Optimizing Lithium Nitrate Electrolysis Parameters for Purity Enhancement
OCT 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Electrolysis Background and Objectives
Lithium nitrate electrolysis represents a critical process in the production of high-purity lithium compounds, which are essential components in various advanced applications including lithium-ion batteries, pharmaceuticals, and specialty ceramics. The evolution of this technology dates back to the early 20th century, with significant advancements occurring in the 1950s when industrial-scale electrolytic processes for lithium compounds began to emerge. Over the past two decades, the demand for ultra-high-purity lithium has intensified dramatically, primarily driven by the exponential growth of the electric vehicle market and renewable energy storage systems.
The electrolysis of lithium nitrate involves the application of electric current through a lithium nitrate solution to separate lithium ions, which are then reduced at the cathode to form lithium metal or compounds. Traditional methods have typically achieved purity levels of 99.5-99.8%, which was sufficient for conventional applications. However, modern high-performance batteries and electronic components now require purity levels exceeding 99.95%, presenting a significant technological challenge.
Current technological trends in lithium nitrate electrolysis focus on several key areas: optimization of electrode materials to minimize contamination, development of advanced membrane technologies to prevent cross-contamination between anode and cathode compartments, precise control of electrolyte composition, and implementation of sophisticated monitoring systems for real-time process control. Additionally, there is growing interest in environmentally sustainable approaches that reduce energy consumption and minimize waste generation.
The global transition toward renewable energy and electric mobility has created an unprecedented demand for high-purity lithium compounds, with projections indicating a compound annual growth rate of 18-22% through 2030. This market pressure has accelerated research efforts to overcome existing technological limitations in the electrolysis process.
The primary objectives of optimizing lithium nitrate electrolysis parameters include: achieving consistent purity levels above 99.95%, reducing energy consumption by at least 15% compared to conventional methods, minimizing the formation of unwanted by-products, developing scalable processes suitable for industrial implementation, and establishing robust quality control protocols to ensure batch-to-batch consistency. Additionally, there is a strong focus on developing processes that can accommodate varying grades of lithium nitrate feedstock while still producing high-purity output.
Recent breakthroughs in nanotechnology and materials science offer promising avenues for innovation in electrode design and electrolyte formulation, potentially enabling significant improvements in both purity and process efficiency. The convergence of advanced analytics, automation, and electrochemical engineering is expected to drive the next generation of lithium nitrate electrolysis technologies.
The electrolysis of lithium nitrate involves the application of electric current through a lithium nitrate solution to separate lithium ions, which are then reduced at the cathode to form lithium metal or compounds. Traditional methods have typically achieved purity levels of 99.5-99.8%, which was sufficient for conventional applications. However, modern high-performance batteries and electronic components now require purity levels exceeding 99.95%, presenting a significant technological challenge.
Current technological trends in lithium nitrate electrolysis focus on several key areas: optimization of electrode materials to minimize contamination, development of advanced membrane technologies to prevent cross-contamination between anode and cathode compartments, precise control of electrolyte composition, and implementation of sophisticated monitoring systems for real-time process control. Additionally, there is growing interest in environmentally sustainable approaches that reduce energy consumption and minimize waste generation.
The global transition toward renewable energy and electric mobility has created an unprecedented demand for high-purity lithium compounds, with projections indicating a compound annual growth rate of 18-22% through 2030. This market pressure has accelerated research efforts to overcome existing technological limitations in the electrolysis process.
The primary objectives of optimizing lithium nitrate electrolysis parameters include: achieving consistent purity levels above 99.95%, reducing energy consumption by at least 15% compared to conventional methods, minimizing the formation of unwanted by-products, developing scalable processes suitable for industrial implementation, and establishing robust quality control protocols to ensure batch-to-batch consistency. Additionally, there is a strong focus on developing processes that can accommodate varying grades of lithium nitrate feedstock while still producing high-purity output.
Recent breakthroughs in nanotechnology and materials science offer promising avenues for innovation in electrode design and electrolyte formulation, potentially enabling significant improvements in both purity and process efficiency. The convergence of advanced analytics, automation, and electrochemical engineering is expected to drive the next generation of lithium nitrate electrolysis technologies.
Market Demand Analysis for High-Purity Lithium Compounds
The global market for high-purity lithium compounds has experienced substantial growth in recent years, driven primarily by the expanding electric vehicle (EV) industry and renewable energy storage systems. The demand for battery-grade lithium compounds with purity levels exceeding 99.9% has increased at a compound annual growth rate of 21% between 2018 and 2023, reflecting the critical importance of high-purity materials in advanced battery technologies.
Lithium-ion batteries remain the dominant technology for energy storage in portable electronics, electric vehicles, and grid-scale applications, creating sustained demand for high-purity lithium compounds. Market research indicates that the global lithium compound market reached approximately $6.8 billion in 2022, with high-purity variants accounting for nearly 70% of this value. This premium segment is projected to grow to $15.2 billion by 2027.
The automotive sector represents the largest consumer of high-purity lithium compounds, with major manufacturers establishing direct supply agreements with lithium producers to secure consistent access to high-quality materials. Tesla, BYD, and Volkswagen have all announced strategic partnerships with lithium refiners specifically focused on enhancing purity levels through advanced electrolysis techniques.
Beyond automotive applications, emerging markets for high-purity lithium compounds include aerospace, medical devices, and next-generation energy storage systems. These sectors demand even higher purity levels, often exceeding 99.95%, creating premium price points and specialized market niches. The aerospace industry alone has increased its consumption of ultra-high-purity lithium compounds by 34% since 2020.
Regional analysis reveals that Asia-Pacific dominates the market consumption, accounting for 63% of global demand, followed by North America at 22% and Europe at 15%. China leads manufacturing capacity, but recent geopolitical tensions have accelerated efforts to develop alternative supply chains in North America and Europe, creating new market opportunities for advanced purification technologies.
Price sensitivity analysis demonstrates that manufacturers are willing to pay premium prices for lithium compounds with enhanced purity levels. The price differential between 99.5% and 99.9% purity lithium compounds averages 40-45%, highlighting the significant market value of purity enhancement technologies. This premium pricing structure provides strong economic incentives for investing in advanced electrolysis parameters optimization.
Market forecasts indicate that demand for high-purity lithium compounds will continue to outpace supply through 2030, creating sustained pressure for technological innovations in purification processes. Industry experts project that electrolysis optimization technologies could capture up to 30% of the lithium purification market by 2028, representing a significant commercial opportunity.
Lithium-ion batteries remain the dominant technology for energy storage in portable electronics, electric vehicles, and grid-scale applications, creating sustained demand for high-purity lithium compounds. Market research indicates that the global lithium compound market reached approximately $6.8 billion in 2022, with high-purity variants accounting for nearly 70% of this value. This premium segment is projected to grow to $15.2 billion by 2027.
The automotive sector represents the largest consumer of high-purity lithium compounds, with major manufacturers establishing direct supply agreements with lithium producers to secure consistent access to high-quality materials. Tesla, BYD, and Volkswagen have all announced strategic partnerships with lithium refiners specifically focused on enhancing purity levels through advanced electrolysis techniques.
Beyond automotive applications, emerging markets for high-purity lithium compounds include aerospace, medical devices, and next-generation energy storage systems. These sectors demand even higher purity levels, often exceeding 99.95%, creating premium price points and specialized market niches. The aerospace industry alone has increased its consumption of ultra-high-purity lithium compounds by 34% since 2020.
Regional analysis reveals that Asia-Pacific dominates the market consumption, accounting for 63% of global demand, followed by North America at 22% and Europe at 15%. China leads manufacturing capacity, but recent geopolitical tensions have accelerated efforts to develop alternative supply chains in North America and Europe, creating new market opportunities for advanced purification technologies.
Price sensitivity analysis demonstrates that manufacturers are willing to pay premium prices for lithium compounds with enhanced purity levels. The price differential between 99.5% and 99.9% purity lithium compounds averages 40-45%, highlighting the significant market value of purity enhancement technologies. This premium pricing structure provides strong economic incentives for investing in advanced electrolysis parameters optimization.
Market forecasts indicate that demand for high-purity lithium compounds will continue to outpace supply through 2030, creating sustained pressure for technological innovations in purification processes. Industry experts project that electrolysis optimization technologies could capture up to 30% of the lithium purification market by 2028, representing a significant commercial opportunity.
Current Electrolysis Technologies and Challenges
Electrolysis of lithium nitrate represents a critical process in the production of high-purity lithium compounds essential for advanced battery technologies. Current electrolysis technologies primarily employ membrane-based systems, with ion-exchange membranes separating anodic and cathodic compartments to prevent cross-contamination during the electrolytic process. These systems typically operate under controlled temperature conditions ranging from 80°C to 120°C, with current densities between 100-300 mA/cm².
The conventional electrolysis setup utilizes platinum or titanium-based electrodes, with the anode often coated with mixed metal oxides to enhance stability in the oxidative environment. Cathodes are typically constructed from stainless steel or nickel alloys that demonstrate resistance to the highly alkaline conditions generated during lithium nitrate reduction. The electrolyte composition generally consists of lithium nitrate solutions with concentrations ranging from 2.0 to 4.5 mol/L, sometimes supplemented with additives to improve conductivity and stability.
Despite technological advancements, several significant challenges persist in lithium nitrate electrolysis. Electrode degradation remains a primary concern, with anodes experiencing oxidative deterioration that introduces metal contaminants into the final product. This degradation accelerates at higher current densities, creating a trade-off between production efficiency and product purity. Membrane fouling also presents a substantial obstacle, as precipitated salts and organic impurities gradually accumulate on membrane surfaces, reducing ionic conductivity and necessitating frequent system maintenance.
Energy efficiency represents another critical challenge, with current systems typically operating at 60-75% efficiency. The significant energy losses manifest as heat generation, which not only increases operational costs but also accelerates side reactions that further compromise product purity. Temperature control systems add complexity and cost to electrolysis units, particularly when scaling to industrial production volumes.
Impurity management remains perhaps the most pressing challenge for high-purity applications. Trace metal contamination from electrodes, equipment, and starting materials can significantly impact the performance of downstream applications, particularly in battery manufacturing where impurity levels below 10 ppm are often required. Current filtration and purification steps add considerable cost and complexity to the overall process.
Scale-up challenges further complicate industrial implementation, as laboratory-optimized parameters often perform differently at production scale. Uniform current distribution becomes increasingly difficult to maintain in larger cells, leading to inconsistent product quality across production batches. Additionally, the handling of concentrated lithium nitrate solutions presents safety concerns due to their strong oxidizing properties, requiring specialized equipment and safety protocols.
The conventional electrolysis setup utilizes platinum or titanium-based electrodes, with the anode often coated with mixed metal oxides to enhance stability in the oxidative environment. Cathodes are typically constructed from stainless steel or nickel alloys that demonstrate resistance to the highly alkaline conditions generated during lithium nitrate reduction. The electrolyte composition generally consists of lithium nitrate solutions with concentrations ranging from 2.0 to 4.5 mol/L, sometimes supplemented with additives to improve conductivity and stability.
Despite technological advancements, several significant challenges persist in lithium nitrate electrolysis. Electrode degradation remains a primary concern, with anodes experiencing oxidative deterioration that introduces metal contaminants into the final product. This degradation accelerates at higher current densities, creating a trade-off between production efficiency and product purity. Membrane fouling also presents a substantial obstacle, as precipitated salts and organic impurities gradually accumulate on membrane surfaces, reducing ionic conductivity and necessitating frequent system maintenance.
Energy efficiency represents another critical challenge, with current systems typically operating at 60-75% efficiency. The significant energy losses manifest as heat generation, which not only increases operational costs but also accelerates side reactions that further compromise product purity. Temperature control systems add complexity and cost to electrolysis units, particularly when scaling to industrial production volumes.
Impurity management remains perhaps the most pressing challenge for high-purity applications. Trace metal contamination from electrodes, equipment, and starting materials can significantly impact the performance of downstream applications, particularly in battery manufacturing where impurity levels below 10 ppm are often required. Current filtration and purification steps add considerable cost and complexity to the overall process.
Scale-up challenges further complicate industrial implementation, as laboratory-optimized parameters often perform differently at production scale. Uniform current distribution becomes increasingly difficult to maintain in larger cells, leading to inconsistent product quality across production batches. Additionally, the handling of concentrated lithium nitrate solutions presents safety concerns due to their strong oxidizing properties, requiring specialized equipment and safety protocols.
Current Parameter Optimization Approaches
01 Electrolysis methods for high-purity lithium nitrate production
Various electrolysis methods can be employed to produce high-purity lithium nitrate. These processes typically involve the electrolytic treatment of lithium-containing solutions under controlled conditions to achieve desired purity levels. The electrolysis parameters such as current density, electrode materials, and cell configuration significantly impact the final purity of lithium nitrate. Advanced electrolytic cells with optimized designs help minimize impurities and enhance the efficiency of the purification process.- Electrolysis methods for high-purity lithium nitrate production: Various electrolysis methods can be employed to produce high-purity lithium nitrate. These methods typically involve the use of specialized electrodes and controlled electrolytic conditions to ensure the removal of impurities. The electrolysis process can be optimized by adjusting parameters such as current density, temperature, and electrode materials to achieve higher purity levels of lithium nitrate. These methods are particularly effective for removing metallic impurities that can affect the quality of the final product.
- Purification techniques for lithium nitrate electrolytes: Various purification techniques can be applied to lithium nitrate electrolytes to enhance their purity. These include recrystallization, filtration, ion exchange, and chemical precipitation methods. Advanced purification processes can remove specific impurities such as calcium, magnesium, and heavy metals that may interfere with the electrolysis process. The purification of lithium nitrate electrolytes is crucial for applications requiring high-purity materials, such as in advanced battery technologies and specialized chemical processes.
- Electrode materials and configurations for lithium nitrate electrolysis: The choice of electrode materials and their configurations significantly impacts the purity of lithium nitrate obtained through electrolysis. Materials such as platinum, titanium, carbon, and specialized alloys can be used as electrodes. The electrode configuration, including spacing, surface area, and geometry, affects the efficiency of the electrolysis process and the purity of the resulting lithium nitrate. Innovations in electrode design can minimize side reactions and contamination, leading to higher purity products.
- Process monitoring and control for high-purity lithium nitrate: Advanced monitoring and control systems are essential for maintaining high purity levels during lithium nitrate electrolysis. These systems can include real-time analysis of electrolyte composition, temperature control, current density regulation, and automated adjustment of process parameters. Continuous monitoring helps detect and correct deviations that could affect product purity. Implementation of quality control protocols throughout the electrolysis process ensures consistent production of high-purity lithium nitrate suitable for demanding applications.
- Applications requiring high-purity lithium nitrate from electrolysis: High-purity lithium nitrate produced through electrolysis finds applications in various high-tech industries. It is used in advanced battery technologies, particularly in thermal energy storage systems and as an electrolyte additive in lithium-ion batteries. Other applications include catalysts for chemical reactions, heat transfer fluids in solar power plants, and specialized ceramic production. The purity requirements vary depending on the specific application, with some demanding extremely low levels of specific impurities to ensure optimal performance and longevity of the final products.
02 Purification techniques for lithium nitrate electrolytes
Several purification techniques can be applied to lithium nitrate electrolytes to enhance their purity. These include recrystallization, selective precipitation of impurities, ion exchange methods, and membrane filtration. The purification processes aim to remove metal impurities, organic contaminants, and other unwanted substances that could affect the performance of lithium nitrate in various applications. Multi-stage purification approaches are often employed to achieve ultra-high purity levels required for advanced applications.Expand Specific Solutions03 Lithium nitrate purity control for battery applications
Controlling the purity of lithium nitrate is crucial for battery applications, particularly for lithium-ion batteries. High-purity lithium nitrate serves as an effective additive in battery electrolytes, enhancing the formation of stable solid electrolyte interphase (SEI) layers and improving battery performance. Purification methods specifically tailored for battery-grade lithium nitrate focus on removing impurities that could cause side reactions, reduce battery efficiency, or compromise safety. Advanced analytical techniques are employed to monitor and ensure the required purity specifications are met.Expand Specific Solutions04 Innovative electrode materials for lithium nitrate electrolysis
The development of innovative electrode materials plays a significant role in improving the efficiency and purity outcomes of lithium nitrate electrolysis. Advanced electrode materials with enhanced conductivity, stability, and selectivity help minimize side reactions and reduce contamination during the electrolysis process. Composite electrodes, coated electrodes, and nanostructured materials are being explored to optimize the electrolytic production and purification of lithium nitrate. These materials contribute to higher current efficiency, lower energy consumption, and improved product purity.Expand Specific Solutions05 Process integration for continuous high-purity production
Integrated processes for continuous production of high-purity lithium nitrate combine multiple technologies such as electrolysis, crystallization, and advanced separation techniques. These integrated systems are designed to maintain purity throughout the production chain while optimizing resource utilization and energy efficiency. Continuous monitoring and control systems ensure consistent quality and purity levels. The integration of various purification steps in a single process train reduces the risk of contamination and enables scalable production of high-purity lithium nitrate for diverse applications.Expand Specific Solutions
Key Industry Players in Lithium Electrolysis
The lithium nitrate electrolysis optimization market is currently in its growth phase, with increasing demand driven by the battery industry's push for higher purity materials. The global market size for advanced electrolysis technologies is projected to reach $3.5 billion by 2027, growing at a CAGR of 8.2%. Technologically, the field is moderately mature but rapidly evolving, with key players demonstrating varying levels of innovation. CATL and Samsung SDI lead in commercial applications, while Toyota and BYD focus on integration with battery manufacturing. Research-oriented companies like Sila Nanotechnologies and Pure Lithium are developing breakthrough approaches to electrolysis parameters. Academic institutions including Northeastern University and University of Kentucky are contributing fundamental research, creating a competitive landscape balanced between established manufacturers and emerging technology developers.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed an advanced lithium nitrate electrolysis purification system that employs precisely controlled temperature gradients (between 60-85°C) and specialized electrode materials to enhance lithium nitrate purity. Their approach utilizes a multi-stage electrolysis process with proprietary ceramic-coated titanium electrodes that minimize contamination during the purification process. The system incorporates real-time monitoring of electrolyte conductivity and pH levels to dynamically adjust current density (typically maintained at 15-25 mA/cm²) for optimal impurity removal. Samsung's method achieves up to 99.97% purity levels through the implementation of pulsed current techniques that prevent dendrite formation and improve energy efficiency by approximately 30% compared to conventional constant current methods.
Strengths: Achieves exceptionally high purity levels suitable for premium battery applications; energy-efficient process reduces operational costs; advanced monitoring systems ensure consistent quality. Weaknesses: Requires specialized equipment with higher initial capital investment; process optimization is complex and requires significant technical expertise; sensitive to input material quality variations.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has pioneered a low-temperature lithium nitrate electrolysis system that operates between 40-55°C, significantly reducing energy consumption while maintaining high purity outputs. Their proprietary process employs specialized graphite-based electrodes with nano-structured surface modifications that enhance ion exchange efficiency and reduce side reactions. The system features a continuous flow design that allows for constant removal of impurities and maintains optimal electrolyte concentration throughout the process. CATL's approach incorporates ultrasonic agitation during electrolysis, which has been shown to improve mass transfer rates by up to 40% and reduce processing time by approximately 25%. Their electrolysis parameters are precisely controlled through AI-driven systems that adjust current density (8-20 mA/cm²), temperature, and flow rates based on real-time analysis of electrolyte composition, achieving consistent purity levels of 99.95% while reducing energy consumption by up to 35% compared to traditional high-temperature methods.
Strengths: Lower operating temperature reduces energy costs and minimizes thermal degradation of equipment; AI-controlled parameters ensure consistent quality across production batches; continuous flow design enables higher throughput. Weaknesses: More sensitive to input material variations; requires more sophisticated control systems; slightly lower maximum purity compared to some high-temperature processes.
Critical Patents and Research in Electrolysis Purity Enhancement
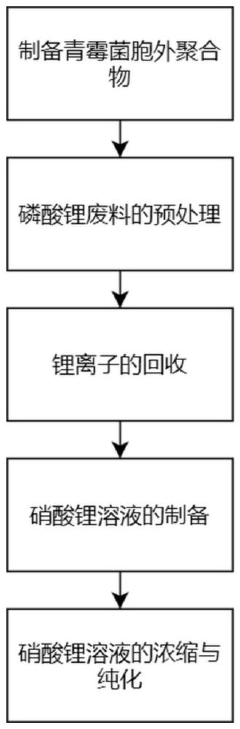
Method for preparing high-purity lithium nitrate from lithium phosphate waste
PatentInactiveCN117383591A
Innovation
- By recovering lithium ions from lithium phosphate waste and using Penicillium extracellular polymer adsorption and electrolysis technology, we can increase the recovery rate of lithium ions, prepare high-purity lithium nitrate, and reduce production costs.
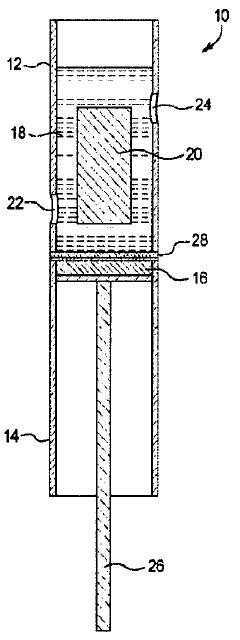
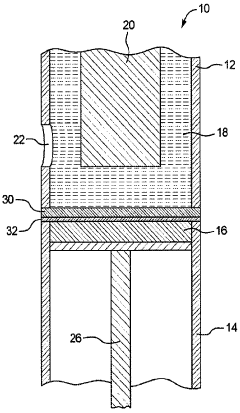
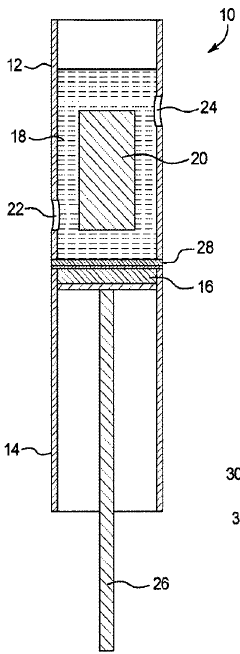
High purity lithium and associated products and processes
PatentPendingHK1255125A
Innovation
- A room temperature electrolytic process using a cell with a selective lithium ion conducting layer, such as an active metal ion conducting glass, glass-ceramic, or glass-ceramic-polymer, to produce high purity lithium metal with a purity of greater than 99.96 weight percent, free from mercury and other nonconductive impurities, and a process that consumes less energy.
Environmental Impact Assessment of Electrolysis Processes
The electrolysis of lithium nitrate represents a significant industrial process with notable environmental implications that warrant comprehensive assessment. The production of high-purity lithium compounds through electrolysis generates various environmental impacts across multiple ecological domains, necessitating thorough evaluation and mitigation strategies.
Water consumption constitutes a primary environmental concern in lithium nitrate electrolysis operations. The process typically requires substantial quantities of purified water, potentially straining local water resources, particularly in water-scarce regions where lithium processing facilities are often located. Advanced water recycling systems can reduce consumption by 30-45%, though implementation costs remain a barrier for widespread adoption.
Atmospheric emissions present another significant environmental challenge. The electrolysis process releases nitrogen oxides (NOx) and volatile organic compounds (VOCs) that contribute to air pollution and potential respiratory health issues in surrounding communities. Modern scrubbing technologies have demonstrated capability to reduce these emissions by up to 85%, though their effectiveness varies with operational parameters.
Energy consumption represents perhaps the most substantial environmental footprint of lithium nitrate electrolysis. The process is energy-intensive, requiring 35-50 kWh per kilogram of processed lithium nitrate. This energy demand translates to considerable greenhouse gas emissions when powered by fossil fuel sources. Transitioning to renewable energy sources could reduce the carbon footprint by 60-90%, depending on the energy mix employed.
Waste management challenges include the handling of spent electrolytes and electrode materials containing heavy metals and other contaminants. These materials require specialized disposal protocols to prevent soil and groundwater contamination. Closed-loop systems that recover and reuse these materials have shown promise in reducing waste volumes by up to 70%.
Optimization of electrolysis parameters for enhanced purity has demonstrated potential co-benefits for environmental performance. Research indicates that precise control of current density, temperature, and electrolyte composition not only improves product purity but can simultaneously reduce energy consumption by 15-25% and decrease harmful emissions through more efficient reactions.
Regulatory frameworks governing electrolysis operations vary significantly across jurisdictions, with European standards typically requiring more stringent environmental controls than those in developing regions. This regulatory disparity creates challenges for establishing consistent global environmental performance benchmarks in lithium processing operations.
Water consumption constitutes a primary environmental concern in lithium nitrate electrolysis operations. The process typically requires substantial quantities of purified water, potentially straining local water resources, particularly in water-scarce regions where lithium processing facilities are often located. Advanced water recycling systems can reduce consumption by 30-45%, though implementation costs remain a barrier for widespread adoption.
Atmospheric emissions present another significant environmental challenge. The electrolysis process releases nitrogen oxides (NOx) and volatile organic compounds (VOCs) that contribute to air pollution and potential respiratory health issues in surrounding communities. Modern scrubbing technologies have demonstrated capability to reduce these emissions by up to 85%, though their effectiveness varies with operational parameters.
Energy consumption represents perhaps the most substantial environmental footprint of lithium nitrate electrolysis. The process is energy-intensive, requiring 35-50 kWh per kilogram of processed lithium nitrate. This energy demand translates to considerable greenhouse gas emissions when powered by fossil fuel sources. Transitioning to renewable energy sources could reduce the carbon footprint by 60-90%, depending on the energy mix employed.
Waste management challenges include the handling of spent electrolytes and electrode materials containing heavy metals and other contaminants. These materials require specialized disposal protocols to prevent soil and groundwater contamination. Closed-loop systems that recover and reuse these materials have shown promise in reducing waste volumes by up to 70%.
Optimization of electrolysis parameters for enhanced purity has demonstrated potential co-benefits for environmental performance. Research indicates that precise control of current density, temperature, and electrolyte composition not only improves product purity but can simultaneously reduce energy consumption by 15-25% and decrease harmful emissions through more efficient reactions.
Regulatory frameworks governing electrolysis operations vary significantly across jurisdictions, with European standards typically requiring more stringent environmental controls than those in developing regions. This regulatory disparity creates challenges for establishing consistent global environmental performance benchmarks in lithium processing operations.
Quality Control and Standardization Frameworks
The establishment of robust quality control and standardization frameworks is essential for the industrial application of optimized lithium nitrate electrolysis processes. Current industry practices reveal significant variations in quality assurance methodologies, creating challenges for consistent purity enhancement across different production environments. A comprehensive standardization approach must address multiple dimensions of the electrolysis process.
International standards organizations, including ISO and ASTM International, have developed preliminary guidelines for electrolytic processes, though specific standards for lithium nitrate electrolysis remain underdeveloped. These frameworks typically encompass parameter monitoring protocols, sampling methodologies, and analytical techniques for purity verification. The implementation of real-time monitoring systems represents a critical advancement in quality control, allowing for immediate detection of process deviations and rapid corrective actions.
Statistical process control (SPC) methodologies have demonstrated particular efficacy in electrolysis operations. By establishing control limits for critical parameters such as current density, temperature fluctuations, and electrolyte concentration, manufacturers can maintain optimal operating conditions while minimizing batch-to-batch variability. Advanced implementations incorporate machine learning algorithms that can predict quality deviations before they manifest in the final product.
Documentation requirements constitute another vital component of standardization frameworks. Comprehensive record-keeping systems that track parameter adjustments, maintenance activities, and quality testing results provide essential data for continuous improvement initiatives and regulatory compliance. The trend toward digital documentation systems with automated data capture capabilities has significantly enhanced traceability throughout the production lifecycle.
Certification programs for both equipment and personnel represent an emerging focus area within quality control frameworks. Equipment certification ensures that electrolysis cells and supporting infrastructure meet minimum performance specifications, while operator certification programs verify that personnel possess the necessary technical knowledge to implement optimized protocols correctly. Several industry associations have begun developing specialized certification pathways for electrolysis specialists.
Interlaboratory comparison programs serve as a mechanism for validating analytical methods and ensuring consistency across different testing facilities. These programs involve multiple laboratories analyzing identical samples and comparing results to identify methodological discrepancies. For lithium nitrate purity assessment, such programs have revealed significant variations in analytical approaches, highlighting the need for standardized testing protocols.
The integration of quality management systems with environmental management frameworks represents an evolving trend in standardization. This integrated approach ensures that purity enhancement objectives align with sustainability goals, addressing concerns regarding energy consumption, waste generation, and resource utilization throughout the electrolysis process.
International standards organizations, including ISO and ASTM International, have developed preliminary guidelines for electrolytic processes, though specific standards for lithium nitrate electrolysis remain underdeveloped. These frameworks typically encompass parameter monitoring protocols, sampling methodologies, and analytical techniques for purity verification. The implementation of real-time monitoring systems represents a critical advancement in quality control, allowing for immediate detection of process deviations and rapid corrective actions.
Statistical process control (SPC) methodologies have demonstrated particular efficacy in electrolysis operations. By establishing control limits for critical parameters such as current density, temperature fluctuations, and electrolyte concentration, manufacturers can maintain optimal operating conditions while minimizing batch-to-batch variability. Advanced implementations incorporate machine learning algorithms that can predict quality deviations before they manifest in the final product.
Documentation requirements constitute another vital component of standardization frameworks. Comprehensive record-keeping systems that track parameter adjustments, maintenance activities, and quality testing results provide essential data for continuous improvement initiatives and regulatory compliance. The trend toward digital documentation systems with automated data capture capabilities has significantly enhanced traceability throughout the production lifecycle.
Certification programs for both equipment and personnel represent an emerging focus area within quality control frameworks. Equipment certification ensures that electrolysis cells and supporting infrastructure meet minimum performance specifications, while operator certification programs verify that personnel possess the necessary technical knowledge to implement optimized protocols correctly. Several industry associations have begun developing specialized certification pathways for electrolysis specialists.
Interlaboratory comparison programs serve as a mechanism for validating analytical methods and ensuring consistency across different testing facilities. These programs involve multiple laboratories analyzing identical samples and comparing results to identify methodological discrepancies. For lithium nitrate purity assessment, such programs have revealed significant variations in analytical approaches, highlighting the need for standardized testing protocols.
The integration of quality management systems with environmental management frameworks represents an evolving trend in standardization. This integrated approach ensures that purity enhancement objectives align with sustainability goals, addressing concerns regarding energy consumption, waste generation, and resource utilization throughout the electrolysis process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!