Reactor Synergies in Electrolytic Cell Innovations
AUG 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cell Evolution and Objectives
Electrolytic cells have undergone significant evolution since their inception in the early 19th century. The fundamental principle of using electrical energy to drive non-spontaneous chemical reactions has remained constant, but the design, efficiency, and applications of these cells have dramatically expanded. Initially developed for metal production, particularly aluminum, electrolytic cells now play crucial roles in various industries, including chlor-alkali production, water treatment, and emerging energy storage technologies.
The evolution of electrolytic cells can be traced through several key phases. The first commercial cells, developed in the late 1800s, were simple and inefficient, primarily used for metal extraction. The 20th century saw rapid advancements in electrode materials, cell design, and process control, leading to improved efficiency and scalability. The introduction of membrane technology in the 1970s marked a significant milestone, enabling more selective and environmentally friendly processes.
Recent decades have witnessed a surge in research focused on novel electrode materials, advanced membranes, and innovative cell configurations. These developments aim to address the growing demands for energy efficiency, sustainability, and versatility in electrolytic processes. The integration of nanotechnology and smart materials has opened new avenues for enhancing cell performance and expanding applications.
The objectives of current research on reactor synergies in electrolytic cell innovations are multifaceted. Primarily, there is a strong focus on improving energy efficiency, as electrolytic processes are often energy-intensive. This involves optimizing electrode materials, reducing overpotentials, and enhancing mass transfer within the cell. Another key objective is to increase the selectivity and yield of desired products, particularly in complex electrolytic reactions involving multiple possible pathways.
Researchers are also exploring ways to integrate electrolytic cells with renewable energy sources, addressing the intermittency challenges associated with solar and wind power. This includes developing flexible and responsive electrolytic systems that can operate efficiently under variable power inputs. Additionally, there is growing interest in scaling down electrolytic cells for decentralized applications, such as on-site chemical production or water treatment.
Environmental considerations form another critical objective, driving research into more sustainable electrolyte materials, minimizing harmful by-products, and developing closed-loop systems that reduce waste and resource consumption. The potential for electrolytic cells in carbon capture and utilization technologies is also being actively explored, aligning with global efforts to combat climate change.
In the context of advanced manufacturing, objectives include developing more compact and modular cell designs, improving durability and longevity of components, and integrating smart monitoring and control systems for optimized performance. The ultimate goal is to create versatile, efficient, and sustainable electrolytic cell technologies that can meet the diverse needs of modern industrial processes and emerging applications in energy and environmental sectors.
The evolution of electrolytic cells can be traced through several key phases. The first commercial cells, developed in the late 1800s, were simple and inefficient, primarily used for metal extraction. The 20th century saw rapid advancements in electrode materials, cell design, and process control, leading to improved efficiency and scalability. The introduction of membrane technology in the 1970s marked a significant milestone, enabling more selective and environmentally friendly processes.
Recent decades have witnessed a surge in research focused on novel electrode materials, advanced membranes, and innovative cell configurations. These developments aim to address the growing demands for energy efficiency, sustainability, and versatility in electrolytic processes. The integration of nanotechnology and smart materials has opened new avenues for enhancing cell performance and expanding applications.
The objectives of current research on reactor synergies in electrolytic cell innovations are multifaceted. Primarily, there is a strong focus on improving energy efficiency, as electrolytic processes are often energy-intensive. This involves optimizing electrode materials, reducing overpotentials, and enhancing mass transfer within the cell. Another key objective is to increase the selectivity and yield of desired products, particularly in complex electrolytic reactions involving multiple possible pathways.
Researchers are also exploring ways to integrate electrolytic cells with renewable energy sources, addressing the intermittency challenges associated with solar and wind power. This includes developing flexible and responsive electrolytic systems that can operate efficiently under variable power inputs. Additionally, there is growing interest in scaling down electrolytic cells for decentralized applications, such as on-site chemical production or water treatment.
Environmental considerations form another critical objective, driving research into more sustainable electrolyte materials, minimizing harmful by-products, and developing closed-loop systems that reduce waste and resource consumption. The potential for electrolytic cells in carbon capture and utilization technologies is also being actively explored, aligning with global efforts to combat climate change.
In the context of advanced manufacturing, objectives include developing more compact and modular cell designs, improving durability and longevity of components, and integrating smart monitoring and control systems for optimized performance. The ultimate goal is to create versatile, efficient, and sustainable electrolytic cell technologies that can meet the diverse needs of modern industrial processes and emerging applications in energy and environmental sectors.
Market Analysis for Advanced Electrolytic Technologies
The market for advanced electrolytic technologies is experiencing significant growth, driven by increasing demand for clean energy solutions and sustainable industrial processes. Electrolytic cells, which use electricity to drive chemical reactions, are finding applications across various sectors, including hydrogen production, water treatment, and metal refining.
In the hydrogen production sector, electrolysis is gaining traction as a key technology for generating green hydrogen. The global green hydrogen market is projected to expand rapidly, with some estimates suggesting a compound annual growth rate (CAGR) of over 50% in the coming years. This growth is fueled by the push for decarbonization in industries such as transportation, power generation, and chemical manufacturing.
Water treatment is another area where advanced electrolytic technologies are seeing increased adoption. Electrochemical water treatment methods offer advantages in removing contaminants and disinfecting water without the use of harmful chemicals. The global water and wastewater treatment market is expected to grow steadily, with electrolytic technologies playing an increasingly important role.
In the metal refining industry, electrolytic processes are essential for producing high-purity metals. As demand for electronics and advanced materials continues to rise, the market for electrolytic refining technologies is also expanding. This is particularly evident in the production of copper, aluminum, and other critical metals used in renewable energy technologies and electric vehicles.
The market for electrolytic technologies is also being shaped by advancements in materials science and reactor design. Innovations in electrode materials, membranes, and cell configurations are improving the efficiency and cost-effectiveness of electrolytic processes. This is opening up new applications and markets for these technologies.
Geographically, North America and Europe are currently leading in the adoption of advanced electrolytic technologies, particularly in the green hydrogen sector. However, Asia-Pacific is expected to see rapid growth in the coming years, driven by increasing industrialization and government initiatives to promote clean technologies.
Key market players in the electrolytic technology space include established industrial giants as well as innovative startups. Companies are investing heavily in research and development to improve the performance and reduce the costs of electrolytic systems. Collaborations between industry and academia are also accelerating the pace of innovation in this field.
Despite the positive market outlook, challenges remain. The high capital costs associated with advanced electrolytic technologies can be a barrier to adoption, particularly in price-sensitive markets. Additionally, the availability of renewable electricity at competitive prices is crucial for the widespread deployment of green hydrogen production through electrolysis.
In the hydrogen production sector, electrolysis is gaining traction as a key technology for generating green hydrogen. The global green hydrogen market is projected to expand rapidly, with some estimates suggesting a compound annual growth rate (CAGR) of over 50% in the coming years. This growth is fueled by the push for decarbonization in industries such as transportation, power generation, and chemical manufacturing.
Water treatment is another area where advanced electrolytic technologies are seeing increased adoption. Electrochemical water treatment methods offer advantages in removing contaminants and disinfecting water without the use of harmful chemicals. The global water and wastewater treatment market is expected to grow steadily, with electrolytic technologies playing an increasingly important role.
In the metal refining industry, electrolytic processes are essential for producing high-purity metals. As demand for electronics and advanced materials continues to rise, the market for electrolytic refining technologies is also expanding. This is particularly evident in the production of copper, aluminum, and other critical metals used in renewable energy technologies and electric vehicles.
The market for electrolytic technologies is also being shaped by advancements in materials science and reactor design. Innovations in electrode materials, membranes, and cell configurations are improving the efficiency and cost-effectiveness of electrolytic processes. This is opening up new applications and markets for these technologies.
Geographically, North America and Europe are currently leading in the adoption of advanced electrolytic technologies, particularly in the green hydrogen sector. However, Asia-Pacific is expected to see rapid growth in the coming years, driven by increasing industrialization and government initiatives to promote clean technologies.
Key market players in the electrolytic technology space include established industrial giants as well as innovative startups. Companies are investing heavily in research and development to improve the performance and reduce the costs of electrolytic systems. Collaborations between industry and academia are also accelerating the pace of innovation in this field.
Despite the positive market outlook, challenges remain. The high capital costs associated with advanced electrolytic technologies can be a barrier to adoption, particularly in price-sensitive markets. Additionally, the availability of renewable electricity at competitive prices is crucial for the widespread deployment of green hydrogen production through electrolysis.
Current Challenges in Reactor Synergies
The field of reactor synergies in electrolytic cell innovations faces several significant challenges that hinder progress and limit the potential for breakthrough advancements. One of the primary obstacles is the complexity of integrating multiple reactor systems within a single electrolytic cell. This integration requires a delicate balance of chemical reactions, heat transfer, and mass transport phenomena, which often leads to unforeseen interactions and reduced overall efficiency.
Another major challenge lies in the scalability of synergistic reactor designs. While laboratory-scale experiments may demonstrate promising results, translating these successes to industrial-scale applications presents numerous engineering hurdles. Issues such as maintaining uniform reaction conditions, managing heat dissipation, and ensuring consistent product quality become increasingly difficult as the scale of operation expands.
Material limitations also pose significant constraints on reactor synergy development. The harsh operating conditions typical in electrolytic cells, including high temperatures, corrosive environments, and strong electric fields, demand materials with exceptional durability and stability. Finding cost-effective materials that can withstand these conditions while maintaining the desired catalytic or electrochemical properties remains a persistent challenge.
Energy efficiency is another critical concern in reactor synergies. The goal of combining multiple processes within a single cell is often to reduce overall energy consumption. However, achieving synergistic effects that result in net energy savings has proven elusive in many cases. Researchers struggle to optimize energy utilization across different reaction pathways without compromising product yield or quality.
Control and monitoring of synergistic processes present additional challenges. The increased complexity of multi-reactor systems requires sophisticated control strategies to maintain optimal operating conditions. Real-time monitoring of key parameters across different reaction zones is essential but technically challenging, particularly in harsh electrolytic environments.
Furthermore, the development of predictive models for synergistic reactor systems lags behind experimental progress. The intricate interplay of various phenomena in these systems often defies accurate simulation, making it difficult to predict performance and guide optimization efforts. This gap between theoretical understanding and practical implementation slows down innovation and increases the reliance on costly trial-and-error approaches.
Lastly, regulatory and safety considerations add another layer of complexity to reactor synergy research. Novel reactor designs that combine multiple processes may fall outside existing regulatory frameworks, necessitating extensive safety studies and potentially lengthy approval processes before commercial implementation can be considered.
Another major challenge lies in the scalability of synergistic reactor designs. While laboratory-scale experiments may demonstrate promising results, translating these successes to industrial-scale applications presents numerous engineering hurdles. Issues such as maintaining uniform reaction conditions, managing heat dissipation, and ensuring consistent product quality become increasingly difficult as the scale of operation expands.
Material limitations also pose significant constraints on reactor synergy development. The harsh operating conditions typical in electrolytic cells, including high temperatures, corrosive environments, and strong electric fields, demand materials with exceptional durability and stability. Finding cost-effective materials that can withstand these conditions while maintaining the desired catalytic or electrochemical properties remains a persistent challenge.
Energy efficiency is another critical concern in reactor synergies. The goal of combining multiple processes within a single cell is often to reduce overall energy consumption. However, achieving synergistic effects that result in net energy savings has proven elusive in many cases. Researchers struggle to optimize energy utilization across different reaction pathways without compromising product yield or quality.
Control and monitoring of synergistic processes present additional challenges. The increased complexity of multi-reactor systems requires sophisticated control strategies to maintain optimal operating conditions. Real-time monitoring of key parameters across different reaction zones is essential but technically challenging, particularly in harsh electrolytic environments.
Furthermore, the development of predictive models for synergistic reactor systems lags behind experimental progress. The intricate interplay of various phenomena in these systems often defies accurate simulation, making it difficult to predict performance and guide optimization efforts. This gap between theoretical understanding and practical implementation slows down innovation and increases the reliance on costly trial-and-error approaches.
Lastly, regulatory and safety considerations add another layer of complexity to reactor synergy research. Novel reactor designs that combine multiple processes may fall outside existing regulatory frameworks, necessitating extensive safety studies and potentially lengthy approval processes before commercial implementation can be considered.
Existing Reactor Synergy Solutions
01 Electrolytic cell design optimization
Innovations in electrolytic cell design focus on improving efficiency and synergy. This includes optimizing electrode configurations, membrane placement, and overall cell geometry to enhance reaction kinetics and product yield. Advanced designs may incorporate features for better mass transfer, reduced energy consumption, and improved scalability.- Electrolytic cell design optimization: Improvements in electrolytic cell design focus on enhancing efficiency and performance. This includes optimizing electrode configurations, membrane placement, and overall cell geometry to maximize reaction rates and minimize energy consumption. Advanced materials and coatings are utilized to improve durability and conductivity, while innovative flow patterns are implemented to ensure uniform distribution of reactants and products.
- Integration of renewable energy sources: Electrolytic cells are increasingly being integrated with renewable energy sources to create sustainable production systems. This synergy allows for the efficient conversion of intermittent renewable energy into storable chemical products or fuels. The integration often involves smart control systems to manage fluctuating power inputs and optimize production rates based on energy availability.
- Multi-functional reactor systems: Development of multi-functional reactor systems that combine electrolytic processes with other chemical or physical processes in a single unit. These integrated systems can perform multiple reactions or separations simultaneously, leading to improved efficiency, reduced footprint, and enhanced product yields. Examples include electrochemical reactors combined with distillation or membrane separation units.
- Advanced catalysts and electrode materials: Research into advanced catalysts and electrode materials aims to enhance the selectivity and efficiency of electrolytic reactions. Novel nanostructured materials, bimetallic catalysts, and surface-modified electrodes are being developed to lower overpotentials, increase current densities, and improve product selectivity. These advancements contribute to more economical and sustainable electrolytic processes.
- Process intensification and scale-up strategies: Innovative approaches to process intensification and scale-up of electrolytic cells are being explored to enhance industrial applicability. This includes the development of modular and stackable cell designs, continuous flow systems, and novel reactor configurations that allow for easier scaling from laboratory to industrial production. These strategies aim to overcome traditional limitations in electrolytic processes and improve overall system efficiency.
02 Integration of multiple reaction processes
Synergistic effects are achieved by integrating multiple reaction processes within a single electrolytic system. This approach combines electrochemical reactions with other processes such as catalysis, photochemistry, or biotechnology. The integration allows for more efficient use of reactants, energy, and space, leading to improved overall performance and product selectivity.Expand Specific Solutions03 Advanced electrode materials and coatings
Development of novel electrode materials and coatings enhances the performance of electrolytic cells. These materials may offer improved conductivity, catalytic activity, or resistance to corrosion. Specialized coatings can also provide selective reactivity, leading to higher product purity and reduced side reactions.Expand Specific Solutions04 Smart control systems and process optimization
Implementation of intelligent control systems and process optimization techniques improves the synergy between different components of electrolytic reactors. This includes real-time monitoring, adaptive control algorithms, and predictive modeling to optimize operating conditions, energy efficiency, and product quality across various reaction stages.Expand Specific Solutions05 Modular and scalable reactor designs
Development of modular and scalable electrolytic reactor designs enables flexible operation and easier scale-up. These designs allow for the addition or removal of reaction units, facilitating maintenance and process optimization. Modular approaches also support the integration of different reaction types within a single system, enhancing overall synergy and efficiency.Expand Specific Solutions
Key Industry Players and Competitors
The research on reactor synergies in electrolytic cell innovations is in a dynamic phase of development, with significant market potential as industries seek more efficient and sustainable energy solutions. The market is experiencing rapid growth, driven by increasing demand for clean energy technologies and environmental regulations. Key players like Intelligent Energy, CEA, and 3M Innovative Properties are at the forefront, leveraging their expertise in fuel cells and materials science. The technology's maturity varies, with established companies like Honda and NGK Insulators advancing commercial applications, while academic institutions such as Kyushu University and Xi'an Jiaotong University contribute cutting-edge research. This collaborative ecosystem of industry leaders and research institutions is accelerating innovation, pushing the boundaries of electrolytic cell technology towards greater efficiency and scalability.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The CEA has developed innovative reactor synergies for electrolytic cells, focusing on high-temperature electrolysis (HTE) for hydrogen production. Their approach combines solid oxide electrolysis cells (SOECs) with nuclear heat sources, achieving efficiencies up to 50% higher than conventional low-temperature electrolysis [1]. The system integrates waste heat recovery from nuclear reactors to preheat steam, reducing the electrical energy required for electrolysis. Additionally, they've implemented advanced electrode materials and cell designs to enhance durability and performance under high-temperature conditions, with some prototypes demonstrating stable operation for over 10,000 hours [2].
Strengths: High efficiency, integration with nuclear energy, long-term stability. Weaknesses: High initial costs, complexity of high-temperature operations.
3M Innovative Properties Co.
Technical Solution: 3M has developed advanced membrane electrode assemblies (MEAs) for proton exchange membrane (PEM) electrolyzers, focusing on improving catalyst utilization and reducing noble metal loading. Their proprietary nanostructured thin film (NSTF) catalyst technology has demonstrated up to 40% reduction in platinum group metal (PGM) usage while maintaining performance [3]. The company has also introduced novel ion exchange membranes with enhanced proton conductivity and mechanical stability, allowing for higher current densities and improved efficiency in electrolytic cells. Recent developments include the integration of advanced coating technologies to enhance the longevity of electrodes in harsh electrolytic environments [4].
Strengths: Reduced material costs, improved efficiency, scalable manufacturing. Weaknesses: Reliance on rare earth materials, potential limitations in extreme pH conditions.
Breakthrough Synergistic Reactor Designs
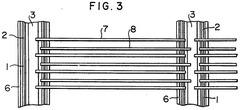
Reactor for the electrochemical treatment of biomass
PatentInactiveEP2090678A1
Innovation
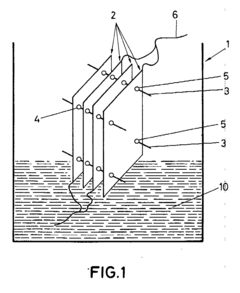
- A reactor design featuring flat-parallel electrodes with a separation distance of less than 1 mm, eliminating the need for extensive separation means and gas separation membranes, utilizing a sandwich arrangement with electrodes made from materials like metal-graphite combinations and coated with catalytic materials, and incorporating a control system for real-time impedance adjustment.
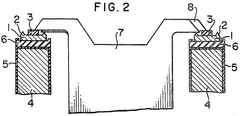
Electrolytic cell having means for supporting the electrodes on the cell wall and means for shorting out the electrodes
PatentInactiveUS3929614A
Innovation
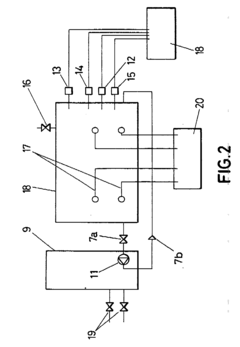
- The implementation of a shorting electric conductor fixed in a vertical plane at the ends of electric conductors on the electrolytic cell wall ensures secure and efficient contact, eliminating sag-related issues and enhancing safety by using vertically oriented connections with bolts, grooves, and conductive connectors.
Environmental Impact Assessment
The environmental impact assessment of reactor synergies in electrolytic cell innovations is a critical aspect of evaluating the sustainability and ecological footprint of these technological advancements. Electrolytic cells, while offering significant potential for clean energy production and industrial processes, also pose environmental challenges that must be carefully considered.
One of the primary environmental concerns associated with electrolytic cell innovations is the consumption of energy. While these cells aim to produce clean energy or facilitate more efficient industrial processes, their operation often requires substantial electrical input. The environmental impact of this energy consumption largely depends on the source of electricity. If powered by renewable energy sources such as solar or wind, the overall environmental footprint can be significantly reduced. However, if reliant on fossil fuel-based power generation, the indirect emissions and resource depletion must be factored into the assessment.
Water usage and management represent another crucial environmental consideration. Many electrolytic processes require substantial amounts of water, both as a reactant and for cooling purposes. The sourcing, treatment, and disposal of this water can have significant impacts on local ecosystems and water resources. Innovations in reactor synergies that improve water efficiency or enable the use of alternative cooling methods could substantially mitigate these impacts.
The production and disposal of electrodes and other cell components also contribute to the environmental footprint. Many electrolytic processes use rare or precious metals as catalysts, the mining and processing of which can have severe environmental consequences. Advancements in reactor synergies that reduce the need for these materials or extend their operational lifespan can help minimize these impacts. Additionally, the development of more recyclable or biodegradable components could significantly reduce waste and resource depletion.
Emissions and byproducts from electrolytic processes must be carefully managed to prevent air and water pollution. While many electrolytic cells are designed to produce specific chemicals or materials, unintended side reactions can result in the release of harmful substances. Innovations in reactor design and process control that minimize these unwanted byproducts are essential for reducing environmental risks. Furthermore, the development of closed-loop systems that capture and reuse emissions could transform potential pollutants into valuable resources.
The long-term environmental effects of scaling up electrolytic cell technologies must also be considered. As these innovations move from laboratory to industrial scale, their cumulative impact on ecosystems, climate, and resource availability becomes more significant. Life cycle assessments that account for raw material extraction, manufacturing, operation, and end-of-life disposal are crucial for understanding the full environmental implications of these technologies.
One of the primary environmental concerns associated with electrolytic cell innovations is the consumption of energy. While these cells aim to produce clean energy or facilitate more efficient industrial processes, their operation often requires substantial electrical input. The environmental impact of this energy consumption largely depends on the source of electricity. If powered by renewable energy sources such as solar or wind, the overall environmental footprint can be significantly reduced. However, if reliant on fossil fuel-based power generation, the indirect emissions and resource depletion must be factored into the assessment.
Water usage and management represent another crucial environmental consideration. Many electrolytic processes require substantial amounts of water, both as a reactant and for cooling purposes. The sourcing, treatment, and disposal of this water can have significant impacts on local ecosystems and water resources. Innovations in reactor synergies that improve water efficiency or enable the use of alternative cooling methods could substantially mitigate these impacts.
The production and disposal of electrodes and other cell components also contribute to the environmental footprint. Many electrolytic processes use rare or precious metals as catalysts, the mining and processing of which can have severe environmental consequences. Advancements in reactor synergies that reduce the need for these materials or extend their operational lifespan can help minimize these impacts. Additionally, the development of more recyclable or biodegradable components could significantly reduce waste and resource depletion.
Emissions and byproducts from electrolytic processes must be carefully managed to prevent air and water pollution. While many electrolytic cells are designed to produce specific chemicals or materials, unintended side reactions can result in the release of harmful substances. Innovations in reactor design and process control that minimize these unwanted byproducts are essential for reducing environmental risks. Furthermore, the development of closed-loop systems that capture and reuse emissions could transform potential pollutants into valuable resources.
The long-term environmental effects of scaling up electrolytic cell technologies must also be considered. As these innovations move from laboratory to industrial scale, their cumulative impact on ecosystems, climate, and resource availability becomes more significant. Life cycle assessments that account for raw material extraction, manufacturing, operation, and end-of-life disposal are crucial for understanding the full environmental implications of these technologies.
Regulatory Framework for Electrolytic Innovations
The regulatory framework for electrolytic innovations plays a crucial role in shaping the development and implementation of new technologies in this field. As electrolytic cell innovations continue to advance, governments and regulatory bodies worldwide are adapting their policies to ensure safety, environmental protection, and fair competition while fostering innovation.
At the international level, organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have established standards for electrolytic processes and equipment. These standards provide a foundation for national regulatory bodies to build upon, ensuring a degree of global consistency in safety and performance requirements.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) are key players in regulating electrolytic processes. The EPA focuses on environmental impacts, particularly concerning the disposal of byproducts and waste materials from electrolytic cells. OSHA, on the other hand, sets standards for workplace safety in facilities utilizing electrolytic technologies.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which affects the use of chemicals in electrolytic processes. This comprehensive framework requires manufacturers to assess and manage the risks associated with substances used in their operations, including those in electrolytic cells.
In Asia, countries like China and Japan have been proactive in developing regulations to support their growing electrolytic industries. China's Ministry of Ecology and Environment has introduced stricter environmental standards for electrolytic processes, particularly in the aluminum and chlor-alkali sectors. Japan's Ministry of Economy, Trade, and Industry has focused on promoting energy efficiency in electrolytic technologies through regulatory incentives.
Emerging economies are also recognizing the importance of regulatory frameworks in this area. India, for example, has been updating its environmental regulations to address the challenges posed by expanding electrolytic industries, particularly in the context of its ambitious renewable energy goals.
As reactor synergies in electrolytic cell innovations continue to evolve, regulatory bodies are faced with the challenge of keeping pace with technological advancements. This has led to an increased emphasis on adaptive regulatory approaches, such as regulatory sandboxes, which allow for controlled testing of new technologies under relaxed regulatory conditions.
The future of regulatory frameworks for electrolytic innovations is likely to focus on balancing innovation with sustainability. This may include the development of more stringent energy efficiency standards, increased scrutiny of raw material sourcing, and greater emphasis on lifecycle assessments of electrolytic technologies. Additionally, as the global focus on carbon neutrality intensifies, regulations are expected to increasingly incentivize the development of electrolytic processes that can contribute to decarbonization efforts across various industries.
At the international level, organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have established standards for electrolytic processes and equipment. These standards provide a foundation for national regulatory bodies to build upon, ensuring a degree of global consistency in safety and performance requirements.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) are key players in regulating electrolytic processes. The EPA focuses on environmental impacts, particularly concerning the disposal of byproducts and waste materials from electrolytic cells. OSHA, on the other hand, sets standards for workplace safety in facilities utilizing electrolytic technologies.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which affects the use of chemicals in electrolytic processes. This comprehensive framework requires manufacturers to assess and manage the risks associated with substances used in their operations, including those in electrolytic cells.
In Asia, countries like China and Japan have been proactive in developing regulations to support their growing electrolytic industries. China's Ministry of Ecology and Environment has introduced stricter environmental standards for electrolytic processes, particularly in the aluminum and chlor-alkali sectors. Japan's Ministry of Economy, Trade, and Industry has focused on promoting energy efficiency in electrolytic technologies through regulatory incentives.
Emerging economies are also recognizing the importance of regulatory frameworks in this area. India, for example, has been updating its environmental regulations to address the challenges posed by expanding electrolytic industries, particularly in the context of its ambitious renewable energy goals.
As reactor synergies in electrolytic cell innovations continue to evolve, regulatory bodies are faced with the challenge of keeping pace with technological advancements. This has led to an increased emphasis on adaptive regulatory approaches, such as regulatory sandboxes, which allow for controlled testing of new technologies under relaxed regulatory conditions.
The future of regulatory frameworks for electrolytic innovations is likely to focus on balancing innovation with sustainability. This may include the development of more stringent energy efficiency standards, increased scrutiny of raw material sourcing, and greater emphasis on lifecycle assessments of electrolytic technologies. Additionally, as the global focus on carbon neutrality intensifies, regulations are expected to increasingly incentivize the development of electrolytic processes that can contribute to decarbonization efforts across various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!