stringent regulations through its Renewable Energy Directive (RED) and Fuel Quality Directive (FQD). These directives set targets for renewable energy use in transportation and mandate reductions in the carbon intensity of fuels. Such regulations create a favorable environment for the development of advanced fuel technologies, including those utilizing isobutane for high-octane alkylate production.
Many countries have adopted their own fuel quality standards, which specify parameters such as octane ratings, sulfur content, and aromatic compound levels. These standards directly impact the production processes and formulations of high-octane fuels, necessitating continuous innovation in alkylation technologies.
Safety regulations also play a significant role in the regulatory framework. Agencies like the Occupational Safety and Health Administration (OSHA) in the U.S. and the European Agency for Safety and Health at Work (EU-OSHA) set guidelines for the safe handling and processing of volatile hydrocarbons like isobutane in industrial settings.
Environmental regulations, particularly those addressing air quality and emissions, have a profound impact on fuel production. These include limits on volatile organic compound (VOC) emissions during fuel production and distribution, as well as regulations on the final fuel composition to minimize harmful exhaust emissions.
As the global focus on sustainability intensifies, many jurisdictions are implementing or considering carbon pricing mechanisms, such as cap-and-trade systems or carbon taxes. These economic instruments can significantly influence the competitiveness of different fuel production technologies, potentially favoring those with lower carbon footprints.
At the international level, organizations such as the United Nations Framework Convention on Climate Change (UNFCCC) and the International Civil Aviation Organization (ICAO) set overarching goals for reducing greenhouse gas emissions from transportation fuels. These global initiatives influence national and regional policies, driving the demand for cleaner, more efficient fuels.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing fuel production and quality. The EPA's Renewable Fuel Standard (RFS) program mandates the blending of renewable fuels into transportation fuels, which indirectly affects the demand for high-octane alkylates. Additionally, the Corporate Average Fuel Economy (CAFE) standards incentivize automakers to produce more fuel-efficient vehicles, potentially increasing the market for high-octane fuels.
The European Union has implemented stringent regulations through its Renewable Energy Directive (RED) and Fuel Quality Directive (FQD). These directives set targets for renewable energy use in transportation and mandate reductions in the carbon intensity of fuels. Such regulations create a favorable environment for the development of advanced fuel technologies, including those utilizing isobutane for high-octane alkylate production.
Many countries have adopted their own fuel quality standards, which specify parameters such as octane ratings, sulfur content, and aromatic compound levels. These standards directly impact the production processes and formulations of high-octane fuels, necessitating continuous innovation in alkylation technologies.
Safety regulations also play a significant role in the regulatory framework. Agencies like the Occupational Safety and Health Administration (OSHA) in the U.S. and the European Agency for Safety and Health at Work (EU-OSHA) set guidelines for the safe handling and processing of volatile hydrocarbons like isobutane in industrial settings.
Environmental regulations, particularly those addressing air quality and emissions, have a profound impact on fuel production. These include limits on volatile organic compound (VOC) emissions during fuel production and distribution, as well as regulations on the final fuel composition to minimize harmful exhaust emissions.
As the global focus on sustainability intensifies, many jurisdictions are implementing or considering carbon pricing mechanisms, such as cap-and-trade systems or carbon taxes. These economic instruments can significantly influence the competitiveness of different fuel production technologies, potentially favoring those with lower carbon footprints.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!