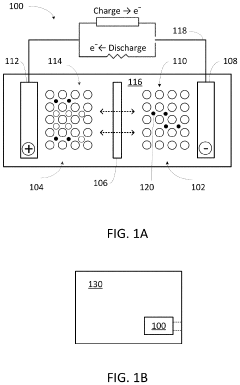
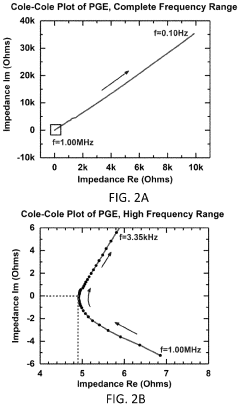
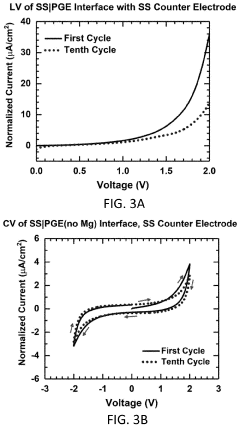
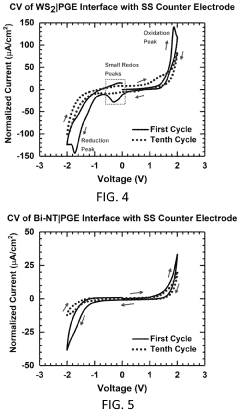
Polymer gel electrolytes for flexible magnesium batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Gel Electrolytes Background and Objectives
Polymer gel electrolytes (PGEs) have emerged as a promising solution for next-generation energy storage systems, particularly for flexible magnesium batteries. The development of these electrolytes represents a convergence of polymer science, electrochemistry, and materials engineering that has evolved significantly over the past three decades. Initially conceived as alternatives to liquid electrolytes in the 1990s, PGEs have progressed from simple polymer-salt complexes to sophisticated multi-component systems incorporating various functional additives.
The evolution of PGEs has been driven by the increasing demand for safer, more reliable, and environmentally friendly energy storage solutions. Traditional liquid electrolytes, while offering high ionic conductivity, present significant safety concerns including flammability and potential leakage. Solid polymer electrolytes addressed these safety issues but suffered from poor room-temperature ionic conductivity. PGEs emerged as a middle-ground solution, combining the safety advantages of solid electrolytes with conductivity values approaching those of liquid systems.
For magnesium batteries specifically, the technical trajectory has been particularly challenging due to the divalent nature of Mg2+ ions, which results in stronger coordination with anions and slower diffusion kinetics compared to monovalent ions like Li+. Early research focused primarily on lithium-based systems, with magnesium receiving significant attention only in the past decade as researchers recognized its potential advantages including higher theoretical volumetric capacity (3833 mAh/cm³ vs. 2062 mAh/cm³ for lithium) and greater natural abundance.
The flexibility aspect of these electrolytes represents a response to the growing market for wearable electronics, flexible displays, and conformable energy storage solutions. This application-driven requirement has pushed researchers to develop novel polymer matrices with enhanced mechanical properties while maintaining electrochemical performance. The incorporation of various plasticizers, nanofillers, and ionic liquids has been instrumental in achieving this balance.
The primary technical objectives for PGEs in flexible magnesium batteries include achieving ionic conductivity exceeding 10⁻⁴ S/cm at room temperature, maintaining mechanical flexibility with Young's modulus below 1 GPa, ensuring electrochemical stability windows wider than 3V, and demonstrating cycling stability beyond 1000 cycles. Additionally, these materials must be environmentally benign, cost-effective, and compatible with scalable manufacturing processes.
Recent breakthroughs in polymer chemistry, particularly the development of block copolymers and polymer blends with nanoscale phase separation, have opened new avenues for achieving these objectives. The integration of computational modeling with experimental approaches has accelerated the discovery and optimization of novel PGE formulations, allowing researchers to predict structure-property relationships and design materials with tailored characteristics for specific applications in flexible magnesium battery systems.
The evolution of PGEs has been driven by the increasing demand for safer, more reliable, and environmentally friendly energy storage solutions. Traditional liquid electrolytes, while offering high ionic conductivity, present significant safety concerns including flammability and potential leakage. Solid polymer electrolytes addressed these safety issues but suffered from poor room-temperature ionic conductivity. PGEs emerged as a middle-ground solution, combining the safety advantages of solid electrolytes with conductivity values approaching those of liquid systems.
For magnesium batteries specifically, the technical trajectory has been particularly challenging due to the divalent nature of Mg2+ ions, which results in stronger coordination with anions and slower diffusion kinetics compared to monovalent ions like Li+. Early research focused primarily on lithium-based systems, with magnesium receiving significant attention only in the past decade as researchers recognized its potential advantages including higher theoretical volumetric capacity (3833 mAh/cm³ vs. 2062 mAh/cm³ for lithium) and greater natural abundance.
The flexibility aspect of these electrolytes represents a response to the growing market for wearable electronics, flexible displays, and conformable energy storage solutions. This application-driven requirement has pushed researchers to develop novel polymer matrices with enhanced mechanical properties while maintaining electrochemical performance. The incorporation of various plasticizers, nanofillers, and ionic liquids has been instrumental in achieving this balance.
The primary technical objectives for PGEs in flexible magnesium batteries include achieving ionic conductivity exceeding 10⁻⁴ S/cm at room temperature, maintaining mechanical flexibility with Young's modulus below 1 GPa, ensuring electrochemical stability windows wider than 3V, and demonstrating cycling stability beyond 1000 cycles. Additionally, these materials must be environmentally benign, cost-effective, and compatible with scalable manufacturing processes.
Recent breakthroughs in polymer chemistry, particularly the development of block copolymers and polymer blends with nanoscale phase separation, have opened new avenues for achieving these objectives. The integration of computational modeling with experimental approaches has accelerated the discovery and optimization of novel PGE formulations, allowing researchers to predict structure-property relationships and design materials with tailored characteristics for specific applications in flexible magnesium battery systems.
Market Analysis for Flexible Mg Battery Applications
The flexible magnesium battery market is experiencing significant growth potential driven by the increasing demand for wearable electronics, IoT devices, and flexible consumer electronics. Current market projections indicate that the global flexible battery market will reach approximately $969 million by 2025, with a compound annual growth rate of 30.0% from 2020. Within this broader market, magnesium-based flexible batteries are emerging as a promising segment due to their superior theoretical energy density, safety profile, and abundant raw material supply compared to lithium-ion alternatives.
The healthcare sector represents one of the most promising application areas, with flexible magnesium batteries being integrated into medical wearables, remote patient monitoring systems, and implantable medical devices. This segment is expected to grow at 35% annually through 2027, driven by increasing healthcare digitalization and remote monitoring adoption.
Consumer electronics constitutes another major market segment, with flexible displays, smart clothing, and foldable devices creating substantial demand for conformable power sources. Major electronics manufacturers including Samsung, LG, and Apple have filed patents related to flexible battery integration, signaling strong industry interest in this technology.
The automotive sector is also exploring applications for flexible magnesium batteries in electric and hybrid vehicles, particularly for supplementary systems and sensors that benefit from form-factor flexibility. This market segment is projected to grow at 25% annually as vehicle electrification accelerates globally.
Regional analysis reveals Asia-Pacific as the dominant market for flexible battery technologies, accounting for approximately 45% of global demand, with China, South Korea, and Japan leading in both production and consumption. North America follows with 30% market share, driven primarily by medical and military applications.
Key market barriers include cost considerations, with current flexible magnesium battery solutions commanding a 40-60% premium over conventional rigid batteries. Manufacturing scalability remains challenging, with production volumes still limited to specialized applications rather than mass market deployment.
Consumer awareness and acceptance represent additional market challenges, as end-users remain largely unfamiliar with magnesium battery technology compared to well-established lithium-ion solutions. However, increasing environmental concerns and sustainability initiatives are creating favorable market conditions for magnesium-based alternatives, which offer reduced environmental impact and improved recyclability compared to lithium-ion technologies.
The healthcare sector represents one of the most promising application areas, with flexible magnesium batteries being integrated into medical wearables, remote patient monitoring systems, and implantable medical devices. This segment is expected to grow at 35% annually through 2027, driven by increasing healthcare digitalization and remote monitoring adoption.
Consumer electronics constitutes another major market segment, with flexible displays, smart clothing, and foldable devices creating substantial demand for conformable power sources. Major electronics manufacturers including Samsung, LG, and Apple have filed patents related to flexible battery integration, signaling strong industry interest in this technology.
The automotive sector is also exploring applications for flexible magnesium batteries in electric and hybrid vehicles, particularly for supplementary systems and sensors that benefit from form-factor flexibility. This market segment is projected to grow at 25% annually as vehicle electrification accelerates globally.
Regional analysis reveals Asia-Pacific as the dominant market for flexible battery technologies, accounting for approximately 45% of global demand, with China, South Korea, and Japan leading in both production and consumption. North America follows with 30% market share, driven primarily by medical and military applications.
Key market barriers include cost considerations, with current flexible magnesium battery solutions commanding a 40-60% premium over conventional rigid batteries. Manufacturing scalability remains challenging, with production volumes still limited to specialized applications rather than mass market deployment.
Consumer awareness and acceptance represent additional market challenges, as end-users remain largely unfamiliar with magnesium battery technology compared to well-established lithium-ion solutions. However, increasing environmental concerns and sustainability initiatives are creating favorable market conditions for magnesium-based alternatives, which offer reduced environmental impact and improved recyclability compared to lithium-ion technologies.
Current Status and Challenges in Polymer Gel Electrolytes
Polymer gel electrolytes (PGEs) have emerged as promising candidates for flexible magnesium batteries due to their unique combination of mechanical flexibility and ionic conductivity. Currently, the development of PGEs for Mg batteries is at an early but rapidly evolving stage, with significant research efforts focused on overcoming the inherent challenges of magnesium electrochemistry.
The global research landscape shows that most advanced work on PGEs for Mg batteries is concentrated in research institutions across North America, Europe, and East Asia, with China, Japan, and the United States leading publication output. Recent technological breakthroughs have primarily focused on enhancing ionic conductivity and electrochemical stability, with several research groups reporting conductivities approaching 10^-3 S/cm at room temperature—a significant improvement over earlier generations.
Despite these advances, PGEs for Mg batteries face several critical challenges. The primary limitation remains the relatively low ionic conductivity compared to liquid electrolytes, typically 1-2 orders of magnitude lower. This conductivity gap significantly impacts battery performance, particularly at high discharge rates. The issue stems from the inherent trade-off between mechanical properties and ionic mobility within polymer matrices.
Another major challenge is the narrow electrochemical stability window of most current PGEs, which limits the operating voltage of Mg batteries and consequently their energy density. Many polymer systems suffer from degradation when exposed to the highly reducing magnesium metal anode or at high operating voltages, leading to capacity fading and shortened cycle life.
Interface compatibility presents another significant hurdle. The formation of passivation layers at the electrolyte-electrode interfaces, particularly with the Mg metal anode, increases interfacial resistance and hinders Mg^2+ transport. This phenomenon is exacerbated by the divalent nature of magnesium ions, which form stronger coordination bonds with polymer chains compared to monovalent ions like lithium.
Long-term stability remains problematic as well. Many PGEs exhibit performance degradation over extended cycling, with issues including polymer chain reorganization, phase separation in gel systems, and gradual loss of plasticizers or solvents. These factors collectively contribute to declining ionic conductivity and mechanical integrity over time.
Manufacturing scalability represents a practical challenge for commercialization. Current laboratory-scale preparation methods for high-performance PGEs often involve complex synthesis procedures that are difficult to scale up cost-effectively. The reproducibility of properties across large-area films and the integration of these electrolytes into practical cell designs remain significant engineering challenges.
The global research landscape shows that most advanced work on PGEs for Mg batteries is concentrated in research institutions across North America, Europe, and East Asia, with China, Japan, and the United States leading publication output. Recent technological breakthroughs have primarily focused on enhancing ionic conductivity and electrochemical stability, with several research groups reporting conductivities approaching 10^-3 S/cm at room temperature—a significant improvement over earlier generations.
Despite these advances, PGEs for Mg batteries face several critical challenges. The primary limitation remains the relatively low ionic conductivity compared to liquid electrolytes, typically 1-2 orders of magnitude lower. This conductivity gap significantly impacts battery performance, particularly at high discharge rates. The issue stems from the inherent trade-off between mechanical properties and ionic mobility within polymer matrices.
Another major challenge is the narrow electrochemical stability window of most current PGEs, which limits the operating voltage of Mg batteries and consequently their energy density. Many polymer systems suffer from degradation when exposed to the highly reducing magnesium metal anode or at high operating voltages, leading to capacity fading and shortened cycle life.
Interface compatibility presents another significant hurdle. The formation of passivation layers at the electrolyte-electrode interfaces, particularly with the Mg metal anode, increases interfacial resistance and hinders Mg^2+ transport. This phenomenon is exacerbated by the divalent nature of magnesium ions, which form stronger coordination bonds with polymer chains compared to monovalent ions like lithium.
Long-term stability remains problematic as well. Many PGEs exhibit performance degradation over extended cycling, with issues including polymer chain reorganization, phase separation in gel systems, and gradual loss of plasticizers or solvents. These factors collectively contribute to declining ionic conductivity and mechanical integrity over time.
Manufacturing scalability represents a practical challenge for commercialization. Current laboratory-scale preparation methods for high-performance PGEs often involve complex synthesis procedures that are difficult to scale up cost-effectively. The reproducibility of properties across large-area films and the integration of these electrolytes into practical cell designs remain significant engineering challenges.
Existing Polymer Gel Electrolyte Solutions for Mg Batteries
01 Polymer gel electrolytes with enhanced flexibility
Polymer gel electrolytes can be formulated to enhance flexibility by incorporating specific polymers and plasticizers. These flexible electrolytes maintain ionic conductivity while allowing for bending and folding, making them suitable for flexible electronic devices. The flexibility is achieved through the optimization of polymer chain mobility and cross-linking density, resulting in a gel network that can withstand mechanical deformation without compromising electrochemical performance.- Polymer gel electrolytes with enhanced mechanical flexibility: Polymer gel electrolytes can be formulated to enhance mechanical flexibility while maintaining ionic conductivity. These electrolytes typically incorporate flexible polymer matrices that can withstand bending and folding without cracking or delamination. The flexibility is achieved through the selection of specific polymer backbones, plasticizers, and cross-linking agents that create a supple network structure while maintaining the electrolyte's functional properties.
- Cross-linked polymer networks for improved flexibility and stability: Cross-linked polymer networks in gel electrolytes provide a balance between mechanical flexibility and dimensional stability. By controlling the degree of cross-linking, the electrolyte can maintain its shape while still allowing for flexibility. These networks often incorporate both chemical and physical cross-links that create a semi-interpenetrating structure, enabling the electrolyte to flex without permanent deformation while preventing excessive swelling or leakage of liquid components.
- Plasticizers and additives for flexibility enhancement: Various plasticizers and additives can be incorporated into polymer gel electrolytes to enhance their flexibility. These components reduce the glass transition temperature of the polymer matrix, increasing chain mobility and improving mechanical properties. Common additives include low molecular weight polyethylene glycol, propylene carbonate, and ionic liquids, which not only improve flexibility but can also enhance ionic conductivity by creating more amorphous regions within the polymer structure.
- Composite and nanocomposite gel electrolytes for flexible applications: Composite and nanocomposite gel electrolytes incorporate inorganic fillers or nanoparticles into the polymer matrix to achieve enhanced flexibility and mechanical strength simultaneously. These fillers, such as silica, alumina, or ceramic nanoparticles, can create additional cross-linking points within the polymer network while maintaining flexibility. The resulting nanocomposite structure provides improved dimensional stability and mechanical properties without sacrificing the flexibility needed for applications in flexible electronic devices.
- Flexible polymer gel electrolytes for bendable energy storage devices: Specialized polymer gel electrolytes have been developed specifically for bendable and flexible energy storage devices such as batteries and supercapacitors. These electrolytes maintain their electrochemical performance even under mechanical deformation, enabling the development of wearable and flexible electronics. The formulations typically balance ionic conductivity, electrochemical stability, and mechanical flexibility through careful selection of polymer types, molecular weights, and additives that preserve interfacial contact with electrodes during bending.
02 Cross-linked polymer networks for mechanical stability
Cross-linked polymer networks in gel electrolytes provide improved mechanical stability while maintaining flexibility. These networks create a three-dimensional structure that prevents leakage and enhances the dimensional stability of the electrolyte. By controlling the degree of cross-linking, the balance between flexibility and mechanical strength can be optimized. This approach allows for the development of gel electrolytes that can withstand mechanical stress while providing efficient ion transport.Expand Specific Solutions03 Composite gel electrolytes with inorganic fillers
Incorporating inorganic fillers into polymer gel electrolytes can enhance both flexibility and mechanical properties. These composite gel electrolytes combine the advantages of organic polymers and inorganic materials, resulting in improved electrochemical performance and physical durability. The inorganic fillers can include nanoparticles, ceramic materials, or other additives that interact with the polymer matrix to create a flexible yet robust electrolyte system suitable for various applications in energy storage devices.Expand Specific Solutions04 Temperature-resistant flexible gel electrolytes
Developing polymer gel electrolytes with temperature resistance while maintaining flexibility involves selecting specific polymer compositions and additives. These electrolytes can operate across a wide temperature range without losing their mechanical properties or ionic conductivity. By incorporating temperature-stabilizing components and optimizing the gel network structure, these electrolytes can withstand thermal cycling and extreme conditions while remaining flexible, making them suitable for applications in harsh environments.Expand Specific Solutions05 Novel polymer blends for high-performance flexible electrolytes
Novel polymer blends can be used to create high-performance flexible gel electrolytes with enhanced ionic conductivity and mechanical properties. These blends combine different polymers with complementary characteristics to achieve synergistic effects. By carefully selecting polymer combinations and optimizing their ratios, electrolytes with superior flexibility, conductivity, and electrochemical stability can be developed. These advanced polymer blends enable the fabrication of next-generation flexible energy storage devices with improved performance and durability.Expand Specific Solutions
Leading Companies and Research Institutions in Mg Battery Field
The polymer gel electrolyte market for flexible magnesium batteries is in an early growth stage, characterized by intensive R&D activities rather than mass commercialization. The global market size remains relatively modest but is projected to expand significantly as magnesium battery technology matures. Currently, the technology demonstrates medium maturity, with key players driving innovation across different sectors. Academic institutions like Ulsan National Institute of Science & Technology, Shanghai Jiao Tong University, and University of Maryland are leading fundamental research, while industrial giants including Samsung SDI, Toyota Motor Corp., and Murata Manufacturing are focusing on practical applications and scalability. Collaboration between research institutions and corporations is accelerating development, with Asian companies particularly active in patenting and prototype development for next-generation flexible energy storage solutions.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed advanced polymer gel electrolytes (PGEs) for flexible magnesium batteries featuring a cross-linked network structure of poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) with magnesium salts. Their proprietary formulation incorporates ionic liquids to enhance ionic conductivity while maintaining mechanical flexibility. The company utilizes a phase inversion technique to create microporous structures that facilitate ion transport while providing structural integrity. Their PGEs demonstrate ionic conductivities of 10^-3 to 10^-4 S/cm at room temperature[1], with excellent electrochemical stability windows exceeding 3.5V vs. Mg/Mg2+. Samsung's technology also features self-healing properties through dynamic cross-linking mechanisms, allowing the electrolyte to recover from mechanical damage during battery flexing operations.
Strengths: Superior ionic conductivity compared to solid polymer electrolytes; excellent mechanical flexibility allowing for various form factors; enhanced safety with reduced leakage risk compared to liquid electrolytes. Weaknesses: Higher manufacturing complexity than conventional liquid electrolytes; potential long-term stability issues under repeated mechanical deformation; relatively higher cost of production compared to traditional electrolyte systems.
Ulsan National Institute of Science & Technology
Technical Solution: UNIST has pioneered a novel approach to polymer gel electrolytes for flexible Mg batteries using a biomimetic design inspired by natural hydrogels. Their technology incorporates a dual-network structure combining synthetic polymers (polyethylene oxide/PMMA blends) with natural polysaccharides to create mechanically robust yet highly flexible electrolyte matrices. The institute's researchers have developed a proprietary plasticizer system that effectively dissolves magnesium salts while maintaining compatibility with the polymer network. Their PGEs demonstrate remarkable stretchability (up to 300% elongation)[2] while maintaining ionic conductivity around 5×10^-4 S/cm at ambient temperature. UNIST's approach also addresses the critical challenge of interfacial stability between the electrolyte and magnesium metal anode through surface modification techniques, significantly reducing interfacial resistance and enabling stable cycling performance even under mechanical deformation.
Strengths: Exceptional mechanical properties including stretchability and flexibility; bio-inspired design offers potential sustainability advantages; excellent interfacial compatibility with magnesium metal anodes. Weaknesses: Complex synthesis procedures may limit large-scale manufacturing; potential degradation of natural polymer components over extended cycling; lower ionic conductivity at sub-ambient temperatures compared to some competing technologies.
Key Patents and Research Breakthroughs in Gel Electrolytes
Mechanically flexible magnesium-ion battery electrodes in a polymer gel perchlorate electrolyte
PatentActiveUS10938022B2
Innovation
- The development of mechanically flexible magnesium-ion batteries using a solid polymer-based anode and cathode, combined with a polymer gel electrolyte containing bismuth nanostructure powder, tungsten disulfide, and specific electrolyte binders such as polyvinylidene fluoride-co-hexafluoropropylene and magnesium perchlorate, which reduce unwanted redox reactions and enhance ionic conductivity.
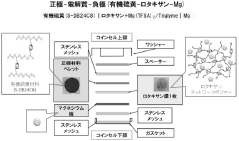
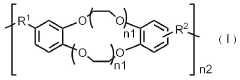
Polymer electrolyte containing rotaxane network polymer and magnesium secondary battery using the same
PatentActiveJP2016162543A
Innovation
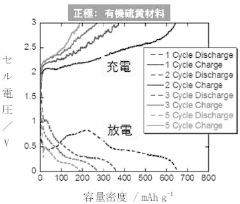
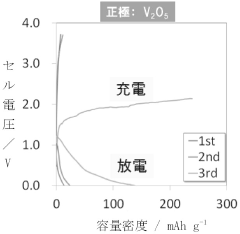
- A polymer gel electrolyte containing a rotaxane network polymer and a magnesium salt solution is used, enabling repeated dissolution and deposition of magnesium ions at room temperature, with an organic sulfur material as the positive electrode for enhanced performance.
Safety and Performance Standards for Flexible Battery Technologies
The development of flexible magnesium batteries with polymer gel electrolytes necessitates robust safety and performance standards to ensure market viability and consumer protection. Currently, the standards landscape for flexible battery technologies remains fragmented, with most regulations derived from conventional rigid battery frameworks such as IEC 62133 and UL 1642, which inadequately address the unique characteristics of flexible power sources.
Safety standards for flexible magnesium batteries must specifically address mechanical deformation scenarios. Unlike traditional batteries, these flexible systems must maintain electrochemical stability and safety while undergoing repeated bending, twisting, and folding operations. Testing protocols should include cyclic deformation tests at various temperatures and humidity levels to simulate real-world usage conditions.
Thermal runaway prevention represents another critical safety consideration. Standards must establish maximum operating temperature thresholds and thermal management requirements specific to polymer gel electrolyte compositions used in magnesium batteries. The interaction between polymer matrices and magnesium ions creates unique thermal behaviors that conventional battery standards fail to address adequately.
Performance standardization for flexible magnesium batteries should encompass energy density metrics that account for the total system weight including flexible substrates and encapsulation materials. Current industry benchmarks target energy densities exceeding 150 Wh/kg and 300 Wh/L for flexible battery systems, with cycle stability requirements of >1000 cycles at 80% capacity retention.
Accelerated aging protocols represent another essential component of performance standards. These should simulate environmental conditions including temperature fluctuations, humidity exposure, and mechanical stress to predict long-term performance degradation. The polymer gel electrolyte's stability under these conditions requires specialized testing methodologies beyond those used for liquid or solid-state systems.
Interoperability standards must also be established to ensure flexible magnesium batteries can interface with existing electronic systems. This includes standardized connection mechanisms that maintain integrity during flexing operations and communication protocols for battery management systems adapted to the unique charging/discharging characteristics of magnesium-based chemistries.
Emerging international efforts, particularly through organizations like IEC TC 21 and IEEE, are beginning to develop flexible battery-specific standards. However, industry consortia comprising battery manufacturers, materials suppliers, and device integrators must collaborate to accelerate standards development that addresses the specific safety and performance requirements of polymer gel electrolyte-based flexible magnesium battery technologies.
Safety standards for flexible magnesium batteries must specifically address mechanical deformation scenarios. Unlike traditional batteries, these flexible systems must maintain electrochemical stability and safety while undergoing repeated bending, twisting, and folding operations. Testing protocols should include cyclic deformation tests at various temperatures and humidity levels to simulate real-world usage conditions.
Thermal runaway prevention represents another critical safety consideration. Standards must establish maximum operating temperature thresholds and thermal management requirements specific to polymer gel electrolyte compositions used in magnesium batteries. The interaction between polymer matrices and magnesium ions creates unique thermal behaviors that conventional battery standards fail to address adequately.
Performance standardization for flexible magnesium batteries should encompass energy density metrics that account for the total system weight including flexible substrates and encapsulation materials. Current industry benchmarks target energy densities exceeding 150 Wh/kg and 300 Wh/L for flexible battery systems, with cycle stability requirements of >1000 cycles at 80% capacity retention.
Accelerated aging protocols represent another essential component of performance standards. These should simulate environmental conditions including temperature fluctuations, humidity exposure, and mechanical stress to predict long-term performance degradation. The polymer gel electrolyte's stability under these conditions requires specialized testing methodologies beyond those used for liquid or solid-state systems.
Interoperability standards must also be established to ensure flexible magnesium batteries can interface with existing electronic systems. This includes standardized connection mechanisms that maintain integrity during flexing operations and communication protocols for battery management systems adapted to the unique charging/discharging characteristics of magnesium-based chemistries.
Emerging international efforts, particularly through organizations like IEC TC 21 and IEEE, are beginning to develop flexible battery-specific standards. However, industry consortia comprising battery manufacturers, materials suppliers, and device integrators must collaborate to accelerate standards development that addresses the specific safety and performance requirements of polymer gel electrolyte-based flexible magnesium battery technologies.
Environmental Impact and Sustainability Considerations
The environmental impact of polymer gel electrolytes for flexible magnesium batteries represents a critical consideration in their development and deployment. Unlike conventional liquid electrolytes that often contain volatile and environmentally harmful organic solvents, polymer gel electrolytes typically incorporate more environmentally benign components. The reduced toxicity profile of many polymer gel systems offers significant advantages from an ecological perspective, particularly regarding end-of-life disposal and potential leakage scenarios.
Manufacturing processes for polymer gel electrolytes generally require lower energy inputs compared to ceramic solid-state alternatives, resulting in reduced carbon footprints during production. Additionally, the elimination of toxic and flammable liquid electrolytes enhances safety profiles while simultaneously reducing environmental hazards associated with battery production, use, and disposal. This alignment with green chemistry principles positions polymer gel electrolytes as promising candidates for sustainable energy storage technologies.
The recyclability of polymer gel electrolyte components presents both opportunities and challenges. While some polymer matrices can be recovered and reprocessed, the composite nature of these electrolytes—often containing various additives, salts, and plasticizers—complicates separation processes. Research into designing polymer gel electrolytes with end-of-life considerations is gaining momentum, with particular focus on biodegradable polymers and environmentally friendly plasticizers that maintain performance while reducing ecological impact.
Life cycle assessment (LCA) studies comparing polymer gel electrolytes to conventional liquid systems in magnesium batteries demonstrate potential environmental benefits, particularly in categories of resource depletion, ecotoxicity, and global warming potential. However, these advantages depend heavily on specific formulations and manufacturing processes. The water footprint of polymer gel electrolyte production generally compares favorably to alternative technologies, though regional water stress factors must be considered in sustainability evaluations.
Regulatory frameworks increasingly emphasize battery sustainability, with initiatives like the European Battery Directive and similar regulations in Asia and North America driving innovation toward greener electrolyte technologies. Compliance with these evolving standards requires continuous improvement in the environmental performance of polymer gel electrolytes, creating both market pressures and opportunities for differentiation through sustainability credentials.
The potential for polymer gel electrolytes to enable longer-lasting, more durable flexible magnesium batteries also contributes to sustainability through extended product lifecycles and reduced waste generation. This longevity factor, combined with the inherent safety improvements, positions polymer gel electrolyte technology as an important contributor to circular economy objectives in the energy storage sector.
Manufacturing processes for polymer gel electrolytes generally require lower energy inputs compared to ceramic solid-state alternatives, resulting in reduced carbon footprints during production. Additionally, the elimination of toxic and flammable liquid electrolytes enhances safety profiles while simultaneously reducing environmental hazards associated with battery production, use, and disposal. This alignment with green chemistry principles positions polymer gel electrolytes as promising candidates for sustainable energy storage technologies.
The recyclability of polymer gel electrolyte components presents both opportunities and challenges. While some polymer matrices can be recovered and reprocessed, the composite nature of these electrolytes—often containing various additives, salts, and plasticizers—complicates separation processes. Research into designing polymer gel electrolytes with end-of-life considerations is gaining momentum, with particular focus on biodegradable polymers and environmentally friendly plasticizers that maintain performance while reducing ecological impact.
Life cycle assessment (LCA) studies comparing polymer gel electrolytes to conventional liquid systems in magnesium batteries demonstrate potential environmental benefits, particularly in categories of resource depletion, ecotoxicity, and global warming potential. However, these advantages depend heavily on specific formulations and manufacturing processes. The water footprint of polymer gel electrolyte production generally compares favorably to alternative technologies, though regional water stress factors must be considered in sustainability evaluations.
Regulatory frameworks increasingly emphasize battery sustainability, with initiatives like the European Battery Directive and similar regulations in Asia and North America driving innovation toward greener electrolyte technologies. Compliance with these evolving standards requires continuous improvement in the environmental performance of polymer gel electrolytes, creating both market pressures and opportunities for differentiation through sustainability credentials.
The potential for polymer gel electrolytes to enable longer-lasting, more durable flexible magnesium batteries also contributes to sustainability through extended product lifecycles and reduced waste generation. This longevity factor, combined with the inherent safety improvements, positions polymer gel electrolyte technology as an important contributor to circular economy objectives in the energy storage sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!