Volumetric 3D printing standards compliance for medical device and aerospace manufacturing
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Volumetric 3D Printing Evolution and Objectives
Volumetric 3D printing represents a paradigm shift in additive manufacturing technology, evolving from traditional layer-by-layer approaches to a revolutionary process that solidifies entire volumes simultaneously. The technology's development can be traced back to the early 2010s when researchers began exploring holographic and tomographic techniques for polymer curing. By 2019, significant breakthroughs emerged with the demonstration of computed axial lithography, enabling the creation of complex structures in seconds rather than hours.
The evolution of volumetric 3D printing has been driven by limitations in conventional additive manufacturing, particularly in medical device and aerospace applications where precision, material properties, and production speed are critical factors. Traditional layer-based methods often introduce mechanical anisotropy and stair-stepping artifacts that compromise structural integrity—unacceptable in high-performance aerospace components or patient-specific medical implants.
Technical advancements have progressed through several key phases: initial proof-of-concept demonstrations, refinement of optical systems for improved resolution, development of specialized photopolymer resins compatible with volumetric curing, and most recently, the integration of real-time monitoring and feedback systems to ensure manufacturing consistency. Each evolutionary stage has incrementally addressed challenges related to resolution, build volume, material compatibility, and process control.
The primary objective of volumetric 3D printing standards compliance for medical and aerospace manufacturing is to establish robust, repeatable processes that meet the stringent regulatory requirements of these highly regulated industries. This includes developing standardized testing protocols for mechanical properties, biocompatibility assessments for medical applications, and qualification procedures for aerospace-grade materials that ensure consistent performance under extreme conditions.
Secondary objectives include increasing manufacturing throughput while maintaining precision, expanding the range of compatible materials beyond photopolymers to include ceramics and metal-polymer composites, and creating closed-loop quality assurance systems that can detect and correct anomalies during the printing process. These capabilities are essential for achieving the certification standards required by regulatory bodies such as the FDA for medical devices and FAA/EASA for aerospace components.
The technology aims to ultimately enable on-demand, distributed manufacturing of complex, high-value components with properties matching or exceeding those produced by conventional methods. For medical applications, this translates to patient-specific implants with optimized mechanical and biological properties; for aerospace, it means lightweight, geometrically optimized components that reduce fuel consumption and enhance performance while meeting rigorous safety standards.
The evolution of volumetric 3D printing has been driven by limitations in conventional additive manufacturing, particularly in medical device and aerospace applications where precision, material properties, and production speed are critical factors. Traditional layer-based methods often introduce mechanical anisotropy and stair-stepping artifacts that compromise structural integrity—unacceptable in high-performance aerospace components or patient-specific medical implants.
Technical advancements have progressed through several key phases: initial proof-of-concept demonstrations, refinement of optical systems for improved resolution, development of specialized photopolymer resins compatible with volumetric curing, and most recently, the integration of real-time monitoring and feedback systems to ensure manufacturing consistency. Each evolutionary stage has incrementally addressed challenges related to resolution, build volume, material compatibility, and process control.
The primary objective of volumetric 3D printing standards compliance for medical and aerospace manufacturing is to establish robust, repeatable processes that meet the stringent regulatory requirements of these highly regulated industries. This includes developing standardized testing protocols for mechanical properties, biocompatibility assessments for medical applications, and qualification procedures for aerospace-grade materials that ensure consistent performance under extreme conditions.
Secondary objectives include increasing manufacturing throughput while maintaining precision, expanding the range of compatible materials beyond photopolymers to include ceramics and metal-polymer composites, and creating closed-loop quality assurance systems that can detect and correct anomalies during the printing process. These capabilities are essential for achieving the certification standards required by regulatory bodies such as the FDA for medical devices and FAA/EASA for aerospace components.
The technology aims to ultimately enable on-demand, distributed manufacturing of complex, high-value components with properties matching or exceeding those produced by conventional methods. For medical applications, this translates to patient-specific implants with optimized mechanical and biological properties; for aerospace, it means lightweight, geometrically optimized components that reduce fuel consumption and enhance performance while meeting rigorous safety standards.
Market Analysis for Medical and Aerospace Applications
The volumetric 3D printing market for medical device and aerospace manufacturing represents a significant growth opportunity, with both sectors increasingly adopting advanced manufacturing technologies to meet their stringent requirements. The global medical 3D printing market was valued at approximately $1.4 billion in 2022 and is projected to grow at a CAGR of 16.2% through 2030, with volumetric printing technologies capturing an expanding share of this market.
In the medical device sector, demand is primarily driven by the need for patient-specific implants, surgical guides, and anatomical models. Hospitals and medical research institutions are increasingly investing in volumetric 3D printing capabilities to produce complex anatomical structures with high precision. The orthopedic segment currently dominates the medical 3D printing market, accounting for roughly 35% of applications, followed by dental prosthetics at 28%.
Regulatory approval pathways are becoming more defined for 3D-printed medical devices, with the FDA having released guidance documents specifically addressing additive manufacturing. This regulatory clarity is encouraging more medical device manufacturers to explore volumetric 3D printing technologies for production applications rather than just prototyping.
The aerospace sector presents another substantial market for volumetric 3D printing, valued at approximately $2.6 billion in 2022 with projected annual growth of 14.8% through 2028. Major aerospace manufacturers including Boeing, Airbus, and GE Aviation have established dedicated additive manufacturing divisions to integrate these technologies into their production workflows.
Key market drivers in aerospace include weight reduction requirements, supply chain optimization, and the ability to produce complex geometries that cannot be manufactured using traditional methods. Components such as fuel nozzles, turbine blades, and structural brackets are increasingly being produced using volumetric 3D printing technologies, offering weight reductions of 30-50% compared to traditionally manufactured parts.
Regional analysis indicates North America currently leads the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to increasing investments in advanced manufacturing capabilities in China, Japan, and South Korea.
Market challenges include the high initial investment costs for volumetric 3D printing systems, which typically range from $500,000 to over $2 million depending on capabilities. Additionally, there remains a skills gap in the workforce trained to operate these advanced systems and design for their unique capabilities, with industry surveys indicating that 68% of manufacturers cite skilled personnel shortages as a significant barrier to adoption.
In the medical device sector, demand is primarily driven by the need for patient-specific implants, surgical guides, and anatomical models. Hospitals and medical research institutions are increasingly investing in volumetric 3D printing capabilities to produce complex anatomical structures with high precision. The orthopedic segment currently dominates the medical 3D printing market, accounting for roughly 35% of applications, followed by dental prosthetics at 28%.
Regulatory approval pathways are becoming more defined for 3D-printed medical devices, with the FDA having released guidance documents specifically addressing additive manufacturing. This regulatory clarity is encouraging more medical device manufacturers to explore volumetric 3D printing technologies for production applications rather than just prototyping.
The aerospace sector presents another substantial market for volumetric 3D printing, valued at approximately $2.6 billion in 2022 with projected annual growth of 14.8% through 2028. Major aerospace manufacturers including Boeing, Airbus, and GE Aviation have established dedicated additive manufacturing divisions to integrate these technologies into their production workflows.
Key market drivers in aerospace include weight reduction requirements, supply chain optimization, and the ability to produce complex geometries that cannot be manufactured using traditional methods. Components such as fuel nozzles, turbine blades, and structural brackets are increasingly being produced using volumetric 3D printing technologies, offering weight reductions of 30-50% compared to traditionally manufactured parts.
Regional analysis indicates North America currently leads the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to increasing investments in advanced manufacturing capabilities in China, Japan, and South Korea.
Market challenges include the high initial investment costs for volumetric 3D printing systems, which typically range from $500,000 to over $2 million depending on capabilities. Additionally, there remains a skills gap in the workforce trained to operate these advanced systems and design for their unique capabilities, with industry surveys indicating that 68% of manufacturers cite skilled personnel shortages as a significant barrier to adoption.
Technical Barriers and Global Standards Landscape
Volumetric 3D printing faces significant technical barriers in achieving standardization for medical device and aerospace applications. The primary challenge lies in material characterization, as the photopolymers and resins used must meet stringent biocompatibility requirements for medical implants or high-performance specifications for aerospace components. Current testing methodologies lack consistency across different printing systems, creating difficulties in establishing universal material property benchmarks.
Resolution limitations present another substantial barrier. While volumetric printing offers advantages in speed, achieving the micron-level precision required for medical devices or aerospace components remains challenging. The trade-off between printing speed and resolution quality has not been fully resolved, particularly when printing complex internal structures that require both dimensional accuracy and mechanical integrity.
The global standards landscape for volumetric 3D printing remains fragmented. ISO/ASTM have developed the ISO/ASTM 52900 series addressing additive manufacturing broadly, but specific standards for volumetric techniques are still emerging. The FDA has published guidance documents for 3D printed medical devices, but these primarily address traditional layer-by-layer approaches rather than volumetric methods. Similarly, aerospace standards bodies like SAE International and ASTM Committee F42 are only beginning to address the unique aspects of volumetric printing.
Regional differences in regulatory frameworks create additional complexity. The European Union's Medical Device Regulation (MDR) and the FAA's aerospace certification requirements differ substantially from their counterparts in Asia and North America, creating a patchwork of compliance requirements that manufacturers must navigate. This regulatory heterogeneity significantly increases validation costs and time-to-market for globally distributed products.
Process validation represents perhaps the most significant technical barrier. Unlike traditional manufacturing methods with established process control parameters, volumetric 3D printing lacks standardized protocols for ensuring repeatability and reliability. The complex interaction between light projection systems, material chemistry, and curing dynamics creates numerous variables that must be controlled to achieve consistent results across different production batches and equipment.
Metrology and quality control standards are similarly underdeveloped. Traditional inspection techniques may not adequately capture the unique geometric features and internal structures possible with volumetric printing. New measurement protocols and acceptance criteria are needed, particularly for critical applications where failure could have catastrophic consequences.
Resolution limitations present another substantial barrier. While volumetric printing offers advantages in speed, achieving the micron-level precision required for medical devices or aerospace components remains challenging. The trade-off between printing speed and resolution quality has not been fully resolved, particularly when printing complex internal structures that require both dimensional accuracy and mechanical integrity.
The global standards landscape for volumetric 3D printing remains fragmented. ISO/ASTM have developed the ISO/ASTM 52900 series addressing additive manufacturing broadly, but specific standards for volumetric techniques are still emerging. The FDA has published guidance documents for 3D printed medical devices, but these primarily address traditional layer-by-layer approaches rather than volumetric methods. Similarly, aerospace standards bodies like SAE International and ASTM Committee F42 are only beginning to address the unique aspects of volumetric printing.
Regional differences in regulatory frameworks create additional complexity. The European Union's Medical Device Regulation (MDR) and the FAA's aerospace certification requirements differ substantially from their counterparts in Asia and North America, creating a patchwork of compliance requirements that manufacturers must navigate. This regulatory heterogeneity significantly increases validation costs and time-to-market for globally distributed products.
Process validation represents perhaps the most significant technical barrier. Unlike traditional manufacturing methods with established process control parameters, volumetric 3D printing lacks standardized protocols for ensuring repeatability and reliability. The complex interaction between light projection systems, material chemistry, and curing dynamics creates numerous variables that must be controlled to achieve consistent results across different production batches and equipment.
Metrology and quality control standards are similarly underdeveloped. Traditional inspection techniques may not adequately capture the unique geometric features and internal structures possible with volumetric printing. New measurement protocols and acceptance criteria are needed, particularly for critical applications where failure could have catastrophic consequences.
Current Compliance Solutions and Methodologies
01 Standardization of volumetric 3D printing processes
Standards for volumetric 3D printing processes ensure consistency and quality in manufacturing. These standards define parameters for printing resolution, material properties, and process validation. Compliance with these standards helps manufacturers achieve repeatable results and ensures that printed objects meet specified requirements for dimensional accuracy and structural integrity.- Standards for volumetric 3D printing quality and accuracy: Standards that ensure the quality and accuracy of volumetric 3D printing processes. These standards define parameters for dimensional accuracy, surface finish, and material properties to ensure consistent print quality. They provide guidelines for calibration procedures, tolerance specifications, and quality control methods to validate that printed objects meet required specifications across different printing systems.
- Compliance frameworks for 3D printing materials and safety: Regulatory frameworks and standards governing the materials used in volumetric 3D printing, focusing on safety, biocompatibility, and environmental impact. These standards specify testing protocols for material characterization, toxicity assessment, and environmental sustainability. They ensure that materials used in various applications, especially medical and food-related, meet safety requirements and are properly certified for their intended use.
- Data format and interoperability standards for volumetric printing: Standards that define file formats, data structures, and communication protocols to ensure interoperability between different volumetric 3D printing systems and software. These standards facilitate seamless data exchange across the 3D printing ecosystem, from design software to printer hardware. They specify requirements for volumetric data representation, slicing algorithms, and printer control commands to ensure consistent interpretation across platforms.
- Certification and validation processes for volumetric 3D printing: Standardized procedures for certifying and validating volumetric 3D printing processes, equipment, and outputs. These standards outline testing methodologies, performance metrics, and documentation requirements for verifying that printing systems meet industry specifications. They establish protocols for system qualification, process validation, and ongoing quality assurance to ensure consistent manufacturing results that comply with relevant industry standards.
- Industry-specific standards for volumetric 3D printing applications: Specialized standards tailored to specific industries utilizing volumetric 3D printing, such as healthcare, aerospace, and automotive. These standards address unique requirements for critical applications, including material traceability, process documentation, and performance testing specific to each industry. They provide guidelines for implementing volumetric 3D printing in regulated environments where product safety, reliability, and consistency are paramount.
02 Quality control and testing protocols for 3D printed components
Quality control standards for volumetric 3D printing include testing protocols to verify the mechanical properties, surface finish, and dimensional accuracy of printed components. These standards establish methods for evaluating print quality, detecting defects, and ensuring that printed objects meet performance requirements. Compliance with these testing protocols is essential for industries where component reliability is critical.Expand Specific Solutions03 Material certification and compatibility standards
Standards for materials used in volumetric 3D printing address composition, purity, and performance characteristics. These standards ensure that printing materials meet specific requirements for safety, biocompatibility, mechanical properties, and environmental impact. Material certification standards are particularly important in medical, aerospace, and automotive applications where material properties directly impact product performance and safety.Expand Specific Solutions04 Data format and interoperability standards
Interoperability standards for volumetric 3D printing define file formats, data structures, and communication protocols that enable seamless information exchange between different software and hardware systems. These standards ensure that design files can be accurately interpreted by various printing systems, facilitating collaboration across different platforms and preventing data loss or corruption during the workflow.Expand Specific Solutions05 Safety and regulatory compliance frameworks
Safety standards for volumetric 3D printing address operator protection, environmental considerations, and end-product safety. These frameworks include guidelines for handling materials, managing emissions, and ensuring electrical safety of printing equipment. Regulatory compliance standards also cover intellectual property protection, liability considerations, and industry-specific requirements for sectors such as healthcare, aerospace, and consumer products.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Volumetric 3D printing for medical device and aerospace manufacturing is currently in a growth phase, with increasing adoption of standardization protocols. The market is expanding rapidly, projected to reach significant scale as compliance frameworks mature. Technical maturity varies across players: established chemical companies like Wacker Chemie AG and Evonik Operations provide advanced materials; research institutions such as ETH Zurich and Massachusetts Eye & Ear Infirmary drive innovation; while specialized manufacturers including Quadratic 3D and Zrapid Technologies develop compliant printing systems. Academic-industry partnerships between universities (Minnesota, Guangzhou Medical) and manufacturers are accelerating standards development, with aerospace and medical sectors leading adoption due to stringent quality requirements.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed advanced silicone-based materials specifically engineered for volumetric 3D printing in medical device manufacturing. Their ELASTOSIL® line of biocompatible silicone resins meets ISO 10993 standards for medical applications and ASTM F3091 for additive manufacturing processes. The company has pioneered a two-component liquid silicone rubber (LSR) system that cures rapidly under volumetric projection, enabling the production of complex medical components with micron-level precision. Their materials undergo rigorous testing to ensure compliance with FDA regulations for implantable and non-implantable medical devices. Wacker's technology allows for the creation of patient-specific medical devices with complex internal structures that would be impossible with traditional manufacturing methods. Their materials maintain mechanical properties and biocompatibility after sterilization processes, including autoclave, gamma radiation, and ethylene oxide treatments, meeting the ASTM F1980 standard for accelerated aging of medical devices.
Strengths: Superior biocompatibility with extensive medical certifications; excellent mechanical stability after sterilization; ability to create complex geometries with high precision. Weaknesses: Higher material costs compared to conventional polymers; limited color options; requires specialized printing equipment optimized for their specific resin formulations.
Quadratic 3D, Inc.
Technical Solution: Quadratic 3D has developed a proprietary volumetric 3D printing technology called Tessellated Volumetric Printing (TVP) that specifically addresses aerospace and medical manufacturing compliance challenges. Their system utilizes a multi-beam approach that projects patterned light into photosensitive resins from multiple angles simultaneously, creating complex 3D structures in seconds rather than hours. The technology achieves ASTM F3091/F3091M compliance for additive manufacturing process characteristics and meets ISO 13485 requirements for medical device production. For aerospace applications, their materials and processes comply with AS9100D quality management standards and ASTM F3301 for spacecraft materials. Quadratic's platform incorporates real-time monitoring with machine vision systems that verify dimensional accuracy to within 10 microns, ensuring part-to-part consistency required for critical applications. Their proprietary software includes a validation module that generates comprehensive documentation for regulatory submissions, including material traceability, process parameters, and quality control metrics required by both FDA and aerospace authorities.
Strengths: Exceptional speed (100x faster than traditional layer-by-layer printing); superior surface finish without visible layer lines; built-in quality assurance systems for regulatory documentation. Weaknesses: Limited material selection compared to established technologies; higher initial equipment investment; technology is relatively new with fewer case studies demonstrating long-term performance in regulated environments.
Critical Patents and Technical Innovations
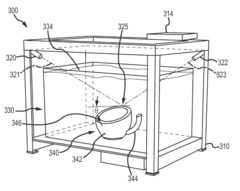
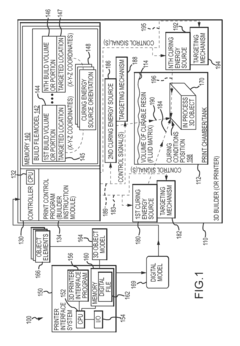
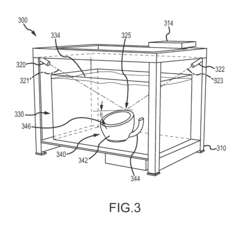
Three dimensional (3D) printing by volumetric addition through selective curing of a fluid matrix
PatentInactiveUS20160067922A1
Innovation
- A 3D printer that uses a tank of curable fluid to selectively and sequentially add volume to the object, eliminating the need for support structures by curing small volumes of resin at targeted locations using multiple curing energy sources, such as lasers or UV radiation, allowing for volumetric addition of the 3D object.
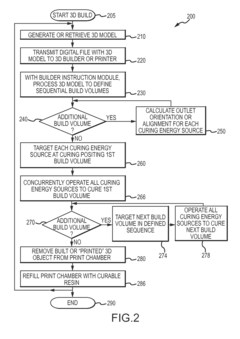
3D Printing Method
PatentActiveUS20240094703A1
Innovation
- The method involves displacing the focal plane relative to the container when imaging projection images, ensuring at least half of the object's extension is within the depth of field range, and adjusting light intensity proportionally to the virtual object material, allowing for larger object production by extending the depth of field coverage.
Regulatory Framework and Certification Processes
Volumetric 3D printing for medical device and aerospace manufacturing operates within a complex regulatory landscape that demands strict adherence to established standards. The FDA in the United States maintains comprehensive frameworks for medical devices through the 510(k) clearance process, Premarket Approval (PMA), and the newer De Novo pathway specifically designed for novel technologies without predicates. These regulatory pathways require extensive validation of materials, processes, and final products to ensure patient safety.
For aerospace applications, the Federal Aviation Administration (FAA) and European Union Aviation Safety Agency (EASA) have developed certification processes that manufacturers must navigate. The AS9100 quality management system standard, which builds upon ISO 9001, serves as the foundation for quality assurance in aerospace manufacturing, with specific provisions addressing additive manufacturing processes.
International standards organizations play a crucial role in establishing technical specifications for volumetric 3D printing. The International Organization for Standardization (ISO) and ASTM International have jointly developed the ISO/ASTM 52900 series, which provides terminology and process classifications for additive manufacturing. Specifically, ISO/ASTM 52904 addresses process qualification for laser-based powder bed fusion, while ASTM F3091 covers specifications for powder bed fusion of plastic materials.
Medical device manufacturers must additionally comply with ISO 13485 for quality management systems and ISO 10993 series for biocompatibility testing. The recent introduction of ISO/ASTM 52904-AM standard specifically addresses qualification principles for additively manufactured medical devices, providing a framework for ensuring consistent quality and performance.
Certification processes typically involve third-party testing laboratories that verify compliance with relevant standards. For medical applications, notified bodies in Europe or FDA-accredited testing facilities in the US conduct rigorous evaluations of both the manufacturing process and final products. These assessments include material characterization, mechanical testing, biocompatibility studies, and validation of sterilization methods.
The aerospace sector implements even more stringent certification requirements, with material qualification programs that can span several years. The National Aerospace and Defense Contractors Accreditation Program (NADCAP) certification represents an industry-managed approach to conformity assessment that has become essential for suppliers in the aerospace supply chain.
Emerging regulatory frameworks are beginning to address the unique challenges of volumetric 3D printing, including process validation, in-process monitoring, and post-processing requirements. The FDA's guidance on Technical Considerations for Additive Manufactured Medical Devices provides recommendations specific to design, manufacturing, and testing considerations, while aerospace authorities are developing similar specialized guidance documents.
For aerospace applications, the Federal Aviation Administration (FAA) and European Union Aviation Safety Agency (EASA) have developed certification processes that manufacturers must navigate. The AS9100 quality management system standard, which builds upon ISO 9001, serves as the foundation for quality assurance in aerospace manufacturing, with specific provisions addressing additive manufacturing processes.
International standards organizations play a crucial role in establishing technical specifications for volumetric 3D printing. The International Organization for Standardization (ISO) and ASTM International have jointly developed the ISO/ASTM 52900 series, which provides terminology and process classifications for additive manufacturing. Specifically, ISO/ASTM 52904 addresses process qualification for laser-based powder bed fusion, while ASTM F3091 covers specifications for powder bed fusion of plastic materials.
Medical device manufacturers must additionally comply with ISO 13485 for quality management systems and ISO 10993 series for biocompatibility testing. The recent introduction of ISO/ASTM 52904-AM standard specifically addresses qualification principles for additively manufactured medical devices, providing a framework for ensuring consistent quality and performance.
Certification processes typically involve third-party testing laboratories that verify compliance with relevant standards. For medical applications, notified bodies in Europe or FDA-accredited testing facilities in the US conduct rigorous evaluations of both the manufacturing process and final products. These assessments include material characterization, mechanical testing, biocompatibility studies, and validation of sterilization methods.
The aerospace sector implements even more stringent certification requirements, with material qualification programs that can span several years. The National Aerospace and Defense Contractors Accreditation Program (NADCAP) certification represents an industry-managed approach to conformity assessment that has become essential for suppliers in the aerospace supply chain.
Emerging regulatory frameworks are beginning to address the unique challenges of volumetric 3D printing, including process validation, in-process monitoring, and post-processing requirements. The FDA's guidance on Technical Considerations for Additive Manufactured Medical Devices provides recommendations specific to design, manufacturing, and testing considerations, while aerospace authorities are developing similar specialized guidance documents.
Material Qualification and Quality Assurance Protocols
Material qualification and quality assurance protocols for volumetric 3D printing in medical device and aerospace manufacturing require rigorous standardization to ensure consistent product performance and safety. These industries demand materials that meet specific mechanical, thermal, and chemical properties while maintaining biocompatibility or aerospace-grade durability.
The qualification process begins with raw material characterization, where suppliers must provide detailed certificates of analysis documenting composition, purity levels, and batch-to-batch consistency. For volumetric 3D printing specifically, photopolymers and resins undergo additional testing for light absorption characteristics, curing kinetics, and viscosity profiles at varying temperatures to ensure compatibility with volumetric printing techniques.
Statistical process control methodologies have been adapted for volumetric 3D printing, establishing acceptance criteria for key material parameters. These include refractive index stability, photoinitiator concentration, and optical clarity—all critical for the precise light patterning required in volumetric printing. Material qualification protocols typically mandate multiple production runs with standardized test specimens to verify reproducibility across different equipment and environmental conditions.
Non-destructive testing protocols have evolved significantly for volumetric printed parts, incorporating micro-CT scanning, optical coherence tomography, and spectroscopic analysis to detect internal defects without compromising structural integrity. These techniques allow for 100% inspection of critical components, particularly important for implantable medical devices and aerospace structural elements where failure is not an option.
Accelerated aging tests simulate long-term material performance under various environmental stressors, including UV exposure, humidity cycling, and temperature fluctuations. For medical applications, biocompatibility testing according to ISO 10993 standards is mandatory, while aerospace components must meet flammability, outgassing, and fluid resistance requirements per industry standards like AS9100 and RTCA DO-160.
Traceability systems have become increasingly sophisticated, with digital material passports tracking each material batch from raw components through processing and final part production. These systems integrate with manufacturing execution systems to create unbroken documentation chains that regulatory bodies increasingly require for both medical and aerospace applications.
Emerging standards specifically addressing volumetric 3D printing are being developed through collaborative efforts between ASTM International, ISO technical committees, and industry consortia. These standards focus on establishing reproducible testing methodologies for unique aspects of volumetric printing, including light dose mapping, volumetric resolution verification, and material property gradients that may occur throughout complex three-dimensional structures.
The qualification process begins with raw material characterization, where suppliers must provide detailed certificates of analysis documenting composition, purity levels, and batch-to-batch consistency. For volumetric 3D printing specifically, photopolymers and resins undergo additional testing for light absorption characteristics, curing kinetics, and viscosity profiles at varying temperatures to ensure compatibility with volumetric printing techniques.
Statistical process control methodologies have been adapted for volumetric 3D printing, establishing acceptance criteria for key material parameters. These include refractive index stability, photoinitiator concentration, and optical clarity—all critical for the precise light patterning required in volumetric printing. Material qualification protocols typically mandate multiple production runs with standardized test specimens to verify reproducibility across different equipment and environmental conditions.
Non-destructive testing protocols have evolved significantly for volumetric printed parts, incorporating micro-CT scanning, optical coherence tomography, and spectroscopic analysis to detect internal defects without compromising structural integrity. These techniques allow for 100% inspection of critical components, particularly important for implantable medical devices and aerospace structural elements where failure is not an option.
Accelerated aging tests simulate long-term material performance under various environmental stressors, including UV exposure, humidity cycling, and temperature fluctuations. For medical applications, biocompatibility testing according to ISO 10993 standards is mandatory, while aerospace components must meet flammability, outgassing, and fluid resistance requirements per industry standards like AS9100 and RTCA DO-160.
Traceability systems have become increasingly sophisticated, with digital material passports tracking each material batch from raw components through processing and final part production. These systems integrate with manufacturing execution systems to create unbroken documentation chains that regulatory bodies increasingly require for both medical and aerospace applications.
Emerging standards specifically addressing volumetric 3D printing are being developed through collaborative efforts between ASTM International, ISO technical committees, and industry consortia. These standards focus on establishing reproducible testing methodologies for unique aspects of volumetric printing, including light dose mapping, volumetric resolution verification, and material property gradients that may occur throughout complex three-dimensional structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!