Reactivity of Ethyl Propanoate in Base-Activated Polymerization
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
Base-activated polymerization of ethyl propanoate represents a significant area of research in polymer chemistry, with implications for various industrial applications. This polymerization process has evolved over several decades, driven by the need for more efficient and controllable methods of producing polymers with specific properties.
The reactivity of ethyl propanoate in base-activated polymerization is influenced by several factors, including the nature of the base catalyst, reaction conditions, and the structure of the monomer itself. Understanding these factors and their interplay is crucial for optimizing the polymerization process and tailoring the resulting polymer properties.
Historically, the study of ethyl propanoate polymerization began in the mid-20th century as part of broader investigations into ester polymerization. Early research focused on identifying suitable catalysts and reaction conditions for initiating and controlling the polymerization process. As the field progressed, researchers began to explore the mechanistic aspects of the reaction, seeking to elucidate the precise pathways by which ethyl propanoate molecules combine to form polymer chains.
The development of more sophisticated analytical techniques in recent decades has allowed for deeper insights into the kinetics and thermodynamics of the polymerization reaction. This has led to a better understanding of how factors such as temperature, pressure, and catalyst concentration affect the rate and extent of polymerization, as well as the molecular weight distribution of the resulting polymers.
One of the key objectives in studying the reactivity of ethyl propanoate in base-activated polymerization is to achieve greater control over the polymer architecture. This includes the ability to produce polymers with specific molecular weights, narrow polydispersity indices, and desired end-group functionalities. Such control is essential for tailoring polymers to meet the requirements of various applications, from packaging materials to biomedical devices.
Another important goal is to improve the efficiency and sustainability of the polymerization process. This involves developing catalysts that are more active and selective, reducing the energy requirements of the reaction, and exploring the use of renewable feedstocks as alternatives to petroleum-based monomers.
Recent trends in the field have focused on the development of "living" polymerization techniques, which allow for precise control over polymer growth and the synthesis of complex architectures such as block copolymers. Additionally, there is growing interest in the use of organocatalysts and metal-free polymerization systems, driven by environmental concerns and the desire for more sustainable chemical processes.
As research in this area continues to advance, the ultimate aim is to develop a comprehensive understanding of the reactivity of ethyl propanoate in base-activated polymerization. This knowledge will enable the design of more efficient and versatile polymerization processes, leading to the production of advanced materials with tailored properties for a wide range of applications.
The reactivity of ethyl propanoate in base-activated polymerization is influenced by several factors, including the nature of the base catalyst, reaction conditions, and the structure of the monomer itself. Understanding these factors and their interplay is crucial for optimizing the polymerization process and tailoring the resulting polymer properties.
Historically, the study of ethyl propanoate polymerization began in the mid-20th century as part of broader investigations into ester polymerization. Early research focused on identifying suitable catalysts and reaction conditions for initiating and controlling the polymerization process. As the field progressed, researchers began to explore the mechanistic aspects of the reaction, seeking to elucidate the precise pathways by which ethyl propanoate molecules combine to form polymer chains.
The development of more sophisticated analytical techniques in recent decades has allowed for deeper insights into the kinetics and thermodynamics of the polymerization reaction. This has led to a better understanding of how factors such as temperature, pressure, and catalyst concentration affect the rate and extent of polymerization, as well as the molecular weight distribution of the resulting polymers.
One of the key objectives in studying the reactivity of ethyl propanoate in base-activated polymerization is to achieve greater control over the polymer architecture. This includes the ability to produce polymers with specific molecular weights, narrow polydispersity indices, and desired end-group functionalities. Such control is essential for tailoring polymers to meet the requirements of various applications, from packaging materials to biomedical devices.
Another important goal is to improve the efficiency and sustainability of the polymerization process. This involves developing catalysts that are more active and selective, reducing the energy requirements of the reaction, and exploring the use of renewable feedstocks as alternatives to petroleum-based monomers.
Recent trends in the field have focused on the development of "living" polymerization techniques, which allow for precise control over polymer growth and the synthesis of complex architectures such as block copolymers. Additionally, there is growing interest in the use of organocatalysts and metal-free polymerization systems, driven by environmental concerns and the desire for more sustainable chemical processes.
As research in this area continues to advance, the ultimate aim is to develop a comprehensive understanding of the reactivity of ethyl propanoate in base-activated polymerization. This knowledge will enable the design of more efficient and versatile polymerization processes, leading to the production of advanced materials with tailored properties for a wide range of applications.
Market Analysis
The market for base-activated polymerization of ethyl propanoate is experiencing significant growth, driven by the increasing demand for sustainable and biodegradable polymers across various industries. This polymerization process offers a promising route to produce polymers with unique properties, making it attractive for applications in packaging, biomedical devices, and specialty materials.
In the packaging industry, there is a growing need for environmentally friendly alternatives to traditional petroleum-based plastics. Polymers derived from ethyl propanoate through base-activated polymerization have shown potential as biodegradable packaging materials, aligning with the global push towards sustainable packaging solutions. This market segment is expected to witness substantial growth as consumers and regulatory bodies increasingly prioritize eco-friendly packaging options.
The biomedical sector presents another significant market opportunity for polymers produced through this process. These materials exhibit biocompatibility and controlled degradation properties, making them suitable for drug delivery systems, tissue engineering scaffolds, and other medical applications. As the healthcare industry continues to advance, the demand for such specialized polymers is projected to increase, driving further research and development in this area.
In the specialty materials market, polymers derived from ethyl propanoate are gaining attention due to their unique chemical and physical properties. These materials find applications in coatings, adhesives, and functional textiles, where their specific characteristics can provide enhanced performance compared to conventional polymers. The growing emphasis on high-performance materials in industries such as automotive, aerospace, and electronics is expected to fuel the demand for these specialized polymers.
The market for base-activated polymerization of ethyl propanoate is also influenced by the broader trend towards circular economy and sustainable chemistry. As industries seek to reduce their environmental footprint and comply with stricter regulations, there is an increasing interest in bio-based and recyclable materials. This trend is likely to create new opportunities for polymers derived from renewable resources like ethyl propanoate.
However, the market faces challenges in terms of production scale-up and cost competitiveness compared to well-established petroleum-based polymers. Overcoming these hurdles will be crucial for widespread adoption across different industries. Additionally, the market's growth is dependent on continued research and development efforts to optimize the polymerization process and expand the range of achievable polymer properties.
Overall, the market for base-activated polymerization of ethyl propanoate shows promising growth potential, driven by the increasing demand for sustainable materials across multiple sectors. As research progresses and production technologies improve, this market is expected to expand, offering innovative solutions to meet evolving industry needs and environmental requirements.
In the packaging industry, there is a growing need for environmentally friendly alternatives to traditional petroleum-based plastics. Polymers derived from ethyl propanoate through base-activated polymerization have shown potential as biodegradable packaging materials, aligning with the global push towards sustainable packaging solutions. This market segment is expected to witness substantial growth as consumers and regulatory bodies increasingly prioritize eco-friendly packaging options.
The biomedical sector presents another significant market opportunity for polymers produced through this process. These materials exhibit biocompatibility and controlled degradation properties, making them suitable for drug delivery systems, tissue engineering scaffolds, and other medical applications. As the healthcare industry continues to advance, the demand for such specialized polymers is projected to increase, driving further research and development in this area.
In the specialty materials market, polymers derived from ethyl propanoate are gaining attention due to their unique chemical and physical properties. These materials find applications in coatings, adhesives, and functional textiles, where their specific characteristics can provide enhanced performance compared to conventional polymers. The growing emphasis on high-performance materials in industries such as automotive, aerospace, and electronics is expected to fuel the demand for these specialized polymers.
The market for base-activated polymerization of ethyl propanoate is also influenced by the broader trend towards circular economy and sustainable chemistry. As industries seek to reduce their environmental footprint and comply with stricter regulations, there is an increasing interest in bio-based and recyclable materials. This trend is likely to create new opportunities for polymers derived from renewable resources like ethyl propanoate.
However, the market faces challenges in terms of production scale-up and cost competitiveness compared to well-established petroleum-based polymers. Overcoming these hurdles will be crucial for widespread adoption across different industries. Additionally, the market's growth is dependent on continued research and development efforts to optimize the polymerization process and expand the range of achievable polymer properties.
Overall, the market for base-activated polymerization of ethyl propanoate shows promising growth potential, driven by the increasing demand for sustainable materials across multiple sectors. As research progresses and production technologies improve, this market is expected to expand, offering innovative solutions to meet evolving industry needs and environmental requirements.
Technical Challenges
The base-activated polymerization of ethyl propanoate presents several technical challenges that researchers and industry professionals must address. One of the primary obstacles is controlling the reaction kinetics and achieving desired molecular weight distributions. The base-catalyzed polymerization tends to proceed rapidly, making it difficult to regulate the chain growth and prevent premature termination or excessive branching.
Another significant challenge lies in managing the side reactions that can occur during the polymerization process. The presence of a strong base can lead to undesired transesterification reactions, potentially altering the polymer structure and properties. Additionally, the base may catalyze hydrolysis of the ester groups, resulting in the formation of carboxylic acid moieties that can interfere with the polymerization mechanism.
The choice of an appropriate base catalyst presents its own set of challenges. While stronger bases generally promote faster reaction rates, they can also increase the likelihood of side reactions and reduce control over the polymerization process. Conversely, weaker bases may provide better control but at the cost of reduced reaction efficiency. Striking the right balance between reactivity and selectivity remains a key challenge in optimizing this polymerization system.
Temperature control during the polymerization process is crucial yet challenging. The exothermic nature of the reaction can lead to localized hot spots, potentially causing degradation of the polymer or loss of control over the molecular weight distribution. Efficient heat dissipation and uniform temperature distribution throughout the reaction mixture are essential for maintaining consistent product quality.
Solvent selection poses another technical hurdle. The solvent must be compatible with both the base catalyst and the growing polymer chains while also facilitating efficient mixing and heat transfer. Finding a solvent system that meets all these criteria without adversely affecting the polymerization kinetics or final product properties can be challenging.
The removal of residual base catalyst and any byproducts from the final polymer product presents additional technical difficulties. Incomplete removal can lead to stability issues or undesired post-polymerization reactions, while excessive purification steps may impact the economic viability of the process.
Scaling up the polymerization process from laboratory to industrial scale introduces further challenges. Maintaining uniform mixing, temperature control, and reaction kinetics in larger reactors requires careful engineering and process optimization. The increased reaction volumes can exacerbate heat transfer issues and potentially lead to greater variability in product quality.
Finally, achieving consistent and reproducible results across different batches remains a significant challenge. Variations in raw material quality, environmental conditions, and subtle differences in processing parameters can all impact the final polymer properties. Developing robust process control strategies and quality assurance protocols is essential for overcoming these challenges and ensuring reliable production of high-quality polymers from ethyl propanoate via base-activated polymerization.
Another significant challenge lies in managing the side reactions that can occur during the polymerization process. The presence of a strong base can lead to undesired transesterification reactions, potentially altering the polymer structure and properties. Additionally, the base may catalyze hydrolysis of the ester groups, resulting in the formation of carboxylic acid moieties that can interfere with the polymerization mechanism.
The choice of an appropriate base catalyst presents its own set of challenges. While stronger bases generally promote faster reaction rates, they can also increase the likelihood of side reactions and reduce control over the polymerization process. Conversely, weaker bases may provide better control but at the cost of reduced reaction efficiency. Striking the right balance between reactivity and selectivity remains a key challenge in optimizing this polymerization system.
Temperature control during the polymerization process is crucial yet challenging. The exothermic nature of the reaction can lead to localized hot spots, potentially causing degradation of the polymer or loss of control over the molecular weight distribution. Efficient heat dissipation and uniform temperature distribution throughout the reaction mixture are essential for maintaining consistent product quality.
Solvent selection poses another technical hurdle. The solvent must be compatible with both the base catalyst and the growing polymer chains while also facilitating efficient mixing and heat transfer. Finding a solvent system that meets all these criteria without adversely affecting the polymerization kinetics or final product properties can be challenging.
The removal of residual base catalyst and any byproducts from the final polymer product presents additional technical difficulties. Incomplete removal can lead to stability issues or undesired post-polymerization reactions, while excessive purification steps may impact the economic viability of the process.
Scaling up the polymerization process from laboratory to industrial scale introduces further challenges. Maintaining uniform mixing, temperature control, and reaction kinetics in larger reactors requires careful engineering and process optimization. The increased reaction volumes can exacerbate heat transfer issues and potentially lead to greater variability in product quality.
Finally, achieving consistent and reproducible results across different batches remains a significant challenge. Variations in raw material quality, environmental conditions, and subtle differences in processing parameters can all impact the final polymer properties. Developing robust process control strategies and quality assurance protocols is essential for overcoming these challenges and ensuring reliable production of high-quality polymers from ethyl propanoate via base-activated polymerization.
Current Methodologies
01 Synthesis and preparation methods
Various methods for synthesizing ethyl propanoate are described, including esterification reactions and catalytic processes. These methods often involve the reaction of propionic acid with ethanol or the use of specific catalysts to improve yield and efficiency.- Synthesis and production methods: Various methods for synthesizing and producing ethyl propanoate are described, including esterification reactions, catalytic processes, and continuous production techniques. These methods aim to improve yield, efficiency, and purity of the final product.
- Applications in chemical industry: Ethyl propanoate finds applications in various chemical processes, including as a solvent, intermediate, or reagent in organic synthesis. Its reactivity is utilized in the production of pharmaceuticals, fragrances, and other specialty chemicals.
- Reactivity in polymer chemistry: The reactivity of ethyl propanoate is explored in polymer chemistry, particularly in the synthesis and modification of various polymers and copolymers. Its ester group can participate in polymerization reactions or be used for polymer functionalization.
- Catalytic transformations: Research focuses on catalytic transformations involving ethyl propanoate, including hydrogenation, decarboxylation, and transesterification reactions. Various catalysts and reaction conditions are investigated to control selectivity and improve yields.
- Environmental and safety considerations: Studies address the environmental impact and safety aspects of ethyl propanoate, including its biodegradability, toxicity, and potential as a green solvent. Research also focuses on developing safer handling and storage methods for this reactive compound.
02 Applications in chemical industry
Ethyl propanoate finds applications in various chemical processes, including as a solvent, flavoring agent, and intermediate in the production of other chemicals. Its reactivity is utilized in the synthesis of more complex compounds and in industrial processes.Expand Specific Solutions03 Reactivity in polymer chemistry
The reactivity of ethyl propanoate is explored in polymer chemistry, where it can be used as a monomer or reagent in polymerization reactions. Its ester group allows for various modifications and incorporations into polymer structures.Expand Specific Solutions04 Catalytic reactions and transformations
Studies on catalytic reactions involving ethyl propanoate, including hydrogenation, decarboxylation, and transesterification, are reported. These reactions demonstrate the compound's versatility in organic synthesis and its potential for producing value-added chemicals.Expand Specific Solutions05 Environmental and safety considerations
Research on the environmental impact and safety aspects of ethyl propanoate use is conducted. This includes studies on biodegradability, toxicity, and potential hazards associated with its reactivity, as well as the development of safer handling and disposal methods.Expand Specific Solutions
Industry Leaders
The competitive landscape for the reactivity of ethyl propanoate in base-activated polymerization is characterized by a mature market with established players and ongoing research. The market size is substantial, driven by applications in various industries including pharmaceuticals, chemicals, and materials science. Key players like BASF SE, Dow Global Technologies, and ExxonMobil Chemical Patents demonstrate the technology's commercial viability. Academic institutions such as MIT and the University of Pittsburgh, along with government research labs like the Naval Research Laboratory, contribute to advancing the fundamental understanding and potential applications of this technology. The involvement of diverse companies across chemical, pharmaceutical, and materials sectors indicates a broad range of industrial applications and ongoing innovation in this field.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has pioneered a sustainable approach to the base-activated polymerization of ethyl propanoate, focusing on green chemistry principles. Their innovative process utilizes bio-based catalysts derived from renewable resources, reducing the environmental impact of polymer production[2]. The company has developed a unique reactor design that enhances mass transfer and heat management during the polymerization reaction, resulting in improved conversion rates and product quality[4]. Additionally, Dow has implemented a solvent recycling system that significantly reduces waste and improves the overall efficiency of the polymerization process[6]. This eco-friendly approach allows for the production of high-quality polymers while minimizing the carbon footprint.
Strengths: Environmentally friendly process, improved sustainability, and potential cost savings through efficient resource use. Weaknesses: May face challenges in scaling up bio-based catalyst production.
BASF SE
Technical Solution: BASF SE has developed a novel approach to base-activated polymerization of ethyl propanoate, utilizing a proprietary catalyst system that enhances reactivity and control over the polymerization process. Their method involves a carefully designed initiator complex that promotes rapid and efficient ring-opening of the ester group, leading to controlled chain growth[1]. The company has also implemented advanced reaction monitoring techniques, such as in-situ FTIR spectroscopy, to precisely track the polymerization kinetics and optimize reaction conditions in real-time[3]. This technology allows for the production of well-defined polymers with tailored molecular weights and narrow polydispersity indices, suitable for various high-performance applications[5].
Strengths: Precise control over polymer properties, high efficiency, and scalability. Weaknesses: Potentially higher production costs due to specialized catalysts and monitoring equipment.
Key Innovations
Norbornene-type polymers having quaternary ammonium functionality
PatentInactiveCN102695741A
Innovation
- Norbornene vinyl addition and ROMP polymers were used to form alkaline anion exchange membranes (AAEM) with high hydroxide ion conductivity and mechanical strength by introducing quaternary ammonium groups and maleimide-alkyl side groups. , improve the chemical stability and water swelling resistance of the membrane and avoid Hofmann decomposition reaction.
Antimicrobial polymer latexes derived from unsaturated quaternary ammonium compounds and antimicrobial coatings, sealants, adhesives and elastomers produced from such latexes
PatentInactiveUS20040092632A1
Innovation
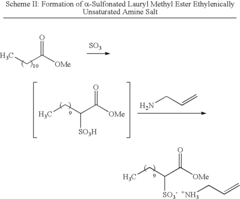
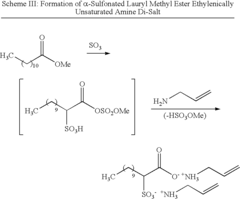
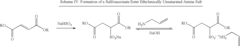
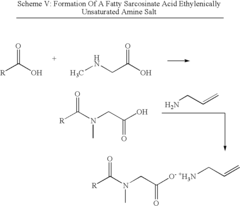
- The use of polymerizable surfactants, specifically ethylenically unsaturated amine salts of sulfonic, phosphoric, and carboxylic acids, which co-polymerize with monomers during the emulsion polymerization process, forming stable polymer particles that improve the hydrophobicity, adhesion, and shear stability of CASE materials.
Reaction Mechanisms
The base-activated polymerization of ethyl propanoate involves several key reaction mechanisms that contribute to the formation of polymer chains. The initial step in this process is the deprotonation of ethyl propanoate by a strong base, typically an alkoxide or hydroxide. This deprotonation occurs at the α-carbon position, adjacent to the carbonyl group, resulting in the formation of an enolate anion.
The enolate anion serves as the active species in the polymerization process. It possesses nucleophilic character and can attack the carbonyl group of another ethyl propanoate molecule, initiating the chain growth mechanism. This nucleophilic addition leads to the formation of a new carbon-carbon bond and generates an alkoxide intermediate.
The alkoxide intermediate then undergoes a proton transfer, either intramolecularly or through interaction with the solvent or unreacted monomer. This proton transfer step regenerates the ester functionality and produces a new enolate anion at the end of the growing polymer chain. The cycle of nucleophilic addition and proton transfer continues, resulting in the propagation of the polymer chain.
Side reactions can occur during the polymerization process, affecting the overall reactivity and product distribution. One such side reaction is the transesterification between the growing polymer chain and unreacted ethyl propanoate monomers. This can lead to the formation of cyclic oligomers or branched structures, potentially impacting the molecular weight distribution and properties of the final polymer.
Another important aspect of the reaction mechanism is the termination step. Termination can occur through various pathways, including protonation of the active enolate by trace amounts of water or other proton sources in the reaction mixture. Additionally, chain transfer reactions may take place, where the growing polymer chain transfers its reactivity to another molecule, effectively terminating one chain while initiating a new one.
The rate and extent of polymerization are influenced by several factors, including the strength and concentration of the base catalyst, reaction temperature, and solvent choice. These parameters can affect the equilibrium between the various reactive species and impact the overall kinetics of the polymerization process.
Understanding these reaction mechanisms is crucial for optimizing the polymerization conditions and controlling the properties of the resulting polymer. By manipulating reaction parameters and potentially introducing additives or co-monomers, it becomes possible to tailor the molecular weight, degree of branching, and functional group distribution of the polymer product.
The enolate anion serves as the active species in the polymerization process. It possesses nucleophilic character and can attack the carbonyl group of another ethyl propanoate molecule, initiating the chain growth mechanism. This nucleophilic addition leads to the formation of a new carbon-carbon bond and generates an alkoxide intermediate.
The alkoxide intermediate then undergoes a proton transfer, either intramolecularly or through interaction with the solvent or unreacted monomer. This proton transfer step regenerates the ester functionality and produces a new enolate anion at the end of the growing polymer chain. The cycle of nucleophilic addition and proton transfer continues, resulting in the propagation of the polymer chain.
Side reactions can occur during the polymerization process, affecting the overall reactivity and product distribution. One such side reaction is the transesterification between the growing polymer chain and unreacted ethyl propanoate monomers. This can lead to the formation of cyclic oligomers or branched structures, potentially impacting the molecular weight distribution and properties of the final polymer.
Another important aspect of the reaction mechanism is the termination step. Termination can occur through various pathways, including protonation of the active enolate by trace amounts of water or other proton sources in the reaction mixture. Additionally, chain transfer reactions may take place, where the growing polymer chain transfers its reactivity to another molecule, effectively terminating one chain while initiating a new one.
The rate and extent of polymerization are influenced by several factors, including the strength and concentration of the base catalyst, reaction temperature, and solvent choice. These parameters can affect the equilibrium between the various reactive species and impact the overall kinetics of the polymerization process.
Understanding these reaction mechanisms is crucial for optimizing the polymerization conditions and controlling the properties of the resulting polymer. By manipulating reaction parameters and potentially introducing additives or co-monomers, it becomes possible to tailor the molecular weight, degree of branching, and functional group distribution of the polymer product.
Environmental Impact
The environmental impact of base-activated polymerization of ethyl propanoate is a crucial aspect to consider in the development and application of this technology. This process, while offering potential benefits in polymer synthesis, also presents several environmental concerns that warrant careful examination.
One of the primary environmental considerations is the use of solvents in the polymerization process. Many base-activated polymerizations require organic solvents, which can be volatile and potentially harmful to the environment if released. These solvents may contribute to air pollution and pose risks to aquatic ecosystems if not properly managed. Additionally, the production and disposal of these solvents can have significant carbon footprints, contributing to overall greenhouse gas emissions.
The base catalysts used in the polymerization of ethyl propanoate also raise environmental concerns. Common bases such as sodium hydroxide or potassium hydroxide, while effective in catalyzing the reaction, can be corrosive and potentially harmful if released into the environment. Proper handling, storage, and disposal of these bases are essential to prevent soil and water contamination.
Energy consumption is another critical factor in assessing the environmental impact of this polymerization process. Base-activated polymerizations often require elevated temperatures or prolonged reaction times, leading to increased energy usage. This energy demand contributes to the overall carbon footprint of the process, especially if non-renewable energy sources are utilized.
The production of ethyl propanoate itself, as the starting material for the polymerization, also has environmental implications. Its synthesis typically involves the esterification of propionic acid with ethanol, both of which are derived from petrochemical sources. This reliance on fossil fuel-based feedstocks raises sustainability concerns and contributes to the depletion of non-renewable resources.
Waste generation and management are significant environmental considerations in this polymerization process. The reaction may produce byproducts or unreacted monomers that require proper disposal. Additionally, the purification of the resulting polymers often involves washing steps that generate aqueous waste streams containing residual bases and organic compounds.
On a positive note, the polymers produced through base-activated polymerization of ethyl propanoate may offer some environmental benefits. Depending on their properties and applications, these polymers could potentially replace less environmentally friendly materials in certain products. For instance, if the resulting polymers are biodegradable or recyclable, they could contribute to reducing plastic waste and promoting a more circular economy.
To mitigate the environmental impact of this polymerization process, several strategies can be explored. These include developing greener solvents or solvent-free processes, utilizing more environmentally benign catalysts, optimizing reaction conditions to reduce energy consumption, and implementing efficient recycling and waste management systems. Additionally, sourcing bio-based feedstocks for ethyl propanoate production could enhance the overall sustainability of the process.
One of the primary environmental considerations is the use of solvents in the polymerization process. Many base-activated polymerizations require organic solvents, which can be volatile and potentially harmful to the environment if released. These solvents may contribute to air pollution and pose risks to aquatic ecosystems if not properly managed. Additionally, the production and disposal of these solvents can have significant carbon footprints, contributing to overall greenhouse gas emissions.
The base catalysts used in the polymerization of ethyl propanoate also raise environmental concerns. Common bases such as sodium hydroxide or potassium hydroxide, while effective in catalyzing the reaction, can be corrosive and potentially harmful if released into the environment. Proper handling, storage, and disposal of these bases are essential to prevent soil and water contamination.
Energy consumption is another critical factor in assessing the environmental impact of this polymerization process. Base-activated polymerizations often require elevated temperatures or prolonged reaction times, leading to increased energy usage. This energy demand contributes to the overall carbon footprint of the process, especially if non-renewable energy sources are utilized.
The production of ethyl propanoate itself, as the starting material for the polymerization, also has environmental implications. Its synthesis typically involves the esterification of propionic acid with ethanol, both of which are derived from petrochemical sources. This reliance on fossil fuel-based feedstocks raises sustainability concerns and contributes to the depletion of non-renewable resources.
Waste generation and management are significant environmental considerations in this polymerization process. The reaction may produce byproducts or unreacted monomers that require proper disposal. Additionally, the purification of the resulting polymers often involves washing steps that generate aqueous waste streams containing residual bases and organic compounds.
On a positive note, the polymers produced through base-activated polymerization of ethyl propanoate may offer some environmental benefits. Depending on their properties and applications, these polymers could potentially replace less environmentally friendly materials in certain products. For instance, if the resulting polymers are biodegradable or recyclable, they could contribute to reducing plastic waste and promoting a more circular economy.
To mitigate the environmental impact of this polymerization process, several strategies can be explored. These include developing greener solvents or solvent-free processes, utilizing more environmentally benign catalysts, optimizing reaction conditions to reduce energy consumption, and implementing efficient recycling and waste management systems. Additionally, sourcing bio-based feedstocks for ethyl propanoate production could enhance the overall sustainability of the process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!