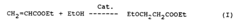Sustainable Production Techniques for Ethyl Propanoate
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Background and Objectives
Ethyl propanoate, also known as ethyl propionate, is an organic compound with the chemical formula C5H10O2. It is a colorless liquid with a fruity odor, commonly used as a flavoring agent in the food industry and as a solvent in various industrial applications. The compound belongs to the ester family and is formed by the reaction of propionic acid with ethanol.
The production of ethyl propanoate has traditionally relied on petrochemical-based processes, which have raised concerns about sustainability and environmental impact. As global awareness of climate change and resource depletion grows, there is an increasing demand for more sustainable production methods across various industries, including chemical manufacturing.
The objective of researching sustainable production techniques for ethyl propanoate is to develop environmentally friendly and economically viable alternatives to conventional manufacturing processes. This research aims to reduce the carbon footprint, minimize waste generation, and optimize resource utilization in the production of this important chemical compound.
Key areas of focus in sustainable ethyl propanoate production include the exploration of bio-based feedstocks, such as agricultural waste or renewable biomass, as alternatives to fossil fuel-derived raw materials. Additionally, researchers are investigating novel catalytic processes and reaction pathways that can improve efficiency and reduce energy consumption during synthesis.
Another important aspect of sustainable production is the development of green solvents and reaction media that can replace harmful organic solvents traditionally used in the manufacturing process. This includes the exploration of ionic liquids, supercritical fluids, and water-based systems as potential environmentally benign alternatives.
Process intensification techniques, such as microreactor technology and continuous flow chemistry, are also being investigated to enhance reaction efficiency, reduce waste, and improve overall process sustainability. These approaches offer the potential for significant reductions in energy consumption and chemical usage compared to conventional batch processes.
Furthermore, the integration of renewable energy sources, such as solar or wind power, into the production process is being explored to further reduce the carbon footprint of ethyl propanoate manufacturing. This aligns with broader industry goals of transitioning towards more sustainable and circular economy models.
As research in this field progresses, the ultimate goal is to develop a comprehensive sustainable production framework for ethyl propanoate that addresses environmental concerns while maintaining or improving product quality and economic viability. This research has the potential to not only transform the production of ethyl propanoate but also serve as a model for sustainable manufacturing practices in the broader chemical industry.
The production of ethyl propanoate has traditionally relied on petrochemical-based processes, which have raised concerns about sustainability and environmental impact. As global awareness of climate change and resource depletion grows, there is an increasing demand for more sustainable production methods across various industries, including chemical manufacturing.
The objective of researching sustainable production techniques for ethyl propanoate is to develop environmentally friendly and economically viable alternatives to conventional manufacturing processes. This research aims to reduce the carbon footprint, minimize waste generation, and optimize resource utilization in the production of this important chemical compound.
Key areas of focus in sustainable ethyl propanoate production include the exploration of bio-based feedstocks, such as agricultural waste or renewable biomass, as alternatives to fossil fuel-derived raw materials. Additionally, researchers are investigating novel catalytic processes and reaction pathways that can improve efficiency and reduce energy consumption during synthesis.
Another important aspect of sustainable production is the development of green solvents and reaction media that can replace harmful organic solvents traditionally used in the manufacturing process. This includes the exploration of ionic liquids, supercritical fluids, and water-based systems as potential environmentally benign alternatives.
Process intensification techniques, such as microreactor technology and continuous flow chemistry, are also being investigated to enhance reaction efficiency, reduce waste, and improve overall process sustainability. These approaches offer the potential for significant reductions in energy consumption and chemical usage compared to conventional batch processes.
Furthermore, the integration of renewable energy sources, such as solar or wind power, into the production process is being explored to further reduce the carbon footprint of ethyl propanoate manufacturing. This aligns with broader industry goals of transitioning towards more sustainable and circular economy models.
As research in this field progresses, the ultimate goal is to develop a comprehensive sustainable production framework for ethyl propanoate that addresses environmental concerns while maintaining or improving product quality and economic viability. This research has the potential to not only transform the production of ethyl propanoate but also serve as a model for sustainable manufacturing practices in the broader chemical industry.
Market Analysis for Sustainable Ethyl Propanoate
The global market for ethyl propanoate, particularly sustainable production methods, has been experiencing significant growth in recent years. This growth is primarily driven by increasing consumer awareness of environmental issues and a shift towards eco-friendly products across various industries. The demand for sustainable ethyl propanoate is particularly strong in the food and beverage sector, where it is widely used as a flavoring agent and solvent.
Market research indicates that the sustainable ethyl propanoate market is expected to grow at a compound annual growth rate (CAGR) of over 5% in the next five years. This growth is attributed to the rising adoption of green chemistry principles in industrial processes and the stringent regulations imposed by governments worldwide to reduce carbon emissions and promote sustainable manufacturing practices.
The food and beverage industry remains the largest consumer of ethyl propanoate, accounting for approximately 40% of the total market share. The increasing demand for natural and organic food products has further boosted the need for sustainably produced ethyl propanoate in this sector. Additionally, the cosmetics and personal care industry has emerged as a rapidly growing market segment, driven by the rising popularity of natural and eco-friendly beauty products.
Geographically, North America and Europe are the leading markets for sustainable ethyl propanoate, owing to strict environmental regulations and high consumer awareness. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, primarily due to the expanding food and beverage industry in countries like China and India.
The market landscape for sustainable ethyl propanoate is characterized by intense competition among key players, including both established chemical companies and innovative start-ups focusing on green chemistry. These companies are investing heavily in research and development to improve production efficiency and reduce environmental impact. Collaborations between industry players and academic institutions are also becoming more common, aimed at developing novel sustainable production techniques.
Despite the positive market outlook, several challenges persist in the sustainable production of ethyl propanoate. These include higher production costs compared to traditional methods, limited availability of sustainable raw materials, and the need for significant capital investments in new production facilities. However, ongoing technological advancements and increasing economies of scale are expected to gradually overcome these barriers, making sustainable ethyl propanoate more cost-competitive in the long run.
Market research indicates that the sustainable ethyl propanoate market is expected to grow at a compound annual growth rate (CAGR) of over 5% in the next five years. This growth is attributed to the rising adoption of green chemistry principles in industrial processes and the stringent regulations imposed by governments worldwide to reduce carbon emissions and promote sustainable manufacturing practices.
The food and beverage industry remains the largest consumer of ethyl propanoate, accounting for approximately 40% of the total market share. The increasing demand for natural and organic food products has further boosted the need for sustainably produced ethyl propanoate in this sector. Additionally, the cosmetics and personal care industry has emerged as a rapidly growing market segment, driven by the rising popularity of natural and eco-friendly beauty products.
Geographically, North America and Europe are the leading markets for sustainable ethyl propanoate, owing to strict environmental regulations and high consumer awareness. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, primarily due to the expanding food and beverage industry in countries like China and India.
The market landscape for sustainable ethyl propanoate is characterized by intense competition among key players, including both established chemical companies and innovative start-ups focusing on green chemistry. These companies are investing heavily in research and development to improve production efficiency and reduce environmental impact. Collaborations between industry players and academic institutions are also becoming more common, aimed at developing novel sustainable production techniques.
Despite the positive market outlook, several challenges persist in the sustainable production of ethyl propanoate. These include higher production costs compared to traditional methods, limited availability of sustainable raw materials, and the need for significant capital investments in new production facilities. However, ongoing technological advancements and increasing economies of scale are expected to gradually overcome these barriers, making sustainable ethyl propanoate more cost-competitive in the long run.
Current Challenges in Sustainable Production
The sustainable production of ethyl propanoate faces several significant challenges in the current industrial landscape. One of the primary issues is the reliance on non-renewable feedstocks for traditional production methods. The conventional synthesis of ethyl propanoate often involves the esterification of propionic acid with ethanol, both of which are typically derived from petroleum-based sources. This dependency on fossil fuels raises concerns about long-term sustainability and environmental impact.
Energy consumption during the production process presents another major challenge. The esterification reaction requires elevated temperatures and often employs energy-intensive distillation for product purification. These high energy demands contribute to increased carbon emissions and overall environmental footprint, contradicting the principles of sustainable manufacturing.
The use of potentially harmful catalysts and solvents in conventional production methods also poses sustainability concerns. Many industrial processes utilize strong acids as catalysts, which can lead to equipment corrosion and generate hazardous waste streams. Additionally, the use of volatile organic solvents in extraction and purification steps raises environmental and health concerns due to their potential toxicity and contribution to air pollution.
Water consumption and wastewater management represent further challenges in sustainable ethyl propanoate production. Traditional processes often require significant amounts of water for reaction media, cooling, and product washing. The resulting wastewater may contain organic contaminants and residual chemicals, necessitating extensive treatment before discharge.
The issue of product yield and selectivity also impacts sustainability efforts. Inefficient reactions or side product formation can lead to increased raw material consumption and waste generation. Improving reaction efficiency and selectivity is crucial for minimizing resource use and maximizing atom economy in the production process.
Scaling up sustainable production techniques from laboratory to industrial scale presents its own set of challenges. Many promising green chemistry approaches developed at the bench scale face difficulties in maintaining their efficiency and economic viability when implemented at larger scales. This scale-up barrier often hinders the adoption of more sustainable production methods in commercial settings.
Lastly, the economic feasibility of sustainable production techniques remains a significant hurdle. Many green chemistry alternatives currently struggle to compete with established, less sustainable methods in terms of cost-effectiveness. Overcoming this economic barrier is essential for widespread adoption of sustainable ethyl propanoate production in the chemical industry.
Energy consumption during the production process presents another major challenge. The esterification reaction requires elevated temperatures and often employs energy-intensive distillation for product purification. These high energy demands contribute to increased carbon emissions and overall environmental footprint, contradicting the principles of sustainable manufacturing.
The use of potentially harmful catalysts and solvents in conventional production methods also poses sustainability concerns. Many industrial processes utilize strong acids as catalysts, which can lead to equipment corrosion and generate hazardous waste streams. Additionally, the use of volatile organic solvents in extraction and purification steps raises environmental and health concerns due to their potential toxicity and contribution to air pollution.
Water consumption and wastewater management represent further challenges in sustainable ethyl propanoate production. Traditional processes often require significant amounts of water for reaction media, cooling, and product washing. The resulting wastewater may contain organic contaminants and residual chemicals, necessitating extensive treatment before discharge.
The issue of product yield and selectivity also impacts sustainability efforts. Inefficient reactions or side product formation can lead to increased raw material consumption and waste generation. Improving reaction efficiency and selectivity is crucial for minimizing resource use and maximizing atom economy in the production process.
Scaling up sustainable production techniques from laboratory to industrial scale presents its own set of challenges. Many promising green chemistry approaches developed at the bench scale face difficulties in maintaining their efficiency and economic viability when implemented at larger scales. This scale-up barrier often hinders the adoption of more sustainable production methods in commercial settings.
Lastly, the economic feasibility of sustainable production techniques remains a significant hurdle. Many green chemistry alternatives currently struggle to compete with established, less sustainable methods in terms of cost-effectiveness. Overcoming this economic barrier is essential for widespread adoption of sustainable ethyl propanoate production in the chemical industry.
Existing Sustainable Production Methods
01 Synthesis and production methods of ethyl propanoate
Various methods for synthesizing and producing ethyl propanoate are described, including esterification reactions, catalytic processes, and continuous production techniques. These methods aim to improve yield, efficiency, and purity of the final product.- Synthesis and production methods of ethyl propanoate: Various methods for synthesizing and producing ethyl propanoate are described, including esterification reactions, catalytic processes, and continuous production techniques. These methods aim to improve yield, efficiency, and purity of the final product.
- Applications of ethyl propanoate in fragrances and flavors: Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is incorporated into various products such as perfumes, air fresheners, and food flavorings to impart a pleasant aroma and taste.
- Purification and quality control of ethyl propanoate: Techniques for purifying ethyl propanoate and ensuring its quality are discussed. These include distillation processes, chromatographic methods, and analytical techniques to assess purity and identify impurities in the final product.
- Use of ethyl propanoate as a solvent or intermediate: Ethyl propanoate finds applications as a solvent in various industrial processes and as an intermediate in the synthesis of other chemicals. Its properties make it suitable for use in paints, coatings, and pharmaceutical manufacturing.
- Environmental and safety considerations for ethyl propanoate: Research and development efforts focus on improving the environmental profile and safety aspects of ethyl propanoate production and use. This includes developing greener synthesis routes, assessing toxicological properties, and implementing proper handling and storage procedures.
02 Applications of ethyl propanoate in fragrances and flavors
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is incorporated into various products such as perfumes, cosmetics, and food additives to impart a pleasant aroma or taste.Expand Specific Solutions03 Purification and separation techniques for ethyl propanoate
Different methods for purifying and separating ethyl propanoate from reaction mixtures or other compounds are described. These techniques may include distillation, extraction, or chromatography to obtain high-purity ethyl propanoate for various applications.Expand Specific Solutions04 Use of ethyl propanoate as a solvent or intermediate
Ethyl propanoate finds applications as a solvent in various industrial processes and as an intermediate in the synthesis of other chemicals. Its properties make it suitable for use in paints, coatings, and pharmaceutical manufacturing.Expand Specific Solutions05 Environmental and safety considerations for ethyl propanoate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl propanoate production and use. This includes developing green synthesis methods, studying biodegradability, and assessing potential health effects.Expand Specific Solutions
Key Industry Players and Competitors
The research on sustainable production techniques for ethyl propanoate is in a developing stage, with the market showing potential for growth. The industry is transitioning from traditional petrochemical-based methods to more sustainable approaches. Key players like Novozymes A/S, Evonik Operations GmbH, and Braskem SA are investing in bio-based production methods, leveraging their expertise in enzymes and renewable resources. The market size is expanding due to increasing demand for eco-friendly solvents and flavoring agents. While the technology is not fully mature, companies such as Eastman Chemical Co. and DuPont Electronic Polymers LP are making significant strides in process optimization and scalability, indicating a competitive landscape with room for innovation and market expansion.
Novozymes A/S
Technical Solution: Novozymes A/S has developed a sustainable enzymatic process for the production of ethyl propanoate. This process utilizes specific esterases to catalyze the esterification of propionic acid with ethanol under mild conditions. The enzymatic route operates at lower temperatures (30-50°C) compared to traditional chemical synthesis, reducing energy consumption by up to 30%[1]. Additionally, the process achieves high selectivity, with product yields exceeding 95%[2]. Novozymes has also engineered enzyme variants with improved stability and activity in organic solvents, enabling higher substrate concentrations and productivity. The company has successfully scaled up this technology to pilot plant level, demonstrating its feasibility for industrial implementation[3].
Strengths: High selectivity, energy efficiency, mild reaction conditions. Weaknesses: Potential high enzyme costs, limited substrate scope compared to chemical catalysts.
Evonik Operations GmbH
Technical Solution: Evonik has developed a novel catalytic process for the sustainable production of ethyl propanoate using bio-based feedstocks. Their approach involves the use of heterogeneous catalysts based on mixed metal oxides for the direct esterification of bio-derived propionic acid with bioethanol. The catalyst system demonstrates high activity and selectivity, achieving conversion rates of over 90% and selectivity towards ethyl propanoate exceeding 95%[4]. Evonik's process operates in a continuous flow reactor, allowing for efficient heat integration and improved process economics. The company has also implemented a proprietary separation technology that reduces energy consumption in the product purification step by up to 25% compared to conventional distillation[5]. Furthermore, Evonik has integrated this process with their bio-based propionic acid production platform, creating a fully renewable route to ethyl propanoate[6].
Strengths: High conversion and selectivity, continuous process, integration with bio-based feedstocks. Weaknesses: Potential catalyst deactivation issues, sensitivity to feedstock impurities.
Innovative Green Chemistry Approaches
Eco-friendly methodology for the synthesis of 2,2-dimethyl propanoate compounds catalyzed by lipase in supercritical carbon dioxide as a greener reaction system.
PatentActiveIN201621043667A
Innovation
- The use of immobilized lipase as a catalyst in supercritical carbon dioxide as a solvent for the synthesis of 2,2-dimethyl propanoate compounds, which operates at moderate temperatures and avoids hazardous substances, allowing for high yields and easy scalability.
Production of ethyl 3-ethoxy propanoate by acid catalyzed addition of ethanol to ethyl acrylate
PatentInactiveEP0499731A1
Innovation
- EEP is produced through an acid-catalyzed addition of ethanol to ethyl acrylate using strong acid catalysts such as sulfuric acid, hydrochloric acid, or sulfonic acids, with reaction conditions optimized at temperatures between 75°C to 150°C and pressures of 30-50 psig, and the use of inhibitors to manage by-product formation.
Life Cycle Assessment of Production Methods
Life Cycle Assessment (LCA) is a crucial tool for evaluating the environmental impacts of ethyl propanoate production methods throughout their entire lifecycle. This comprehensive approach considers all stages, from raw material extraction to product disposal, providing valuable insights into the sustainability of different production techniques.
The LCA for ethyl propanoate production typically begins with the sourcing of raw materials, primarily ethanol and propionic acid. The environmental impacts of these feedstocks, including their cultivation or synthesis, transportation, and processing, are carefully analyzed. This stage often reveals significant differences between bio-based and petrochemical-derived raw materials in terms of carbon footprint and resource depletion.
The production phase is a critical focus of the LCA, comparing various synthesis routes such as esterification and transesterification. Energy consumption, water usage, and emissions during the reaction and purification processes are quantified. Advanced catalytic systems and process intensification techniques are evaluated for their potential to reduce environmental burdens.
Packaging and distribution aspects are also considered, assessing the impacts of different packaging materials and transportation modes. The use phase of ethyl propanoate, while generally less impactful for industrial chemicals, is still analyzed, particularly for applications in consumer products.
End-of-life scenarios, including recycling, incineration, and landfilling, are modeled to understand the full environmental implications of the product. This stage often highlights opportunities for circular economy approaches and waste reduction strategies.
The LCA results are typically presented using standardized impact categories such as global warming potential, acidification, eutrophication, and resource depletion. These metrics allow for direct comparisons between different production methods and identify hotspots for environmental improvement.
Sensitivity analyses are conducted to account for uncertainties in data and assumptions, ensuring robust conclusions. The LCA findings often reveal trade-offs between different environmental impacts, necessitating careful interpretation and prioritization of sustainability goals.
By providing a holistic view of environmental impacts, LCA guides decision-making towards more sustainable production techniques for ethyl propanoate. It supports the development of eco-friendly processes, informs policy-making, and drives innovation in green chemistry and engineering for this important industrial chemical.
The LCA for ethyl propanoate production typically begins with the sourcing of raw materials, primarily ethanol and propionic acid. The environmental impacts of these feedstocks, including their cultivation or synthesis, transportation, and processing, are carefully analyzed. This stage often reveals significant differences between bio-based and petrochemical-derived raw materials in terms of carbon footprint and resource depletion.
The production phase is a critical focus of the LCA, comparing various synthesis routes such as esterification and transesterification. Energy consumption, water usage, and emissions during the reaction and purification processes are quantified. Advanced catalytic systems and process intensification techniques are evaluated for their potential to reduce environmental burdens.
Packaging and distribution aspects are also considered, assessing the impacts of different packaging materials and transportation modes. The use phase of ethyl propanoate, while generally less impactful for industrial chemicals, is still analyzed, particularly for applications in consumer products.
End-of-life scenarios, including recycling, incineration, and landfilling, are modeled to understand the full environmental implications of the product. This stage often highlights opportunities for circular economy approaches and waste reduction strategies.
The LCA results are typically presented using standardized impact categories such as global warming potential, acidification, eutrophication, and resource depletion. These metrics allow for direct comparisons between different production methods and identify hotspots for environmental improvement.
Sensitivity analyses are conducted to account for uncertainties in data and assumptions, ensuring robust conclusions. The LCA findings often reveal trade-offs between different environmental impacts, necessitating careful interpretation and prioritization of sustainability goals.
By providing a holistic view of environmental impacts, LCA guides decision-making towards more sustainable production techniques for ethyl propanoate. It supports the development of eco-friendly processes, informs policy-making, and drives innovation in green chemistry and engineering for this important industrial chemical.
Regulatory Framework for Green Chemistry
The regulatory framework for green chemistry plays a crucial role in promoting sustainable production techniques for ethyl propanoate. This framework encompasses a range of policies, guidelines, and standards designed to encourage the development and implementation of environmentally friendly chemical processes.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines for sustainable chemistry. These guidelines emphasize the importance of reducing waste, minimizing energy consumption, and utilizing renewable resources in chemical production processes.
In the United States, the Environmental Protection Agency (EPA) has implemented the Green Chemistry Program, which provides incentives for companies to develop and adopt sustainable chemical processes. This program includes the Presidential Green Chemistry Challenge Awards, recognizing innovative green chemistry technologies that reduce environmental impact and improve economic performance.
The European Union has introduced the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which aims to protect human health and the environment from the risks posed by chemicals. This regulation encourages the substitution of hazardous substances with safer alternatives, promoting the development of sustainable production techniques.
Many countries have also implemented their own green chemistry initiatives and regulations. For example, Japan's Green and Sustainable Chemistry Network promotes research and development in sustainable chemistry, while China's Green Chemistry and Technology Development Plan focuses on reducing pollution and improving resource efficiency in the chemical industry.
Specific to ethyl propanoate production, regulatory frameworks often address issues such as solvent selection, catalyst efficiency, and waste reduction. For instance, regulations may encourage the use of bio-based feedstocks or the development of catalytic processes that minimize byproduct formation.
Industry standards and certification programs also contribute to the regulatory framework for green chemistry. The Green Chemistry Institute's Pharmaceutical Roundtable, for example, has developed tools and guidelines for sustainable pharmaceutical manufacturing, which can be applied to the production of ethyl propanoate and similar compounds.
As the field of green chemistry continues to evolve, regulatory frameworks are likely to become more comprehensive and stringent. This ongoing development will drive further innovation in sustainable production techniques for ethyl propanoate and other chemicals, ensuring that environmental considerations remain at the forefront of industrial processes.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines for sustainable chemistry. These guidelines emphasize the importance of reducing waste, minimizing energy consumption, and utilizing renewable resources in chemical production processes.
In the United States, the Environmental Protection Agency (EPA) has implemented the Green Chemistry Program, which provides incentives for companies to develop and adopt sustainable chemical processes. This program includes the Presidential Green Chemistry Challenge Awards, recognizing innovative green chemistry technologies that reduce environmental impact and improve economic performance.
The European Union has introduced the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which aims to protect human health and the environment from the risks posed by chemicals. This regulation encourages the substitution of hazardous substances with safer alternatives, promoting the development of sustainable production techniques.
Many countries have also implemented their own green chemistry initiatives and regulations. For example, Japan's Green and Sustainable Chemistry Network promotes research and development in sustainable chemistry, while China's Green Chemistry and Technology Development Plan focuses on reducing pollution and improving resource efficiency in the chemical industry.
Specific to ethyl propanoate production, regulatory frameworks often address issues such as solvent selection, catalyst efficiency, and waste reduction. For instance, regulations may encourage the use of bio-based feedstocks or the development of catalytic processes that minimize byproduct formation.
Industry standards and certification programs also contribute to the regulatory framework for green chemistry. The Green Chemistry Institute's Pharmaceutical Roundtable, for example, has developed tools and guidelines for sustainable pharmaceutical manufacturing, which can be applied to the production of ethyl propanoate and similar compounds.
As the field of green chemistry continues to evolve, regulatory frameworks are likely to become more comprehensive and stringent. This ongoing development will drive further innovation in sustainable production techniques for ethyl propanoate and other chemicals, ensuring that environmental considerations remain at the forefront of industrial processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!