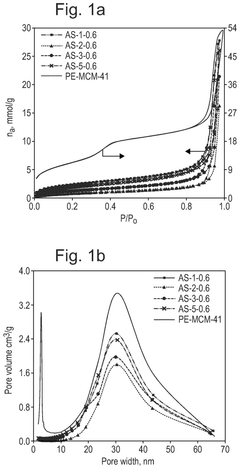
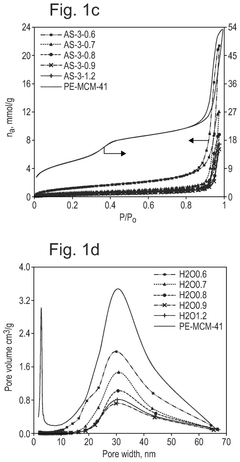
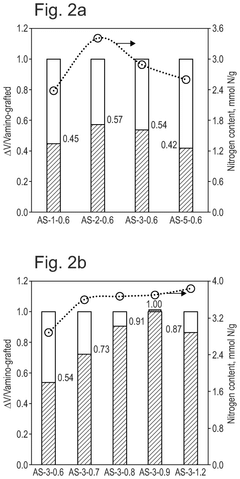
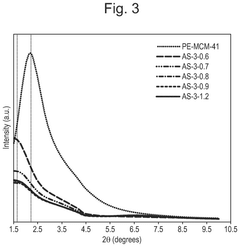
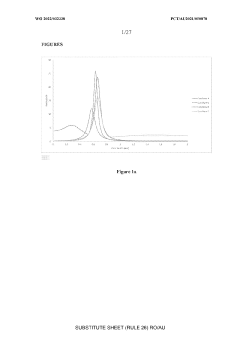
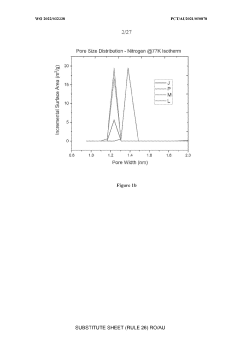
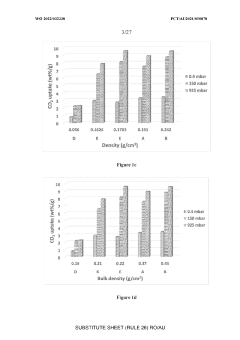
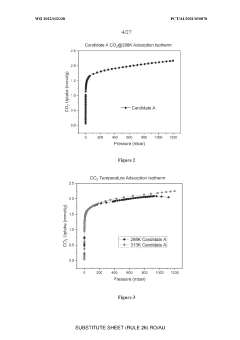
What material innovations improve Solid sorbents for CO2 capture adsorption and regeneration
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. The journey began with conventional absorption methods using liquid amines in the 1930s, which while effective, presented challenges including high energy consumption during regeneration and equipment corrosion. The 1990s marked a pivotal shift toward solid sorbents as researchers recognized their potential advantages in energy efficiency and operational stability.
Solid sorbents for CO2 capture have progressed through several generations. First-generation materials included activated carbons and zeolites, which offered moderate CO2 selectivity but suffered from moisture sensitivity and limited capacity. The early 2000s witnessed the emergence of second-generation materials such as metal-organic frameworks (MOFs) and amine-functionalized silicas, which demonstrated significantly improved adsorption capacities and selectivity.
The current technological landscape is dominated by third-generation hybrid materials that combine the advantages of different sorbent classes. These include hierarchical porous structures, composite materials integrating organic and inorganic components, and stimuli-responsive sorbents that can be triggered by external factors such as temperature, pressure, or light to release captured CO2.
Recent advancements have focused on enhancing the fundamental properties that determine sorbent performance: adsorption capacity, selectivity, kinetics, stability, and regeneration energy. Particular attention has been directed toward developing materials with rapid adsorption-desorption cycles and minimal capacity degradation over thousands of cycles – critical factors for industrial implementation.
The primary objective of current research is to develop solid sorbents that can achieve CO2 capture costs below $30 per ton while maintaining high performance under real-world conditions. This includes tolerance to flue gas contaminants such as SOx, NOx, and water vapor, as well as mechanical stability under pressure and temperature fluctuations typical in industrial settings.
Looking forward, the field aims to design "programmable" sorbents with tunable properties that can be optimized for specific capture scenarios, from power plants to direct air capture. Researchers are increasingly employing computational methods, including machine learning and high-throughput screening, to accelerate material discovery and optimization. The ultimate goal remains developing materials that enable economically viable carbon capture at scale, supporting global decarbonization efforts across various industries.
The convergence of materials science, chemistry, and engineering disciplines has created unprecedented opportunities for breakthrough innovations in this field, with particular promise shown by bio-inspired materials and nanoscale engineering approaches that mimic natural carbon fixation processes.
Solid sorbents for CO2 capture have progressed through several generations. First-generation materials included activated carbons and zeolites, which offered moderate CO2 selectivity but suffered from moisture sensitivity and limited capacity. The early 2000s witnessed the emergence of second-generation materials such as metal-organic frameworks (MOFs) and amine-functionalized silicas, which demonstrated significantly improved adsorption capacities and selectivity.
The current technological landscape is dominated by third-generation hybrid materials that combine the advantages of different sorbent classes. These include hierarchical porous structures, composite materials integrating organic and inorganic components, and stimuli-responsive sorbents that can be triggered by external factors such as temperature, pressure, or light to release captured CO2.
Recent advancements have focused on enhancing the fundamental properties that determine sorbent performance: adsorption capacity, selectivity, kinetics, stability, and regeneration energy. Particular attention has been directed toward developing materials with rapid adsorption-desorption cycles and minimal capacity degradation over thousands of cycles – critical factors for industrial implementation.
The primary objective of current research is to develop solid sorbents that can achieve CO2 capture costs below $30 per ton while maintaining high performance under real-world conditions. This includes tolerance to flue gas contaminants such as SOx, NOx, and water vapor, as well as mechanical stability under pressure and temperature fluctuations typical in industrial settings.
Looking forward, the field aims to design "programmable" sorbents with tunable properties that can be optimized for specific capture scenarios, from power plants to direct air capture. Researchers are increasingly employing computational methods, including machine learning and high-throughput screening, to accelerate material discovery and optimization. The ultimate goal remains developing materials that enable economically viable carbon capture at scale, supporting global decarbonization efforts across various industries.
The convergence of materials science, chemistry, and engineering disciplines has created unprecedented opportunities for breakthrough innovations in this field, with particular promise shown by bio-inspired materials and nanoscale engineering approaches that mimic natural carbon fixation processes.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating a compound annual growth rate of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade.
Solid sorbent technologies for CO2 capture represent a rapidly expanding segment within this market, currently accounting for about 15% of carbon capture technologies but expected to grow substantially due to their operational advantages over traditional liquid solvent systems. The demand for advanced solid sorbents is particularly strong in power generation, cement production, and industrial manufacturing sectors, which collectively contribute over 60% of global CO2 emissions.
Regionally, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. China and India are emerging as critical growth markets due to their heavy reliance on coal-fired power plants and increasing regulatory pressure to reduce emissions. The Middle East, despite being a smaller market currently, shows promising growth potential as oil-producing nations diversify their economies and invest in cleaner technologies.
Investment patterns reveal increasing venture capital interest in solid sorbent innovations, with funding for startups in this space growing by 145% between 2020 and 2023. Corporate R&D spending by established industrial gas companies and chemical manufacturers has similarly increased, with major players allocating 12-18% of their research budgets to advanced sorbent development.
Customer segments for solid sorbent technologies include utility companies (35% of market demand), industrial manufacturers (30%), oil and gas operators (20%), and emerging direct air capture ventures (15%). The utility sector shows the highest growth potential as coal and natural gas plants face stricter emission regulations globally.
Price sensitivity varies significantly by application, with industrial users demonstrating willingness to pay premium prices for sorbents that offer lower regeneration energy requirements and longer operational lifespans. Current market pricing for advanced solid sorbents ranges from $2,000 to $8,000 per ton, depending on performance characteristics and application specifics.
Market barriers include high initial capital costs, technical uncertainties regarding long-term performance, and competition from alternative carbon reduction strategies. However, policy incentives such as carbon pricing mechanisms, tax credits for carbon capture (like the 45Q tax credit in the US), and emerging carbon border adjustment mechanisms are creating favorable market conditions for accelerated adoption of advanced solid sorbent technologies.
Solid sorbent technologies for CO2 capture represent a rapidly expanding segment within this market, currently accounting for about 15% of carbon capture technologies but expected to grow substantially due to their operational advantages over traditional liquid solvent systems. The demand for advanced solid sorbents is particularly strong in power generation, cement production, and industrial manufacturing sectors, which collectively contribute over 60% of global CO2 emissions.
Regionally, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. China and India are emerging as critical growth markets due to their heavy reliance on coal-fired power plants and increasing regulatory pressure to reduce emissions. The Middle East, despite being a smaller market currently, shows promising growth potential as oil-producing nations diversify their economies and invest in cleaner technologies.
Investment patterns reveal increasing venture capital interest in solid sorbent innovations, with funding for startups in this space growing by 145% between 2020 and 2023. Corporate R&D spending by established industrial gas companies and chemical manufacturers has similarly increased, with major players allocating 12-18% of their research budgets to advanced sorbent development.
Customer segments for solid sorbent technologies include utility companies (35% of market demand), industrial manufacturers (30%), oil and gas operators (20%), and emerging direct air capture ventures (15%). The utility sector shows the highest growth potential as coal and natural gas plants face stricter emission regulations globally.
Price sensitivity varies significantly by application, with industrial users demonstrating willingness to pay premium prices for sorbents that offer lower regeneration energy requirements and longer operational lifespans. Current market pricing for advanced solid sorbents ranges from $2,000 to $8,000 per ton, depending on performance characteristics and application specifics.
Market barriers include high initial capital costs, technical uncertainties regarding long-term performance, and competition from alternative carbon reduction strategies. However, policy incentives such as carbon pricing mechanisms, tax credits for carbon capture (like the 45Q tax credit in the US), and emerging carbon border adjustment mechanisms are creating favorable market conditions for accelerated adoption of advanced solid sorbent technologies.
Current Solid Sorbent Technologies and Limitations
Solid sorbent technologies for CO2 capture have emerged as promising alternatives to conventional liquid amine scrubbing processes due to their potential for lower energy requirements and reduced environmental impact. Currently, several classes of solid sorbents dominate the research landscape, each with distinct advantages and limitations that affect their commercial viability.
Activated carbons represent one of the most established solid sorbent technologies, offering high surface areas and relatively low production costs. However, they typically exhibit low CO2 selectivity and modest adsorption capacities under practical operating conditions, particularly at atmospheric pressure. Their performance deteriorates significantly in humid conditions, limiting their application in real-world flue gas environments.
Metal-organic frameworks (MOFs) have garnered substantial attention due to their exceptional porosity and tunable chemical properties. While certain MOFs demonstrate impressive CO2 uptake capacities exceeding 300 mg/g, they face critical challenges including hydrothermal instability, high synthesis costs, and mechanical fragility during cycling operations. These limitations have hindered their transition from laboratory success to industrial implementation.
Zeolites, particularly those containing alkali and alkaline earth metals, show good CO2 adsorption properties and benefit from established manufacturing processes. Nevertheless, their performance is severely compromised by moisture, requiring extensive pre-drying of flue gas streams. Additionally, their regeneration typically demands high temperatures, resulting in substantial energy penalties.
Amine-functionalized silica materials combine the physical adsorption benefits of porous supports with the chemical selectivity of amine groups. While these materials demonstrate excellent CO2 selectivity even at low partial pressures, they suffer from amine leaching during cycling, thermal degradation at higher temperatures, and oxidative degradation in the presence of oxygen. Their production costs also remain prohibitively high for large-scale deployment.
Hydrotalcite-based sorbents offer good stability at elevated temperatures, making them suitable for pre-combustion capture scenarios. However, they exhibit relatively low CO2 capacities at temperatures below 300°C and require high regeneration temperatures, limiting their energy efficiency.
Across all solid sorbent technologies, common limitations include insufficient working capacities under practical conditions, inadequate stability during multiple adsorption-desorption cycles, and challenges in heat management during the exothermic adsorption process. Material synthesis scalability and manufacturing costs represent additional barriers to commercialization, as most advanced materials remain confined to laboratory-scale production.
The gap between theoretical performance and practical application persists as a significant challenge, with most materials showing dramatically reduced performance when exposed to real flue gas conditions containing moisture, SOx, NOx, and other contaminants. Bridging this gap requires innovative approaches to material design that address multiple performance criteria simultaneously.
Activated carbons represent one of the most established solid sorbent technologies, offering high surface areas and relatively low production costs. However, they typically exhibit low CO2 selectivity and modest adsorption capacities under practical operating conditions, particularly at atmospheric pressure. Their performance deteriorates significantly in humid conditions, limiting their application in real-world flue gas environments.
Metal-organic frameworks (MOFs) have garnered substantial attention due to their exceptional porosity and tunable chemical properties. While certain MOFs demonstrate impressive CO2 uptake capacities exceeding 300 mg/g, they face critical challenges including hydrothermal instability, high synthesis costs, and mechanical fragility during cycling operations. These limitations have hindered their transition from laboratory success to industrial implementation.
Zeolites, particularly those containing alkali and alkaline earth metals, show good CO2 adsorption properties and benefit from established manufacturing processes. Nevertheless, their performance is severely compromised by moisture, requiring extensive pre-drying of flue gas streams. Additionally, their regeneration typically demands high temperatures, resulting in substantial energy penalties.
Amine-functionalized silica materials combine the physical adsorption benefits of porous supports with the chemical selectivity of amine groups. While these materials demonstrate excellent CO2 selectivity even at low partial pressures, they suffer from amine leaching during cycling, thermal degradation at higher temperatures, and oxidative degradation in the presence of oxygen. Their production costs also remain prohibitively high for large-scale deployment.
Hydrotalcite-based sorbents offer good stability at elevated temperatures, making them suitable for pre-combustion capture scenarios. However, they exhibit relatively low CO2 capacities at temperatures below 300°C and require high regeneration temperatures, limiting their energy efficiency.
Across all solid sorbent technologies, common limitations include insufficient working capacities under practical conditions, inadequate stability during multiple adsorption-desorption cycles, and challenges in heat management during the exothermic adsorption process. Material synthesis scalability and manufacturing costs represent additional barriers to commercialization, as most advanced materials remain confined to laboratory-scale production.
The gap between theoretical performance and practical application persists as a significant challenge, with most materials showing dramatically reduced performance when exposed to real flue gas conditions containing moisture, SOx, NOx, and other contaminants. Bridging this gap requires innovative approaches to material design that address multiple performance criteria simultaneously.
State-of-the-Art Solid Sorbent Solutions
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They have emerged as promising solid sorbents for CO2 capture due to their high surface area, tunable pore size, and adjustable chemical functionality. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and adsorption capacity. Their regeneration can be achieved through temperature or pressure swing processes, with relatively low energy requirements compared to traditional sorbents.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands, forming one-, two-, or three-dimensional structures. These materials have high surface areas, tunable pore sizes, and chemical functionality that make them effective for selective CO2 adsorption. MOFs can be designed with specific metal centers and organic linkers to enhance CO2 binding affinity and selectivity. Their regeneration typically involves temperature or pressure swing processes, with some advanced MOFs showing excellent stability over multiple adsorption-desorption cycles.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of CO2 sorbents that operate through chemical adsorption mechanisms. These materials typically consist of amines grafted onto high-surface-area supports such as silica, activated carbon, or polymeric substrates. The amine groups form carbamates or carbonates with CO2, enabling high selectivity even at low CO2 concentrations. Regeneration of these sorbents generally requires moderate heating (70-120°C) to reverse the chemical bonds. Multi-layered amine structures and optimized amine densities can significantly improve both capture capacity and regeneration efficiency.
- Zeolites and molecular sieves for CO2 adsorption: Zeolites and molecular sieves are aluminosilicate materials with well-defined microporous structures that enable molecular sieving and selective adsorption of CO2. These materials function primarily through physical adsorption mechanisms based on pore size exclusion and electrostatic interactions. The adsorption capacity depends on the Si/Al ratio, cation type, and framework structure. Regeneration can be achieved through pressure swing adsorption (PSA), temperature swing adsorption (TSA), or vacuum swing adsorption (VSA) processes. Modified zeolites with tailored pore sizes and surface chemistry show enhanced CO2 selectivity over nitrogen and other gases in flue gas streams.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbons, carbon molecular sieves, graphene-based materials, and carbon nanotubes, offer versatile platforms for CO2 capture. These materials can be produced from various precursors including biomass, coal, and polymers. Their effectiveness stems from high surface areas, tunable pore structures, and surface chemistry that can be modified through activation processes or chemical treatments. Nitrogen-doped carbon materials show particularly enhanced CO2 adsorption properties. Regeneration typically requires less energy compared to amine-based systems, with most carbon sorbents regenerable through moderate temperature or pressure changes while maintaining structural integrity over multiple cycles.
- Hybrid and composite sorbents for enhanced CO2 capture: Hybrid and composite sorbents combine multiple materials to leverage complementary properties for improved CO2 capture performance. These may include MOF-polymer composites, amine-grafted MOFs, zeolite-carbon composites, or layered double hydroxides with functional additives. The synergistic effects often result in enhanced adsorption capacity, improved selectivity, better mechanical stability, and more efficient regeneration. These materials can be designed for specific operating conditions, such as high humidity environments or varying CO2 concentrations. Advanced regeneration strategies for these composites include microwave-assisted desorption, electrical swing adsorption, and combined pressure-temperature swing processes that minimize energy requirements while maximizing working capacity.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine a porous support structure with amine groups that chemically bind to CO2 through carbamate formation. Common supports include silica, activated carbon, and polymeric materials. The amine functionality provides high selectivity for CO2 over other gases and good adsorption capacity even at low CO2 partial pressures. Regeneration typically requires moderate heating to break the chemical bonds between the amine groups and captured CO2.Expand Specific Solutions03 Zeolites and molecular sieves for selective CO2 adsorption
Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving effects for selective CO2 capture. These materials can separate CO2 from gas mixtures based on differences in molecular size, shape, and polarity. The adsorption mechanism primarily involves physisorption, with CO2 molecules interacting with cations and framework oxygen atoms within the zeolite structure. Regeneration can be accomplished through temperature or pressure swing processes, with the advantage of high thermal stability allowing for multiple adsorption-desorption cycles.Expand Specific Solutions04 Carbon-based adsorbents for CO2 capture
Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, serve as effective CO2 adsorbents. These materials offer advantages such as high surface area, hydrophobicity, and chemical stability. Their surface properties can be modified through chemical treatments to enhance CO2 selectivity and capacity. The primarily physical adsorption mechanism allows for relatively easy regeneration through pressure or temperature changes. Carbon-based sorbents are particularly attractive due to their low cost, availability, and environmental compatibility.Expand Specific Solutions05 Novel regeneration techniques for CO2 sorbents
Advanced regeneration methods are being developed to improve the energy efficiency and longevity of CO2 capture systems. These include vacuum swing adsorption, electrical swing adsorption, microwave-assisted regeneration, and hybrid approaches combining multiple desorption mechanisms. Some techniques utilize steam or inert gas stripping to enhance CO2 release from the sorbent surface. Novel regeneration approaches aim to reduce the energy penalty associated with sorbent regeneration while maintaining high working capacity and minimizing sorbent degradation over multiple adsorption-desorption cycles.Expand Specific Solutions
Leading Organizations in CO2 Capture Materials Research
The CO2 capture adsorption market is in a growth phase, characterized by increasing investments in material innovations for solid sorbents. The global market is expanding rapidly due to stringent carbon reduction policies and net-zero commitments. Technologically, the field shows varying maturity levels across different approaches. Leading players include established energy corporations like ExxonMobil, China Petroleum & Chemical Corp., and Korea Electric Power Corp., alongside specialized carbon capture companies such as Climeworks AG and Global Thermostat. Academic institutions including University of California, Arizona State University, and National University of Singapore are driving fundamental research advancements. The competitive landscape features collaboration between industry and academia, with research organizations like CSIRO and A*STAR contributing significantly to sorbent material development and process optimization for improved adsorption capacity and regeneration efficiency.
Climeworks AG
Technical Solution: Climeworks has developed a Direct Air Capture (DAC) technology using solid sorbents that selectively capture CO2 from ambient air. Their proprietary adsorbent material consists of modified amine-functionalized filters that bind with CO2 molecules. The technology operates in a cyclic process where ambient air passes through the collector, CO2 binds to the sorbent surface, and then the material is heated to approximately 100°C to release concentrated CO2 for storage or utilization. Climeworks has implemented a modular design with stackable "CO2 collectors" that can be scaled according to capture requirements. Their latest innovation includes optimizing the regeneration process to use low-grade waste heat (80-100°C), significantly improving energy efficiency. The company has deployed commercial plants in Switzerland, Iceland (Orca plant), and is developing their larger Mammoth plant with 36,000 tons/year capacity using geothermal energy for regeneration.
Strengths: Modular, scalable design allows for flexible deployment; utilizes low-temperature heat for regeneration (80-100°C); demonstrated commercial viability with operational plants; integration with renewable energy sources. Weaknesses: Higher cost compared to point-source capture ($600-800/ton CO2); relatively low capture capacity per unit area; requires significant energy input for the temperature swing regeneration process.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced solid sorbent materials for CO2 capture focusing on mesoporous silica-supported polyethyleneimine (PEI) adsorbents. Their proprietary technology features hierarchical pore structures that maximize the dispersion of amine groups while minimizing diffusion limitations. Sinopec's approach incorporates thermal and chemical stability enhancements through cross-linking techniques and metal oxide additives that prevent amine leaching and degradation during multiple adsorption-regeneration cycles. Their latest innovation includes a dual-function catalyst-sorbent system that can simultaneously capture CO2 and convert it to valuable chemicals when regenerated under specific conditions. The company has demonstrated these materials at pilot scale in several of their refineries, achieving CO2 capture capacities of 3-4 mmol/g with regeneration temperatures as low as 90°C. Sinopec has also developed a novel vacuum-temperature swing adsorption process that reduces energy consumption by approximately 30% compared to conventional temperature swing methods. Their integrated system design allows for heat recovery from industrial processes to power the regeneration step.
Strengths: High CO2 adsorption capacity (3-4 mmol/g); relatively low regeneration temperature requirements; excellent stability over multiple cycles (>1000 cycles with <10% capacity loss); integration with existing industrial infrastructure. Weaknesses: Performance degradation in the presence of moisture and SOx/NOx contaminants; energy intensity of the regeneration process still presents efficiency challenges; manufacturing scale-up complexity for specialized hierarchical materials.
Key Innovations in Sorbent Material Engineering
sorbent
PatentPendingUS20250281904A1
Innovation
- Development of a solid sorbent comprising a silica support with covalently attached secondary amines confined inside its pores, optimized through controlled grafting and polymerization, achieving high amine density and stability.
Microporous aerogel
PatentWO2022032338A1
Innovation
- A silica-based microporous aerogel with a high percentage of pores less than 2 nm in diameter, composed of a reaction product of amino substituted silane, silicate, and optionally alkyl substituted silane, is developed for efficient CO2 capture and regeneration, suitable for low CO2 concentrations and scalable for industrial applications.
Environmental Impact Assessment of Sorbent Materials
The environmental impact of solid sorbent materials for CO2 capture extends far beyond their primary function of carbon sequestration. A comprehensive assessment reveals that while these materials contribute positively to climate change mitigation, their production, use, and disposal present complex environmental trade-offs that must be carefully evaluated.
Life cycle assessment (LCA) studies indicate that amine-based solid sorbents, particularly those utilizing polyethyleneimine (PEI), demonstrate significant carbon reduction potential but may contribute to acidification and eutrophication during manufacturing processes. The synthesis of these materials often requires energy-intensive procedures and potentially toxic precursors, creating an environmental burden that partially offsets their carbon capture benefits.
Metal-organic frameworks (MOFs), despite their exceptional CO2 adsorption capacities, present environmental concerns related to metal leaching and persistence in ecosystems. Recent research indicates that zirconium-based MOFs exhibit lower ecotoxicity compared to copper-based alternatives, though their synthesis typically demands substantial solvent usage, contributing to their environmental footprint.
Water consumption represents another critical environmental parameter, particularly for zeolite-based sorbents requiring hydrothermal synthesis conditions. Activated carbon materials generally demonstrate lower water intensity but may contribute to particulate matter emissions during production and regeneration cycles. Innovative water-efficient synthesis routes have emerged, reducing freshwater requirements by up to 40% compared to conventional methods.
Regeneration processes significantly influence the overall environmental profile of sorbent materials. Temperature swing adsorption (TSA) systems utilizing renewable energy sources can substantially reduce the environmental impact compared to steam-based regeneration approaches. Recent innovations in microwave and electrical swing adsorption demonstrate promising reductions in regeneration energy requirements, potentially decreasing associated emissions by 25-30%.
End-of-life considerations reveal that most current sorbent materials lack established recycling pathways. Silica-supported amine sorbents show potential for component recovery and reuse, while certain MOFs can be chemically decomposed to recover valuable metal constituents. However, the environmental impacts of disposal remain inadequately characterized, particularly regarding long-term leaching behavior in landfill environments.
Emerging bio-based sorbents derived from agricultural waste streams offer promising environmental profiles, with substantially reduced production impacts compared to synthetic alternatives. These materials present opportunities for circular economy approaches, though challenges remain in achieving comparable performance metrics to conventional sorbents.
Life cycle assessment (LCA) studies indicate that amine-based solid sorbents, particularly those utilizing polyethyleneimine (PEI), demonstrate significant carbon reduction potential but may contribute to acidification and eutrophication during manufacturing processes. The synthesis of these materials often requires energy-intensive procedures and potentially toxic precursors, creating an environmental burden that partially offsets their carbon capture benefits.
Metal-organic frameworks (MOFs), despite their exceptional CO2 adsorption capacities, present environmental concerns related to metal leaching and persistence in ecosystems. Recent research indicates that zirconium-based MOFs exhibit lower ecotoxicity compared to copper-based alternatives, though their synthesis typically demands substantial solvent usage, contributing to their environmental footprint.
Water consumption represents another critical environmental parameter, particularly for zeolite-based sorbents requiring hydrothermal synthesis conditions. Activated carbon materials generally demonstrate lower water intensity but may contribute to particulate matter emissions during production and regeneration cycles. Innovative water-efficient synthesis routes have emerged, reducing freshwater requirements by up to 40% compared to conventional methods.
Regeneration processes significantly influence the overall environmental profile of sorbent materials. Temperature swing adsorption (TSA) systems utilizing renewable energy sources can substantially reduce the environmental impact compared to steam-based regeneration approaches. Recent innovations in microwave and electrical swing adsorption demonstrate promising reductions in regeneration energy requirements, potentially decreasing associated emissions by 25-30%.
End-of-life considerations reveal that most current sorbent materials lack established recycling pathways. Silica-supported amine sorbents show potential for component recovery and reuse, while certain MOFs can be chemically decomposed to recover valuable metal constituents. However, the environmental impacts of disposal remain inadequately characterized, particularly regarding long-term leaching behavior in landfill environments.
Emerging bio-based sorbents derived from agricultural waste streams offer promising environmental profiles, with substantially reduced production impacts compared to synthetic alternatives. These materials present opportunities for circular economy approaches, though challenges remain in achieving comparable performance metrics to conventional sorbents.
Techno-Economic Analysis of Advanced Sorbent Systems
The techno-economic analysis of advanced sorbent systems for CO2 capture reveals significant cost implications across the carbon capture value chain. Current estimates indicate that solid sorbent technologies could potentially reduce capture costs by 30-40% compared to conventional amine scrubbing processes, with capital expenditure reductions of approximately 25% and operating cost savings of up to 45% depending on implementation scale.
Energy requirements for regeneration represent a critical economic factor, with thermal energy consumption ranging from 2.0-3.5 GJ/tonne CO2 for advanced metal-organic frameworks (MOFs) and 2.5-4.0 GJ/tonne CO2 for functionalized porous polymers. These values compare favorably against the 3.5-5.0 GJ/tonne CO2 typically required by liquid amine systems, translating to substantial operational savings over facility lifetimes.
Material durability significantly impacts long-term economics, with current generation solid sorbents demonstrating performance degradation of 0.5-2% per cycle. Advanced materials incorporating stability-enhancing functional groups have extended useful lifetimes to 1,000-5,000 cycles, dramatically reducing replacement costs that previously represented 15-25% of operational expenditures.
Manufacturing scalability presents both challenges and opportunities, with current production costs for advanced sorbents ranging from $15-80/kg depending on material complexity. Economic modeling suggests that economies of scale could reduce these costs by 60-75% at industrial production volumes, making widespread deployment increasingly viable.
Integration costs for retrofitting existing facilities with solid sorbent systems vary significantly based on facility configuration, with estimates ranging from $400-900/kW for power generation applications. Greenfield implementations demonstrate more favorable economics, with integration costs approximately 30% lower than retrofit scenarios.
Sensitivity analysis indicates that sorbent capacity, regeneration energy, and material lifetime represent the most influential parameters affecting levelized cost of capture. A 20% improvement in any of these parameters typically yields a 5-15% reduction in overall capture costs, highlighting the economic value of continued materials innovation.
Market projections suggest that with continued development, advanced solid sorbent systems could achieve capture costs below $40/tonne CO2 by 2030, representing a critical threshold for widespread commercial adoption across multiple industrial sectors beyond power generation, including cement, steel, and chemical manufacturing.
Energy requirements for regeneration represent a critical economic factor, with thermal energy consumption ranging from 2.0-3.5 GJ/tonne CO2 for advanced metal-organic frameworks (MOFs) and 2.5-4.0 GJ/tonne CO2 for functionalized porous polymers. These values compare favorably against the 3.5-5.0 GJ/tonne CO2 typically required by liquid amine systems, translating to substantial operational savings over facility lifetimes.
Material durability significantly impacts long-term economics, with current generation solid sorbents demonstrating performance degradation of 0.5-2% per cycle. Advanced materials incorporating stability-enhancing functional groups have extended useful lifetimes to 1,000-5,000 cycles, dramatically reducing replacement costs that previously represented 15-25% of operational expenditures.
Manufacturing scalability presents both challenges and opportunities, with current production costs for advanced sorbents ranging from $15-80/kg depending on material complexity. Economic modeling suggests that economies of scale could reduce these costs by 60-75% at industrial production volumes, making widespread deployment increasingly viable.
Integration costs for retrofitting existing facilities with solid sorbent systems vary significantly based on facility configuration, with estimates ranging from $400-900/kW for power generation applications. Greenfield implementations demonstrate more favorable economics, with integration costs approximately 30% lower than retrofit scenarios.
Sensitivity analysis indicates that sorbent capacity, regeneration energy, and material lifetime represent the most influential parameters affecting levelized cost of capture. A 20% improvement in any of these parameters typically yields a 5-15% reduction in overall capture costs, highlighting the economic value of continued materials innovation.
Market projections suggest that with continued development, advanced solid sorbent systems could achieve capture costs below $40/tonne CO2 by 2030, representing a critical threshold for widespread commercial adoption across multiple industrial sectors beyond power generation, including cement, steel, and chemical manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!