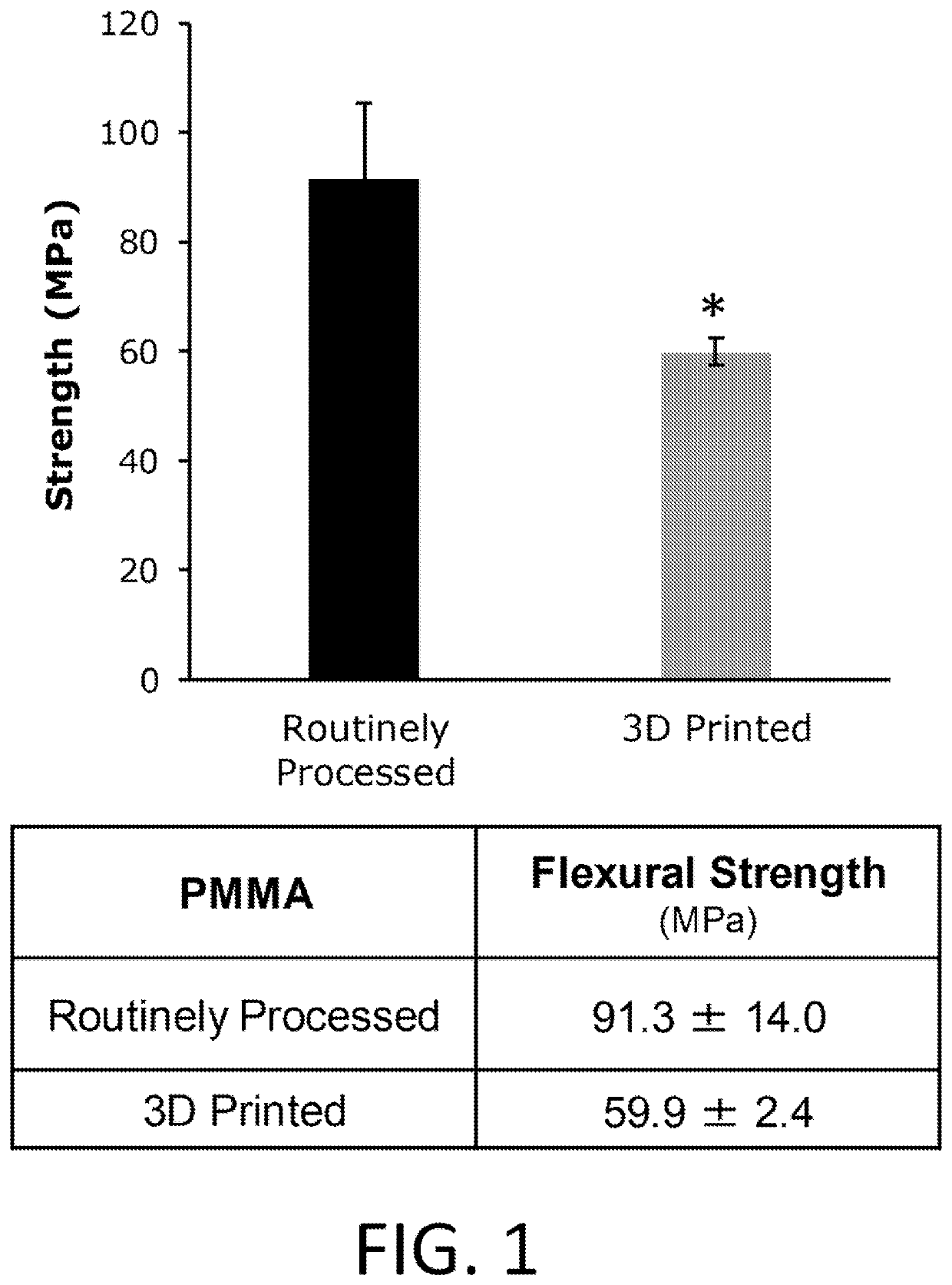
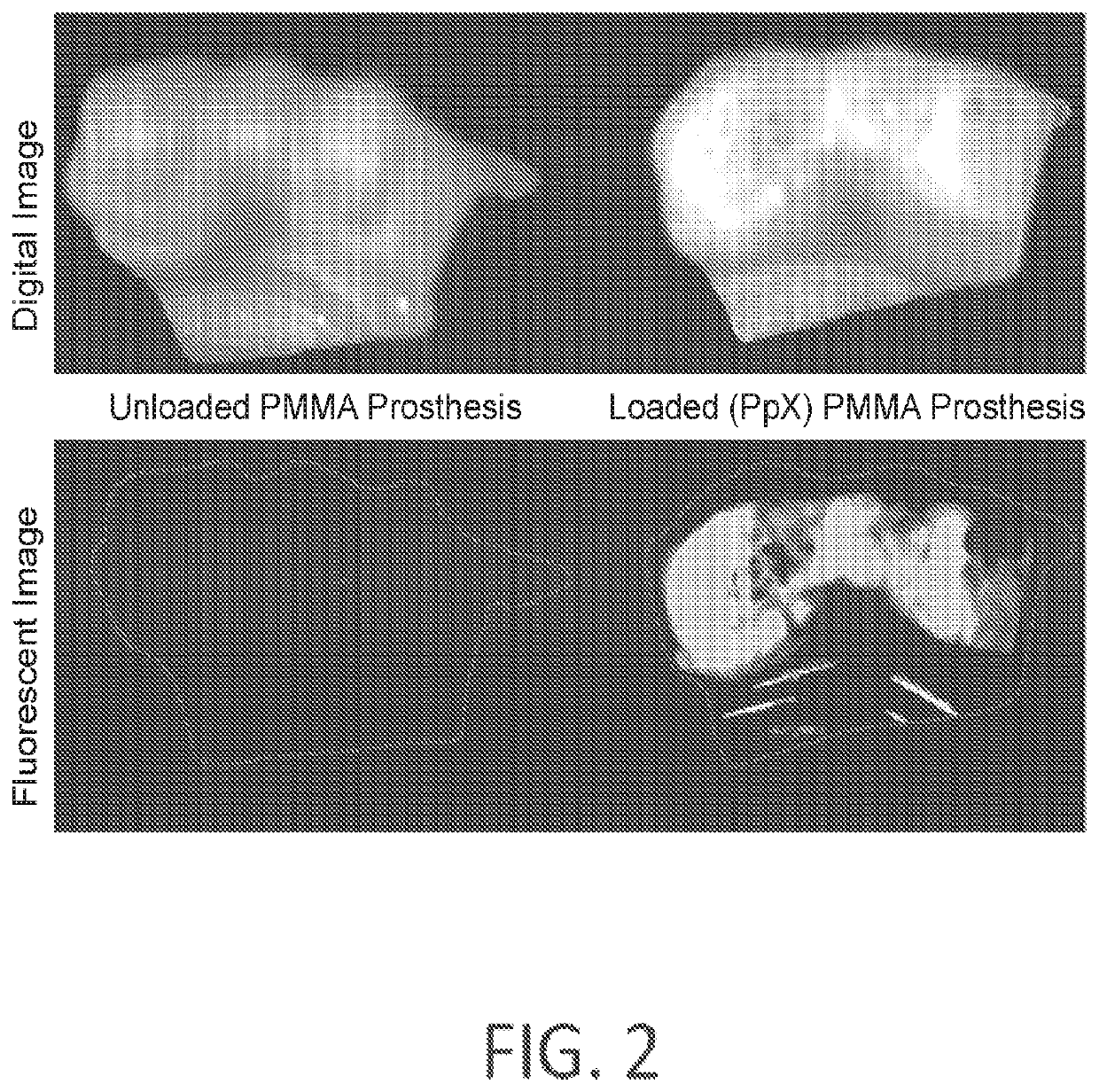
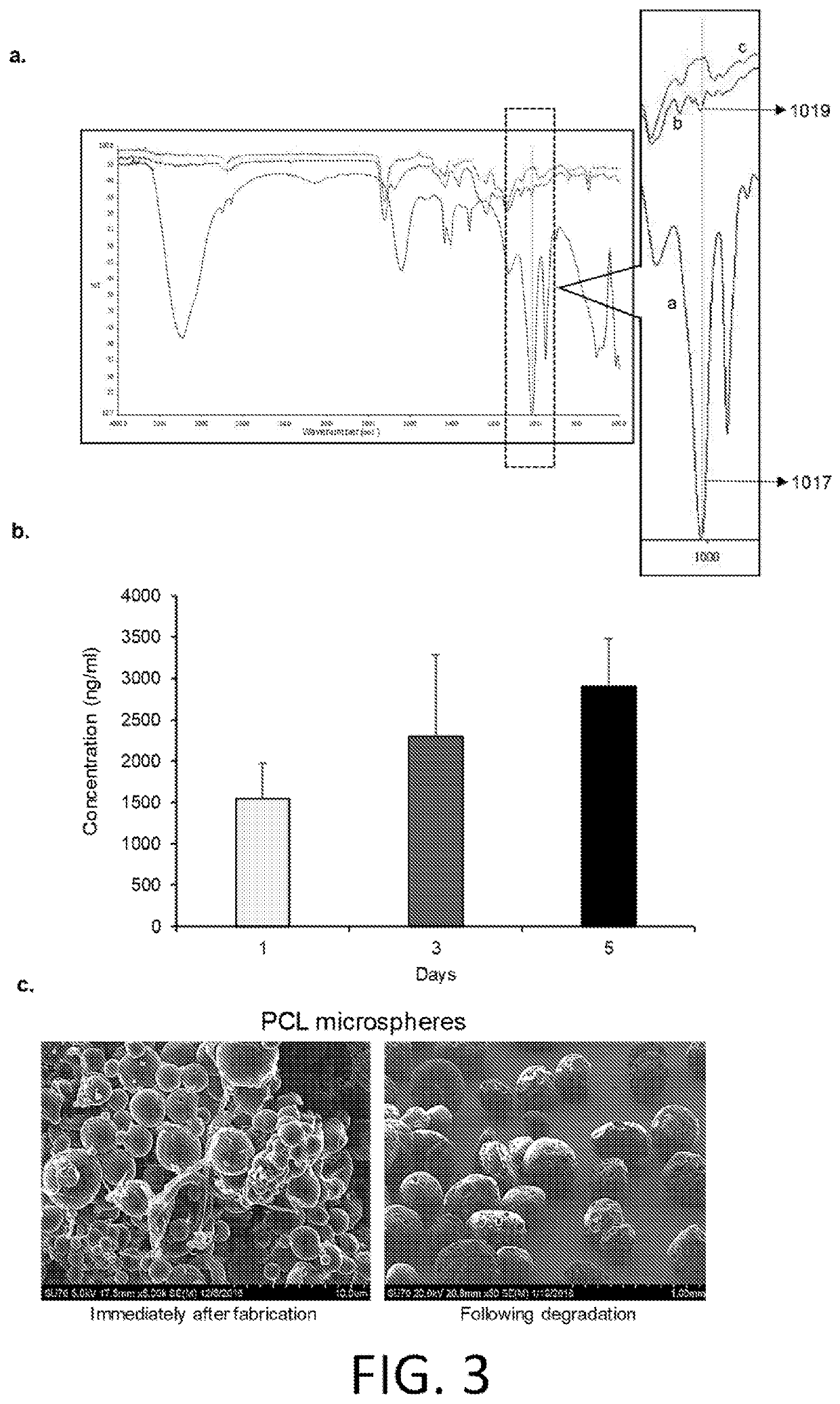
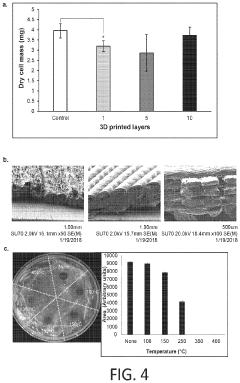
Research on Volumetric 3D printing for high throughput dental and biomedical applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Volumetric 3D Printing Evolution and Objectives
Volumetric 3D printing represents a paradigm shift in additive manufacturing technology, evolving from traditional layer-by-layer approaches to a revolutionary technique that creates entire objects simultaneously. This evolution began in the early 2010s with the theoretical exploration of holographic and tomographic printing methods, but significant practical breakthroughs emerged only in the late 2010s. The technology leverages principles of computed tomography in reverse, projecting carefully calculated light patterns into photosensitive resins to solidify complete 3D structures in seconds rather than hours.
The historical trajectory of volumetric printing shows a clear acceleration in development over the past five years, with key milestones including the 2019 publication in Science demonstrating the first commercially viable volumetric printing system capable of creating complex structures in under 30 seconds. This represented a dramatic improvement over traditional stereolithography, which might require hours for comparable objects.
In the dental and biomedical domains specifically, volumetric printing has evolved from academic curiosity to practical application. Early implementations focused on simple dental models, while recent advances have enabled the production of patient-specific dental prosthetics, surgical guides, and even scaffold structures for tissue engineering with unprecedented speed and precision.
The primary technical objective of volumetric 3D printing research for dental and biomedical applications is to achieve high-throughput production of biocompatible, patient-specific components with micron-level precision. This includes developing systems capable of printing complete dental aligners, crowns, or surgical guides in minutes rather than hours, while maintaining or exceeding the material properties and dimensional accuracy of traditional manufacturing methods.
Secondary objectives include expanding the range of compatible biomaterials, increasing build volumes while maintaining resolution, and integrating real-time quality control systems. The technology aims to enable point-of-care manufacturing in clinical settings, reducing patient wait times from days or weeks to potentially minutes or hours for custom medical devices.
The long-term vision encompasses the development of multi-material volumetric printing capabilities that could revolutionize tissue engineering by creating complex, heterogeneous structures with embedded functional gradients mimicking natural tissues. This would potentially enable the rapid fabrication of tissue constructs with spatially controlled mechanical properties and biochemical compositions, addressing critical challenges in regenerative medicine.
The historical trajectory of volumetric printing shows a clear acceleration in development over the past five years, with key milestones including the 2019 publication in Science demonstrating the first commercially viable volumetric printing system capable of creating complex structures in under 30 seconds. This represented a dramatic improvement over traditional stereolithography, which might require hours for comparable objects.
In the dental and biomedical domains specifically, volumetric printing has evolved from academic curiosity to practical application. Early implementations focused on simple dental models, while recent advances have enabled the production of patient-specific dental prosthetics, surgical guides, and even scaffold structures for tissue engineering with unprecedented speed and precision.
The primary technical objective of volumetric 3D printing research for dental and biomedical applications is to achieve high-throughput production of biocompatible, patient-specific components with micron-level precision. This includes developing systems capable of printing complete dental aligners, crowns, or surgical guides in minutes rather than hours, while maintaining or exceeding the material properties and dimensional accuracy of traditional manufacturing methods.
Secondary objectives include expanding the range of compatible biomaterials, increasing build volumes while maintaining resolution, and integrating real-time quality control systems. The technology aims to enable point-of-care manufacturing in clinical settings, reducing patient wait times from days or weeks to potentially minutes or hours for custom medical devices.
The long-term vision encompasses the development of multi-material volumetric printing capabilities that could revolutionize tissue engineering by creating complex, heterogeneous structures with embedded functional gradients mimicking natural tissues. This would potentially enable the rapid fabrication of tissue constructs with spatially controlled mechanical properties and biochemical compositions, addressing critical challenges in regenerative medicine.
Dental and Biomedical Market Demand Analysis
The dental and biomedical markets represent significant growth opportunities for volumetric 3D printing technologies, driven by increasing demand for personalized healthcare solutions and efficient manufacturing processes. The global dental 3D printing market was valued at $2.9 billion in 2022 and is projected to reach $7.9 billion by 2028, growing at a CAGR of approximately 18.4%. This substantial growth reflects the industry's rapid adoption of advanced manufacturing technologies.
In the dental sector, there is a strong demand for high-throughput solutions capable of producing custom dental prosthetics, aligners, crowns, bridges, and surgical guides. Traditional manufacturing methods often involve labor-intensive processes with multiple steps, creating bottlenecks in production. Dental laboratories and clinics are actively seeking technologies that can reduce turnaround time while maintaining high precision and biocompatibility standards.
The biomedical market similarly shows robust demand for volumetric 3D printing applications. The global biomedical 3D printing market was valued at $1.4 billion in 2022 and is expected to grow to $4.5 billion by 2027, representing a CAGR of 26.2%. This growth is fueled by increasing applications in tissue engineering, personalized implants, drug delivery systems, and anatomical models for surgical planning.
Healthcare providers are particularly interested in solutions that can produce patient-specific implants and prosthetics with complex geometries that closely mimic natural tissues. The ability to rapidly prototype and manufacture these devices significantly improves patient outcomes while reducing surgical complications and recovery times.
Market research indicates that end-users are willing to invest in volumetric 3D printing technologies that demonstrate clear advantages in throughput, resolution, and material compatibility. A survey of dental laboratories revealed that 78% consider production speed a critical factor in technology adoption decisions, while 82% prioritize accuracy and surface finish quality.
Regulatory considerations are also shaping market demand. As healthcare regulations evolve to accommodate 3D printed medical devices, manufacturers are seeking technologies with validated workflows that can meet stringent quality control requirements and documentation standards. This regulatory landscape creates both challenges and opportunities for volumetric 3D printing technologies.
Geographically, North America currently leads the dental and biomedical 3D printing market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness of advanced manufacturing technologies, and expanding dental tourism in countries like China, India, and South Korea.
In the dental sector, there is a strong demand for high-throughput solutions capable of producing custom dental prosthetics, aligners, crowns, bridges, and surgical guides. Traditional manufacturing methods often involve labor-intensive processes with multiple steps, creating bottlenecks in production. Dental laboratories and clinics are actively seeking technologies that can reduce turnaround time while maintaining high precision and biocompatibility standards.
The biomedical market similarly shows robust demand for volumetric 3D printing applications. The global biomedical 3D printing market was valued at $1.4 billion in 2022 and is expected to grow to $4.5 billion by 2027, representing a CAGR of 26.2%. This growth is fueled by increasing applications in tissue engineering, personalized implants, drug delivery systems, and anatomical models for surgical planning.
Healthcare providers are particularly interested in solutions that can produce patient-specific implants and prosthetics with complex geometries that closely mimic natural tissues. The ability to rapidly prototype and manufacture these devices significantly improves patient outcomes while reducing surgical complications and recovery times.
Market research indicates that end-users are willing to invest in volumetric 3D printing technologies that demonstrate clear advantages in throughput, resolution, and material compatibility. A survey of dental laboratories revealed that 78% consider production speed a critical factor in technology adoption decisions, while 82% prioritize accuracy and surface finish quality.
Regulatory considerations are also shaping market demand. As healthcare regulations evolve to accommodate 3D printed medical devices, manufacturers are seeking technologies with validated workflows that can meet stringent quality control requirements and documentation standards. This regulatory landscape creates both challenges and opportunities for volumetric 3D printing technologies.
Geographically, North America currently leads the dental and biomedical 3D printing market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness of advanced manufacturing technologies, and expanding dental tourism in countries like China, India, and South Korea.
Current Limitations in High-Throughput Bioprinting
Despite significant advancements in bioprinting technologies, high-throughput volumetric 3D printing for dental and biomedical applications faces several critical limitations that impede its widespread adoption and clinical translation. Traditional layer-by-layer bioprinting methods suffer from inherently slow processing speeds, with complex structures often requiring hours to complete, making them impractical for high-volume production environments such as dental clinics or biomedical manufacturing facilities.
Resolution constraints represent another significant challenge, particularly for dental applications where sub-micron precision is often required for proper fit and functionality. Current volumetric printing systems struggle to achieve the necessary resolution while maintaining high throughput, creating a technological bottleneck that limits application scope. This resolution-speed tradeoff remains one of the most persistent challenges in the field.
Material compatibility issues further complicate high-throughput bioprinting. The photopolymerization process central to volumetric printing requires biocompatible resins with specific optical properties, yet many biocompatible materials exhibit poor light penetration characteristics or insufficient mechanical properties. For dental applications, materials must simultaneously satisfy biocompatibility requirements while providing appropriate mechanical strength and aesthetic qualities.
Scalability concerns also plague current systems. Many promising volumetric printing approaches demonstrate excellent results at laboratory scales but encounter significant engineering challenges when scaled to industrial production volumes. These challenges include maintaining optical precision across larger build volumes and ensuring consistent material properties throughout printed structures.
Cell viability and functionality represent critical limitations for biomedical applications. The high-energy light exposure necessary for rapid volumetric printing can damage embedded cells or biological molecules, compromising the biological functionality of printed constructs. Current systems struggle to balance printing speed with maintaining cellular viability, particularly for complex tissue constructs containing multiple cell types.
Post-processing requirements further reduce effective throughput. Many volumetric printed structures require extensive cleaning, curing, or other post-processing steps that can become bottlenecks in production workflows. These additional steps often require manual intervention, limiting the true throughput potential of the technology.
Regulatory hurdles also present significant barriers, particularly for medical applications. Novel materials and processes must undergo rigorous safety testing and validation before clinical implementation, a process that can take years and millions in investment. The lack of standardized testing protocols specifically designed for volumetric bioprinting further complicates regulatory approval pathways.
Resolution constraints represent another significant challenge, particularly for dental applications where sub-micron precision is often required for proper fit and functionality. Current volumetric printing systems struggle to achieve the necessary resolution while maintaining high throughput, creating a technological bottleneck that limits application scope. This resolution-speed tradeoff remains one of the most persistent challenges in the field.
Material compatibility issues further complicate high-throughput bioprinting. The photopolymerization process central to volumetric printing requires biocompatible resins with specific optical properties, yet many biocompatible materials exhibit poor light penetration characteristics or insufficient mechanical properties. For dental applications, materials must simultaneously satisfy biocompatibility requirements while providing appropriate mechanical strength and aesthetic qualities.
Scalability concerns also plague current systems. Many promising volumetric printing approaches demonstrate excellent results at laboratory scales but encounter significant engineering challenges when scaled to industrial production volumes. These challenges include maintaining optical precision across larger build volumes and ensuring consistent material properties throughout printed structures.
Cell viability and functionality represent critical limitations for biomedical applications. The high-energy light exposure necessary for rapid volumetric printing can damage embedded cells or biological molecules, compromising the biological functionality of printed constructs. Current systems struggle to balance printing speed with maintaining cellular viability, particularly for complex tissue constructs containing multiple cell types.
Post-processing requirements further reduce effective throughput. Many volumetric printed structures require extensive cleaning, curing, or other post-processing steps that can become bottlenecks in production workflows. These additional steps often require manual intervention, limiting the true throughput potential of the technology.
Regulatory hurdles also present significant barriers, particularly for medical applications. Novel materials and processes must undergo rigorous safety testing and validation before clinical implementation, a process that can take years and millions in investment. The lack of standardized testing protocols specifically designed for volumetric bioprinting further complicates regulatory approval pathways.
Current High-Throughput Volumetric Printing Solutions
01 Volumetric 3D printing techniques for high throughput manufacturing
Volumetric 3D printing enables high throughput manufacturing by creating entire objects simultaneously rather than layer-by-layer. This approach uses techniques such as computed tomography (CT) and optical projection to cure entire volumes of photosensitive resin at once, significantly reducing production time compared to conventional methods. These techniques allow for complex geometries to be produced rapidly while maintaining high resolution and structural integrity.- Volumetric 3D printing techniques for high throughput manufacturing: Volumetric 3D printing enables high throughput manufacturing by simultaneously curing entire volumes of photopolymer resin, rather than building objects layer by layer. This approach significantly reduces production time while maintaining high resolution. Various techniques such as computed axial lithography and tomographic volumetric additive manufacturing allow for the rapid fabrication of complex structures without mechanical movement of the build platform, enabling continuous production processes.
- Advanced light projection systems for volumetric printing: High throughput volumetric 3D printing relies on sophisticated light projection systems that can precisely control light patterns in three-dimensional space. These systems utilize digital light processing technology, spatial light modulators, and specialized optics to project computed patterns that selectively cure resin at specific points within the volume. The synchronization of multiple light sources from different angles enables the creation of complex geometries with high resolution and speed.
- Materials and formulations for high-speed volumetric printing: Specialized photopolymer formulations are essential for high throughput volumetric 3D printing. These materials feature carefully balanced photoinitiators, absorbers, and rheological modifiers that enable rapid curing while maintaining optical clarity throughout the printing process. Advanced resin systems with tunable viscosity and curing thresholds allow for faster printing speeds and improved feature resolution, contributing to higher manufacturing throughput.
- Computational methods for volumetric image processing and optimization: High throughput volumetric 3D printing depends on sophisticated computational algorithms for image processing and optimization. These methods include tomographic reconstruction techniques, real-time feedback systems, and machine learning approaches that calculate optimal light patterns for complex geometries. Advanced computational frameworks enable the precise control of energy distribution within the resin volume, resulting in faster print speeds and higher accuracy.
- System integration for industrial-scale volumetric printing: Achieving high throughput with volumetric 3D printing requires sophisticated system integration that combines hardware, software, and materials science. These integrated systems incorporate automated material handling, in-process quality monitoring, and parallel processing capabilities to maximize production efficiency. Advanced control systems coordinate multiple subsystems to enable continuous operation, reducing downtime between print jobs and scaling production capacity for industrial applications.
02 Light-based volumetric printing systems for increased production speed
Advanced light-based systems for volumetric 3D printing utilize specialized light sources, projection techniques, and optical arrangements to achieve high throughput manufacturing. These systems employ technologies such as digital light processing (DLP), stereolithography (SLA), or holographic projections to cure photopolymers rapidly. By optimizing light delivery and controlling curing parameters, these systems can significantly increase production speed while maintaining precision in the printed objects.Expand Specific Solutions03 Materials and formulations for high-speed volumetric printing
Specialized materials and formulations are crucial for high throughput volumetric 3D printing. These include fast-curing photopolymers, hybrid resins with enhanced mechanical properties, and materials with controlled viscosity for rapid flow and settling. Advanced formulations may incorporate nanoparticles or other additives to improve curing speed, resolution, and final part properties, enabling faster production cycles without compromising quality.Expand Specific Solutions04 Computational methods for optimizing volumetric printing processes
Sophisticated computational methods are employed to optimize volumetric 3D printing for high throughput. These include advanced algorithms for tomographic reconstruction, real-time process monitoring, and machine learning approaches for print parameter optimization. Computational techniques help in predicting and compensating for optical distortions, ensuring uniform curing throughout the volume, and minimizing print defects, thereby enhancing both speed and quality of the printing process.Expand Specific Solutions05 Multi-material and parallel processing in volumetric printing
Multi-material capabilities and parallel processing techniques enhance the throughput of volumetric 3D printing. These approaches involve simultaneous printing of different materials or concurrent processing of multiple objects. Advanced systems may incorporate multiple light sources, rotating build platforms, or segmented resin vats to enable parallel fabrication. These techniques not only increase production speed but also expand the range of applications by allowing for functionally graded materials and complex multi-component structures.Expand Specific Solutions
Leading Companies in Biomedical 3D Printing
Volumetric 3D printing for dental and biomedical applications is currently in the growth phase, with an expanding market driven by increasing demand for personalized medical solutions. The global market is projected to reach significant scale as healthcare providers seek higher throughput manufacturing capabilities. Technologically, the field shows varying maturity levels across players: established dental companies like Align Technology, Ivoclar Vivadent, and 3Shape lead with commercial applications, while research institutions (EPFL, KU Leuven, HKU) advance fundamental capabilities. Companies like Regenhu and polySpectra are developing specialized materials and printing systems, while emerging players from China (Guangzhou Haige, Shanghai Zhengya) are rapidly gaining ground with cost-effective solutions. The convergence of academic research and industrial implementation suggests the technology is approaching mainstream adoption for high-volume dental applications, with biomedical applications following closely behind.
Align Technology, Inc.
Technical Solution: Align Technology has pioneered volumetric 3D printing technology specifically for dental applications, focusing on their Digital Aligner Manufacturing (DAM) system. Their approach utilizes continuous liquid interface production (CLIP) technology, which enables the creation of complex dental models and aligners at significantly higher speeds than traditional layer-by-layer printing. The company has developed proprietary photopolymer resins optimized for biocompatibility and mechanical properties required for dental applications. Their volumetric printing process achieves throughput rates up to 20 times faster than conventional methods by projecting multiple cross-sectional images simultaneously into a photosensitive resin, allowing entire dental models to be formed in minutes rather than hours. This technology has been integrated into their end-to-end digital workflow, from intraoral scanning to final aligner production, enabling mass customization of orthodontic devices with reduced production time and costs.
Strengths: Industry-leading throughput rates for dental applications; proprietary biocompatible materials; seamless integration with existing digital workflow systems; high precision suitable for orthodontic requirements. Weaknesses: High initial equipment investment; limited to specific dental applications; requires specialized training for operation and maintenance.
3Shape A/S
Technical Solution: 3Shape has integrated volumetric 3D printing technology into their comprehensive digital dentistry ecosystem. Their approach combines tomographic volumetric additive manufacturing (TVAM) with their industry-leading dental scanning and CAD software. The system utilizes synchronized multi-angle light projection to cure entire dental models, crowns, and surgical guides simultaneously throughout a photosensitive resin volume. This technology achieves printing speeds up to 50 times faster than conventional layer-by-layer methods while maintaining accuracy within 25 microns. 3Shape has developed proprietary algorithms that calculate optimal projection patterns to minimize optical distortion and ensure uniform curing throughout the volume. Their system is particularly effective for high-throughput production environments, capable of producing up to 300 unique dental models daily on a single machine. The technology has been optimized for compatibility with their TRIOS intraoral scanners and dental design software, creating an end-to-end digital workflow from patient scan to final printed product.
Strengths: Seamless integration with existing digital dental workflows; high accuracy suitable for precision dental applications; exceptional throughput capabilities; reduced material waste compared to traditional methods. Weaknesses: Limited material selection compared to conventional manufacturing; higher initial investment costs; requires specialized training; technology still evolving for certain complex dental structures.
Critical Patents in Dental and Biomedical 3D Printing
Functionalized prosthetic interfaces for the prevention and treatment of dental conditions
PatentPendingUS20220160488A1
Innovation
- 3D printing of dentures using PMMA polymer filaments with antimicrobial agents encapsulated in biocompatible and biodegradable PCL microspheres, which are layered onto the surface for sustained drug release, providing an antimicrobial interface while maintaining the mechanical properties of PMMA.
Biocompatibility and Material Science Considerations
Biocompatibility represents a critical consideration in volumetric 3D printing for dental and biomedical applications, requiring materials that can safely interface with human tissues without triggering adverse immune responses. The materials used must meet stringent regulatory standards, including ISO 10993 for biomedical devices and FDA requirements for dental applications, which evaluate cytotoxicity, sensitization, irritation, and systemic toxicity.
Material science considerations for volumetric 3D printing extend beyond biocompatibility to include mechanical properties that match target tissues. For dental applications, materials must withstand masticatory forces while maintaining dimensional stability, with elastic moduli ranging from 1-20 GPa depending on the specific application. Biomedical implants require tailored mechanical properties that mimic surrounding tissues to prevent stress shielding and promote proper integration.
Photopolymerizable resins dominate the volumetric 3D printing landscape, with methacrylate and acrylate-based systems being particularly prevalent. Recent advances have introduced thiol-ene chemistry and hybrid ceramic-polymer composites that offer improved biocompatibility profiles. These materials demonstrate reduced cytotoxicity compared to traditional photopolymers while maintaining the rapid curing characteristics essential for volumetric printing techniques.
Degradation kinetics represent another crucial consideration, particularly for temporary implants or scaffolds. Controlled biodegradation rates must align with tissue regeneration timelines, typically ranging from weeks to months depending on the application. Materials science innovations have enabled precise tuning of degradation profiles through molecular weight adjustments and incorporation of enzymatically cleavable linkages.
Surface chemistry modifications have emerged as a promising approach to enhance biocompatibility while maintaining bulk material properties. Techniques such as plasma treatment, layer-by-layer deposition, and biomolecule grafting can create cell-friendly interfaces without compromising structural integrity. These modifications can promote specific cellular responses, including improved adhesion, proliferation, and differentiation of relevant cell types.
The integration of bioactive components into printable materials represents a frontier in volumetric 3D printing research. Incorporation of growth factors, antimicrobial agents, and cell-signaling molecules can enhance therapeutic outcomes. However, these additives must maintain stability during the printing process, which often involves exposure to high-energy light sources that could potentially denature bioactive components.
Material science considerations for volumetric 3D printing extend beyond biocompatibility to include mechanical properties that match target tissues. For dental applications, materials must withstand masticatory forces while maintaining dimensional stability, with elastic moduli ranging from 1-20 GPa depending on the specific application. Biomedical implants require tailored mechanical properties that mimic surrounding tissues to prevent stress shielding and promote proper integration.
Photopolymerizable resins dominate the volumetric 3D printing landscape, with methacrylate and acrylate-based systems being particularly prevalent. Recent advances have introduced thiol-ene chemistry and hybrid ceramic-polymer composites that offer improved biocompatibility profiles. These materials demonstrate reduced cytotoxicity compared to traditional photopolymers while maintaining the rapid curing characteristics essential for volumetric printing techniques.
Degradation kinetics represent another crucial consideration, particularly for temporary implants or scaffolds. Controlled biodegradation rates must align with tissue regeneration timelines, typically ranging from weeks to months depending on the application. Materials science innovations have enabled precise tuning of degradation profiles through molecular weight adjustments and incorporation of enzymatically cleavable linkages.
Surface chemistry modifications have emerged as a promising approach to enhance biocompatibility while maintaining bulk material properties. Techniques such as plasma treatment, layer-by-layer deposition, and biomolecule grafting can create cell-friendly interfaces without compromising structural integrity. These modifications can promote specific cellular responses, including improved adhesion, proliferation, and differentiation of relevant cell types.
The integration of bioactive components into printable materials represents a frontier in volumetric 3D printing research. Incorporation of growth factors, antimicrobial agents, and cell-signaling molecules can enhance therapeutic outcomes. However, these additives must maintain stability during the printing process, which often involves exposure to high-energy light sources that could potentially denature bioactive components.
Regulatory Pathway for Medical 3D Printed Products
The regulatory landscape for medical 3D printed products, particularly those produced through volumetric 3D printing for dental and biomedical applications, presents a complex framework that manufacturers must navigate. The U.S. Food and Drug Administration (FDA) has established specific pathways for these innovative technologies, categorizing them based on risk classification and intended use rather than manufacturing method.
For volumetric 3D printed dental and biomedical products, manufacturers typically follow one of three regulatory routes: 510(k) premarket notification, De Novo classification, or Premarket Approval (PMA). The 510(k) pathway, most common for moderate-risk devices, requires demonstrating substantial equivalence to a legally marketed predicate device. This presents challenges for novel volumetric printing technologies that may not have clear predicates.
The FDA's guidance document "Technical Considerations for Additive Manufactured Medical Devices" provides a framework specifically addressing quality system requirements, device testing considerations, and validation processes for 3D printed medical products. However, volumetric 3D printing's unique capabilities for high-throughput production introduce additional regulatory considerations regarding batch definition, process validation, and quality control.
International regulatory bodies have developed varying approaches. The European Union's Medical Device Regulation (MDR) classifies 3D printed medical products based on risk categories and requires CE marking. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for additive manufacturing technologies in medical applications, while China's National Medical Products Administration (NMPA) continues to evolve its regulatory framework for these technologies.
Point-of-care manufacturing, particularly relevant for high-throughput dental applications, introduces additional regulatory complexities. The FDA has begun addressing this through discussion papers on point-of-care manufacturing, requiring clear delineation of responsibilities between technology providers and healthcare facilities implementing these systems.
For biomedical applications involving living cells or tissues, volumetric 3D printing faces additional regulatory hurdles under combination product frameworks or advanced therapy medicinal product (ATMP) regulations in various jurisdictions. These applications often require extensive preclinical and clinical data demonstrating both safety and efficacy.
Manufacturers pursuing volumetric 3D printing technologies must implement robust quality management systems that address the unique aspects of this manufacturing process, including material characterization, process validation, and final product verification. Early engagement with regulatory authorities through pre-submission consultations is highly recommended to establish appropriate testing protocols and data requirements for these innovative manufacturing approaches.
For volumetric 3D printed dental and biomedical products, manufacturers typically follow one of three regulatory routes: 510(k) premarket notification, De Novo classification, or Premarket Approval (PMA). The 510(k) pathway, most common for moderate-risk devices, requires demonstrating substantial equivalence to a legally marketed predicate device. This presents challenges for novel volumetric printing technologies that may not have clear predicates.
The FDA's guidance document "Technical Considerations for Additive Manufactured Medical Devices" provides a framework specifically addressing quality system requirements, device testing considerations, and validation processes for 3D printed medical products. However, volumetric 3D printing's unique capabilities for high-throughput production introduce additional regulatory considerations regarding batch definition, process validation, and quality control.
International regulatory bodies have developed varying approaches. The European Union's Medical Device Regulation (MDR) classifies 3D printed medical products based on risk categories and requires CE marking. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for additive manufacturing technologies in medical applications, while China's National Medical Products Administration (NMPA) continues to evolve its regulatory framework for these technologies.
Point-of-care manufacturing, particularly relevant for high-throughput dental applications, introduces additional regulatory complexities. The FDA has begun addressing this through discussion papers on point-of-care manufacturing, requiring clear delineation of responsibilities between technology providers and healthcare facilities implementing these systems.
For biomedical applications involving living cells or tissues, volumetric 3D printing faces additional regulatory hurdles under combination product frameworks or advanced therapy medicinal product (ATMP) regulations in various jurisdictions. These applications often require extensive preclinical and clinical data demonstrating both safety and efficacy.
Manufacturers pursuing volumetric 3D printing technologies must implement robust quality management systems that address the unique aspects of this manufacturing process, including material characterization, process validation, and final product verification. Early engagement with regulatory authorities through pre-submission consultations is highly recommended to establish appropriate testing protocols and data requirements for these innovative manufacturing approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!