Volumetric 3D printing for biomedical scaffolds and personalized medical devices
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical 3D Printing Evolution and Objectives
The evolution of biomedical 3D printing represents a transformative journey in healthcare manufacturing, progressing from basic prototyping to advanced functional tissue engineering. Initially emerging in the 1990s, biomedical 3D printing focused primarily on anatomical models for surgical planning and education. The early 2000s witnessed significant advancements with the introduction of powder bed fusion and material extrusion techniques, enabling the creation of custom implants and prosthetics with improved biocompatibility.
A pivotal shift occurred around 2010 with the development of bioprinting technologies capable of depositing living cells alongside supporting biomaterials. This breakthrough expanded the application scope from static medical devices to potentially viable tissue constructs. The integration of medical imaging data with 3D printing workflows further revolutionized personalized medicine, allowing for patient-specific solutions based on CT or MRI scans.
Volumetric 3D printing represents the latest evolutionary milestone in this field. Unlike traditional layer-by-layer approaches, volumetric techniques generate entire structures simultaneously through photopolymerization within a volume of resin. This methodology offers unprecedented advantages for biomedical applications, including dramatically reduced production times, elimination of layer lines that compromise mechanical integrity, and the ability to create complex internal architectures essential for tissue scaffolds.
The primary objectives of volumetric 3D printing in the biomedical sector encompass several ambitious goals. First, developing high-resolution printing capabilities that can accurately replicate the intricate microstructures found in natural tissues, including vascular networks and cellular niches. Second, expanding the range of biocompatible and biodegradable materials compatible with volumetric techniques to better mimic the mechanical and biological properties of native tissues.
Another critical objective involves optimizing cell viability during the printing process, as the simultaneous exposure approach presents unique challenges for incorporating living components. Researchers aim to achieve functional tissue constructs with appropriate cellular organization and vascularization to support nutrient delivery and waste removal. For personalized medical devices, objectives include streamlining the workflow from patient imaging to final product while maintaining precision and reproducibility.
Long-term goals extend to creating implantable tissues and organs with proper function, addressing the global organ shortage crisis. The technology also aims to revolutionize drug testing by providing more physiologically relevant tissue models than traditional cell cultures, potentially reducing animal testing and accelerating pharmaceutical development.
A pivotal shift occurred around 2010 with the development of bioprinting technologies capable of depositing living cells alongside supporting biomaterials. This breakthrough expanded the application scope from static medical devices to potentially viable tissue constructs. The integration of medical imaging data with 3D printing workflows further revolutionized personalized medicine, allowing for patient-specific solutions based on CT or MRI scans.
Volumetric 3D printing represents the latest evolutionary milestone in this field. Unlike traditional layer-by-layer approaches, volumetric techniques generate entire structures simultaneously through photopolymerization within a volume of resin. This methodology offers unprecedented advantages for biomedical applications, including dramatically reduced production times, elimination of layer lines that compromise mechanical integrity, and the ability to create complex internal architectures essential for tissue scaffolds.
The primary objectives of volumetric 3D printing in the biomedical sector encompass several ambitious goals. First, developing high-resolution printing capabilities that can accurately replicate the intricate microstructures found in natural tissues, including vascular networks and cellular niches. Second, expanding the range of biocompatible and biodegradable materials compatible with volumetric techniques to better mimic the mechanical and biological properties of native tissues.
Another critical objective involves optimizing cell viability during the printing process, as the simultaneous exposure approach presents unique challenges for incorporating living components. Researchers aim to achieve functional tissue constructs with appropriate cellular organization and vascularization to support nutrient delivery and waste removal. For personalized medical devices, objectives include streamlining the workflow from patient imaging to final product while maintaining precision and reproducibility.
Long-term goals extend to creating implantable tissues and organs with proper function, addressing the global organ shortage crisis. The technology also aims to revolutionize drug testing by providing more physiologically relevant tissue models than traditional cell cultures, potentially reducing animal testing and accelerating pharmaceutical development.
Market Analysis for Volumetric Bioprinting Applications
The volumetric bioprinting market is experiencing rapid growth, driven by increasing demand for personalized medical solutions and tissue engineering applications. Current market valuations indicate the global 3D bioprinting sector reached approximately $1.3 billion in 2022, with volumetric bioprinting representing an emerging segment poised for accelerated adoption. Industry forecasts project a compound annual growth rate of 15-20% for the broader bioprinting market through 2030, with volumetric techniques potentially capturing significant market share due to their superior speed and resolution capabilities.
Healthcare institutions represent the largest customer segment, particularly academic medical centers and research hospitals investing in advanced regenerative medicine capabilities. Pharmaceutical companies constitute the second-largest market segment, utilizing bioprinted tissues for drug testing and development, potentially reducing animal testing requirements and accelerating clinical trials.
Geographically, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and Singapore, demonstrates the fastest growth trajectory due to substantial government investments in biomedical research infrastructure and supportive regulatory frameworks.
Key market drivers include the aging global population increasing demand for organ transplants and tissue replacements, rising prevalence of chronic diseases requiring personalized treatment approaches, and growing adoption of regenerative medicine techniques. The shortage of donor organs represents a particularly significant market opportunity, with over 100,000 patients on organ transplant waiting lists in the United States alone.
Regulatory considerations remain a critical market factor, with the FDA and equivalent international bodies developing frameworks specifically for bioprinted products. Recent regulatory approvals for bioprinted skin substitutes and cartilage implants have established precedents that may accelerate future commercialization pathways.
Reimbursement models are evolving to accommodate these novel therapies, with several insurance providers beginning to cover certain bioprinted tissue applications. This trend is expected to expand as clinical evidence accumulates demonstrating improved patient outcomes and potential long-term cost savings compared to traditional treatments.
Market barriers include high equipment costs, with advanced volumetric bioprinting systems typically ranging from $200,000 to $500,000, limiting adoption to well-funded institutions. Technical challenges in achieving vascularization of complex tissues and scaling production also constrain immediate market growth, though these limitations are being addressed through ongoing research and development efforts.
Healthcare institutions represent the largest customer segment, particularly academic medical centers and research hospitals investing in advanced regenerative medicine capabilities. Pharmaceutical companies constitute the second-largest market segment, utilizing bioprinted tissues for drug testing and development, potentially reducing animal testing requirements and accelerating clinical trials.
Geographically, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and Singapore, demonstrates the fastest growth trajectory due to substantial government investments in biomedical research infrastructure and supportive regulatory frameworks.
Key market drivers include the aging global population increasing demand for organ transplants and tissue replacements, rising prevalence of chronic diseases requiring personalized treatment approaches, and growing adoption of regenerative medicine techniques. The shortage of donor organs represents a particularly significant market opportunity, with over 100,000 patients on organ transplant waiting lists in the United States alone.
Regulatory considerations remain a critical market factor, with the FDA and equivalent international bodies developing frameworks specifically for bioprinted products. Recent regulatory approvals for bioprinted skin substitutes and cartilage implants have established precedents that may accelerate future commercialization pathways.
Reimbursement models are evolving to accommodate these novel therapies, with several insurance providers beginning to cover certain bioprinted tissue applications. This trend is expected to expand as clinical evidence accumulates demonstrating improved patient outcomes and potential long-term cost savings compared to traditional treatments.
Market barriers include high equipment costs, with advanced volumetric bioprinting systems typically ranging from $200,000 to $500,000, limiting adoption to well-funded institutions. Technical challenges in achieving vascularization of complex tissues and scaling production also constrain immediate market growth, though these limitations are being addressed through ongoing research and development efforts.
Technical Barriers in Volumetric 3D Bioprinting
Despite significant advancements in volumetric 3D bioprinting technology, several critical technical barriers continue to impede its widespread adoption for biomedical scaffolds and personalized medical devices. The foremost challenge lies in achieving sufficient resolution for complex biological structures. Current volumetric printing systems typically offer resolution in the range of 50-100 μm, which remains inadequate for replicating the intricate microarchitecture of many biological tissues that require features at the 1-10 μm scale.
Material limitations represent another substantial hurdle. The photosensitive bioinks must simultaneously satisfy contradictory requirements: they need to be sufficiently transparent to allow light penetration throughout the volume while containing enough photoinitiator to enable rapid polymerization. Additionally, these materials must maintain biocompatibility and provide appropriate mechanical properties for the intended application, creating a complex multi-parameter optimization problem that has not been fully resolved.
The polymerization kinetics in volumetric printing presents unique challenges compared to layer-by-layer approaches. The need for precise control over the gelation process throughout the entire volume simultaneously demands sophisticated light delivery systems and carefully calibrated photoinitiator concentrations. Achieving uniform crosslinking density throughout the printed structure remains problematic, often resulting in mechanical property gradients that can compromise structural integrity.
Cell viability during the printing process constitutes a significant concern. The high-intensity light exposure necessary for rapid volumetric polymerization can generate cytotoxic free radicals and localized heating effects that damage encapsulated cells. Studies have reported variable cell survival rates ranging from 40% to 85% depending on printing parameters, significantly lower than the >90% viability often achieved with extrusion-based bioprinting methods.
Computational limitations further constrain progress in this field. The generation of precise holographic light patterns or synchronized multi-angle projections requires substantial computational resources and sophisticated algorithms. Current software solutions struggle to efficiently calculate optimal light delivery patterns for complex biological geometries while accounting for light scattering and absorption effects within the bioink volume.
Scalability issues persist in transitioning from laboratory demonstrations to clinically relevant applications. Most volumetric bioprinting systems are limited to relatively small build volumes (typically <10 cm³), restricting their utility for larger tissue constructs or organ-scale applications. Additionally, the specialized equipment and expertise required for volumetric bioprinting operations present barriers to widespread clinical implementation.
Material limitations represent another substantial hurdle. The photosensitive bioinks must simultaneously satisfy contradictory requirements: they need to be sufficiently transparent to allow light penetration throughout the volume while containing enough photoinitiator to enable rapid polymerization. Additionally, these materials must maintain biocompatibility and provide appropriate mechanical properties for the intended application, creating a complex multi-parameter optimization problem that has not been fully resolved.
The polymerization kinetics in volumetric printing presents unique challenges compared to layer-by-layer approaches. The need for precise control over the gelation process throughout the entire volume simultaneously demands sophisticated light delivery systems and carefully calibrated photoinitiator concentrations. Achieving uniform crosslinking density throughout the printed structure remains problematic, often resulting in mechanical property gradients that can compromise structural integrity.
Cell viability during the printing process constitutes a significant concern. The high-intensity light exposure necessary for rapid volumetric polymerization can generate cytotoxic free radicals and localized heating effects that damage encapsulated cells. Studies have reported variable cell survival rates ranging from 40% to 85% depending on printing parameters, significantly lower than the >90% viability often achieved with extrusion-based bioprinting methods.
Computational limitations further constrain progress in this field. The generation of precise holographic light patterns or synchronized multi-angle projections requires substantial computational resources and sophisticated algorithms. Current software solutions struggle to efficiently calculate optimal light delivery patterns for complex biological geometries while accounting for light scattering and absorption effects within the bioink volume.
Scalability issues persist in transitioning from laboratory demonstrations to clinically relevant applications. Most volumetric bioprinting systems are limited to relatively small build volumes (typically <10 cm³), restricting their utility for larger tissue constructs or organ-scale applications. Additionally, the specialized equipment and expertise required for volumetric bioprinting operations present barriers to widespread clinical implementation.
Current Volumetric Bioprinting Methodologies
01 Light-based volumetric 3D printing techniques
Volumetric 3D printing can be achieved using various light-based techniques where photosensitive resins are cured by controlled light patterns. These methods include computed axial lithography (CAL), tomographic volumetric additive manufacturing, and holographic printing approaches. By projecting specific light patterns into a volume of photocurable material, entire 3D objects can be formed simultaneously rather than layer-by-layer, significantly reducing printing time and eliminating the need for support structures.- Light-based volumetric 3D printing techniques: Volumetric 3D printing can be achieved using various light-based techniques where patterns of light are projected into photosensitive resins to cure entire volumes simultaneously. These methods include computed axial lithography (CAL), holographic techniques, and multi-beam interference patterns that create complex 3D structures without the need for layer-by-layer processing. This approach significantly reduces printing time and enables the creation of structures that would be difficult to produce with conventional methods.
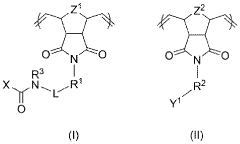
- Materials and formulations for volumetric printing: Specialized photopolymer resins and materials have been developed specifically for volumetric 3D printing applications. These materials feature carefully tuned absorption profiles, photoinitiators, and inhibitors that allow for controlled solidification throughout a volume rather than just at surfaces. Some formulations incorporate nanoparticles or other additives to enhance mechanical properties, optical characteristics, or to enable specific functionalities in the printed objects.
- Tomographic approaches to volumetric printing: Tomographic volumetric printing uses rotating projection systems where multiple 2D images are projected into a photosensitive resin from different angles. As the container rotates, these projections combine to create a 3D structure in a single continuous process. This approach allows for the creation of complex geometries without support structures and can produce objects with exceptional surface quality and internal features that would be challenging with conventional layer-by-layer methods.
- Hardware systems and apparatus for volumetric printing: Specialized hardware systems have been developed for volumetric 3D printing, including optical setups with spatial light modulators, digital micromirror devices, and synchronized projection systems. These systems often incorporate precise rotation mechanisms, temperature control systems, and specialized resin vats designed to optimize the volumetric printing process. Advanced control systems coordinate the timing of light exposure with material properties to achieve high-resolution prints with minimal distortion.
- Applications and advancements in volumetric printing: Volumetric 3D printing has found applications in various fields including biomedical engineering, where it enables the rapid fabrication of tissue scaffolds and anatomical models with complex internal structures. Other applications include microfluidics, optical components, and customized consumer products. Recent advancements have focused on increasing resolution, expanding the range of printable materials, and developing hybrid approaches that combine volumetric techniques with traditional methods to leverage the advantages of both.
02 Materials and formulations for volumetric printing
Specialized photopolymer resins and materials have been developed specifically for volumetric 3D printing applications. These materials feature carefully balanced photoinitiators, absorbers, and other additives that enable controlled solidification throughout a volume. Advanced formulations allow for gradient properties, multi-material printing, and specific mechanical characteristics in the final printed objects. The development of these materials is crucial for expanding the applications of volumetric printing technologies.Expand Specific Solutions03 Volumetric printing systems and hardware configurations
Volumetric 3D printing systems incorporate specialized hardware components including multiple light sources, rotating stages, spatial light modulators, and advanced optics. These systems are designed to project controlled patterns of light into photosensitive materials from multiple angles or in specific sequences. The hardware configurations enable precise control over energy delivery throughout the printing volume, allowing for the creation of complex geometries with high resolution and accuracy.Expand Specific Solutions04 Computational methods for volumetric printing
Advanced computational algorithms are essential for volumetric 3D printing to calculate the precise light patterns needed to create desired 3D structures. These algorithms include tomographic reconstruction techniques, holographic pattern generation, and optimization methods that account for light scattering and absorption within the printing medium. Machine learning approaches have also been developed to improve print quality and speed by predicting optimal exposure patterns and compensating for optical distortions in the printing system.Expand Specific Solutions05 Applications and specialized implementations of volumetric printing
Volumetric 3D printing has been adapted for various specialized applications including bioprinting of tissue constructs, manufacturing of microfluidic devices, production of optical components, and creation of complex mechanical parts. The technology has been implemented in medical fields for creating patient-specific implants, in electronics for embedding components within printed structures, and in rapid prototyping for significantly reduced production times. These specialized implementations often combine volumetric printing with other manufacturing techniques to achieve unique capabilities.Expand Specific Solutions
Industry Leaders in Medical 3D Printing
Volumetric 3D bioprinting for biomedical scaffolds and personalized medical devices is currently in the growth phase of industry development, with an estimated market size of $1.5-2 billion and projected annual growth of 15-20%. The technology is advancing from early research to commercial applications, with varying degrees of maturity across players. Academic institutions like Columbia University, Northwestern University, and Duke University are establishing fundamental research frameworks, while companies such as Theradaptive, CollPlant, and Cellbricks are developing commercial applications. Established players like Covestro and Hon Hai Precision Industry are leveraging their manufacturing expertise to scale production. The competitive landscape shows a healthy mix of academic innovation and commercial development, with increasing focus on regulatory approval pathways for clinical applications.
Brinter, Inc.
Technical Solution: Brinter has developed a modular bioprinting platform specifically designed for volumetric 3D printing of personalized medical devices and tissue scaffolds. Their system incorporates multiple printing technologies within a single platform, including digital light processing for volumetric printing, enabling rapid production of complex structures with high resolution. The company's proprietary software allows for seamless integration of medical imaging data to create patient-specific designs with optimized internal architectures. Brinter's technology supports a wide range of biomaterials, from synthetic polymers to natural hydrogels, providing versatility for different tissue engineering applications. Their printing process incorporates real-time monitoring systems that ensure quality control throughout fabrication, critical for medical device manufacturing. The company has demonstrated successful applications in cartilage regeneration, drug-releasing implants, and personalized orthopedic devices. Brinter's platform enables the creation of gradient structures with varying mechanical properties and bioactive factor concentrations, mimicking the heterogeneous nature of natural tissues. Their volumetric approach significantly reduces production time compared to traditional layer-by-layer methods while maintaining high precision.
Strengths: Modular platform offers flexibility for different applications; supports wide range of biomaterials; integrated quality control systems ensure consistency. Weaknesses: As a smaller company, may have limited resources for extensive clinical validation; modular approach may increase complexity of operation compared to dedicated systems.
Theradaptive, Inc.
Technical Solution: Theradaptive has developed a proprietary platform technology called "AMP2" (Activated Matrix Platform) for volumetric 3D bioprinting that enables precise spatial control of bioactive proteins within scaffolds. Their approach involves binding regenerative proteins directly to implantable medical devices and scaffolds, creating materials that can actively promote tissue regeneration. The company uses computational design to optimize scaffold architecture for specific anatomical requirements while incorporating their protein-binding technology. This allows for the creation of personalized implants that can induce targeted tissue formation exactly where needed. Their volumetric printing technique enables rapid production of complex structures with embedded bioactive components in a single step, significantly reducing manufacturing time compared to traditional layer-by-layer approaches. Theradaptive's technology has shown promising results in orthopedic and craniomaxillofacial applications, with clinical trials demonstrating enhanced bone regeneration capabilities.
Strengths: Superior protein binding technology allows for precise spatial control of bioactive factors; volumetric approach enables faster production of complex structures; demonstrated clinical efficacy in bone regeneration applications. Weaknesses: May require specialized equipment and materials; technology primarily focused on bone applications with less development in soft tissue applications.
Breakthrough Patents in Scaffold Fabrication
A method of producing a bioactive polymer filament, the bioactive polymer filament and printing methods using the same
PatentWO2023033730A2
Innovation
- A method involving the production of a bioactive polymer filament by mixing a base polymer powder with a bioactive copolymer, obtained through ring-opening metathesis polymerization, and extruding it at specific temperature profiles, followed by thermal analysis to ensure onset degradation assessment, suitable for use in fused filament fabrication (FFF) or fused deposition modeling (FDM) 3D printing.
Biomaterial Compatibility and Selection Criteria
The selection of appropriate biomaterials for volumetric 3D printing in biomedical applications requires careful consideration of multiple factors to ensure both functionality and biocompatibility. Biomaterials must meet stringent requirements regarding their interaction with biological systems while maintaining the physical and chemical properties necessary for successful printing and subsequent application.
Primary biocompatibility considerations include cytotoxicity, immunogenicity, and inflammatory response. Materials must not release toxic compounds during degradation or induce adverse immune reactions when implanted. For scaffold applications, the material should promote cell adhesion, proliferation, and differentiation while maintaining appropriate mechanical properties that match the target tissue.
Photopolymerizable hydrogels have emerged as leading candidates for volumetric bioprinting due to their tunable properties and compatibility with light-based curing systems. These include modified gelatin methacrylate (GelMA), polyethylene glycol diacrylate (PEGDA), and hyaluronic acid derivatives. Each offers distinct advantages: GelMA provides excellent cell adhesion properties, PEGDA offers superior mechanical tunability, and hyaluronic acid derivatives enhance tissue integration.
Material selection criteria must also account for the specific requirements of volumetric printing technology. Light penetration depth, curing kinetics, and refractive index matching are critical parameters that influence printing resolution and fidelity. Materials must exhibit appropriate viscosity before curing while achieving adequate mechanical strength post-polymerization to maintain structural integrity.
Degradation kinetics represent another crucial selection criterion. Ideal scaffold materials should degrade at rates that complement tissue regeneration, providing initial support while gradually transferring load to newly formed tissue. Controllable degradation pathways that avoid acidic byproducts are particularly valuable for preventing localized inflammation.
For personalized medical devices, additional considerations include sterilizability, shelf-life stability, and compatibility with imaging modalities for post-implantation monitoring. Materials must withstand sterilization processes without compromising their mechanical or biological properties. Furthermore, regulatory approval pathways must be considered during material selection, with preference given to materials with established safety profiles.
Recent advances have focused on developing composite biomaterials that combine synthetic polymers for mechanical strength with natural components for enhanced bioactivity. These hybrid systems aim to overcome the limitations of single-component materials while leveraging the advantages of each constituent. Additionally, the incorporation of bioactive molecules and growth factors into printable biomaterials represents a promising approach to enhance tissue regeneration and integration.
Primary biocompatibility considerations include cytotoxicity, immunogenicity, and inflammatory response. Materials must not release toxic compounds during degradation or induce adverse immune reactions when implanted. For scaffold applications, the material should promote cell adhesion, proliferation, and differentiation while maintaining appropriate mechanical properties that match the target tissue.
Photopolymerizable hydrogels have emerged as leading candidates for volumetric bioprinting due to their tunable properties and compatibility with light-based curing systems. These include modified gelatin methacrylate (GelMA), polyethylene glycol diacrylate (PEGDA), and hyaluronic acid derivatives. Each offers distinct advantages: GelMA provides excellent cell adhesion properties, PEGDA offers superior mechanical tunability, and hyaluronic acid derivatives enhance tissue integration.
Material selection criteria must also account for the specific requirements of volumetric printing technology. Light penetration depth, curing kinetics, and refractive index matching are critical parameters that influence printing resolution and fidelity. Materials must exhibit appropriate viscosity before curing while achieving adequate mechanical strength post-polymerization to maintain structural integrity.
Degradation kinetics represent another crucial selection criterion. Ideal scaffold materials should degrade at rates that complement tissue regeneration, providing initial support while gradually transferring load to newly formed tissue. Controllable degradation pathways that avoid acidic byproducts are particularly valuable for preventing localized inflammation.
For personalized medical devices, additional considerations include sterilizability, shelf-life stability, and compatibility with imaging modalities for post-implantation monitoring. Materials must withstand sterilization processes without compromising their mechanical or biological properties. Furthermore, regulatory approval pathways must be considered during material selection, with preference given to materials with established safety profiles.
Recent advances have focused on developing composite biomaterials that combine synthetic polymers for mechanical strength with natural components for enhanced bioactivity. These hybrid systems aim to overcome the limitations of single-component materials while leveraging the advantages of each constituent. Additionally, the incorporation of bioactive molecules and growth factors into printable biomaterials represents a promising approach to enhance tissue regeneration and integration.
Regulatory Pathway for Custom Medical Devices
The regulatory landscape for volumetric 3D printing in medical applications presents unique challenges due to the personalized nature of these devices. The FDA has established a framework for regulating medical devices through various pathways, with custom devices falling under specific provisions that acknowledge their individualized characteristics.
For volumetric 3D-printed biomedical scaffolds and personalized medical devices, manufacturers typically navigate between the custom device exemption (21 CFR 812.3(b)) and the premarket approval (PMA) or 510(k) clearance processes. The custom device exemption allows for limited production of devices designed for specific patients without requiring full premarket review, provided they meet strict criteria including documentation of unique patient needs.
The FDA's guidance document "Technical Considerations for Additive Manufactured Medical Devices" (2017) specifically addresses 3D printing technologies, though volumetric printing's novel approach may require additional considerations. This guidance outlines design, manufacturing, and testing expectations, emphasizing the importance of process validation and quality control systems.
For patient-matched devices produced through volumetric 3D printing, manufacturers must implement robust quality management systems that ensure consistency despite the inherent variability in personalized production. This includes validation of the printing process, material characterization, and post-processing procedures that maintain biocompatibility and mechanical integrity.
International regulatory bodies have varying approaches to custom medical devices. The European Union's Medical Device Regulation (MDR) includes specific provisions for custom-made devices under Article 52, requiring manufacturers to prepare documentation demonstrating compliance with general safety and performance requirements while exempting them from certain conformity assessment procedures.
Regulatory submissions for volumetric 3D-printed devices typically require comprehensive data packages including design controls, risk analysis, biocompatibility testing, and clinical evidence where applicable. The FDA's "leap-frog" guidance initiatives aim to address emerging technologies like volumetric printing, potentially offering accelerated pathways for innovative devices that demonstrate substantial benefits.
Manufacturers pursuing commercialization must consider establishing master files that document their manufacturing processes, allowing reference in multiple submissions while protecting proprietary information. Additionally, post-market surveillance requirements are particularly important for these novel devices to monitor long-term performance and safety in diverse patient populations.
For volumetric 3D-printed biomedical scaffolds and personalized medical devices, manufacturers typically navigate between the custom device exemption (21 CFR 812.3(b)) and the premarket approval (PMA) or 510(k) clearance processes. The custom device exemption allows for limited production of devices designed for specific patients without requiring full premarket review, provided they meet strict criteria including documentation of unique patient needs.
The FDA's guidance document "Technical Considerations for Additive Manufactured Medical Devices" (2017) specifically addresses 3D printing technologies, though volumetric printing's novel approach may require additional considerations. This guidance outlines design, manufacturing, and testing expectations, emphasizing the importance of process validation and quality control systems.
For patient-matched devices produced through volumetric 3D printing, manufacturers must implement robust quality management systems that ensure consistency despite the inherent variability in personalized production. This includes validation of the printing process, material characterization, and post-processing procedures that maintain biocompatibility and mechanical integrity.
International regulatory bodies have varying approaches to custom medical devices. The European Union's Medical Device Regulation (MDR) includes specific provisions for custom-made devices under Article 52, requiring manufacturers to prepare documentation demonstrating compliance with general safety and performance requirements while exempting them from certain conformity assessment procedures.
Regulatory submissions for volumetric 3D-printed devices typically require comprehensive data packages including design controls, risk analysis, biocompatibility testing, and clinical evidence where applicable. The FDA's "leap-frog" guidance initiatives aim to address emerging technologies like volumetric printing, potentially offering accelerated pathways for innovative devices that demonstrate substantial benefits.
Manufacturers pursuing commercialization must consider establishing master files that document their manufacturing processes, allowing reference in multiple submissions while protecting proprietary information. Additionally, post-market surveillance requirements are particularly important for these novel devices to monitor long-term performance and safety in diverse patient populations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!