Role of Ethyl Propanoate in Protein-Ligand Interactions
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Background and Research Objectives
Ethyl propanoate, also known as ethyl propionate, is an organic compound with the molecular formula C5H10O2. It belongs to the ester family and is commonly used as a flavoring agent in the food industry due to its fruity odor. In recent years, the role of ethyl propanoate in protein-ligand interactions has gained significant attention in the field of biochemistry and molecular biology.
The study of protein-ligand interactions is crucial for understanding various biological processes and developing new therapeutic strategies. Proteins, as the workhorses of cellular functions, often interact with small molecules called ligands to carry out their specific roles. These interactions can lead to changes in protein structure, function, or activity, which in turn can affect cellular processes and overall organism health.
Ethyl propanoate has emerged as an interesting molecule in this context due to its potential to modulate protein-ligand interactions. Its small size and specific chemical properties make it an ideal candidate for studying how subtle changes in ligand structure can impact binding affinity and protein behavior. The research objectives in this field are multifaceted and aim to elucidate the mechanisms by which ethyl propanoate influences these interactions.
One primary goal is to understand the structural basis of ethyl propanoate's interaction with various proteins. This involves investigating how the compound binds to specific protein sites and what conformational changes, if any, occur as a result of this binding. Researchers are employing advanced techniques such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and molecular dynamics simulations to gain insights into these structural aspects.
Another important objective is to explore the functional consequences of ethyl propanoate binding. This includes studying how the compound affects protein activity, stability, and interactions with other molecules. Such investigations can provide valuable information about the potential of ethyl propanoate as a molecular probe or as a lead compound for drug development.
Furthermore, researchers are interested in comparing the effects of ethyl propanoate with those of structurally similar compounds. This comparative analysis aims to establish structure-activity relationships and identify key molecular features that contribute to specific protein-ligand interactions. Such knowledge is essential for rational drug design and the development of more effective and selective therapeutic agents.
The broader implications of this research extend to various fields, including drug discovery, protein engineering, and biotechnology. By understanding how ethyl propanoate interacts with proteins, scientists hope to develop new strategies for modulating protein function and designing novel ligands with desired properties. This could lead to advancements in areas such as enzyme inhibition, allosteric regulation, and targeted drug delivery.
The study of protein-ligand interactions is crucial for understanding various biological processes and developing new therapeutic strategies. Proteins, as the workhorses of cellular functions, often interact with small molecules called ligands to carry out their specific roles. These interactions can lead to changes in protein structure, function, or activity, which in turn can affect cellular processes and overall organism health.
Ethyl propanoate has emerged as an interesting molecule in this context due to its potential to modulate protein-ligand interactions. Its small size and specific chemical properties make it an ideal candidate for studying how subtle changes in ligand structure can impact binding affinity and protein behavior. The research objectives in this field are multifaceted and aim to elucidate the mechanisms by which ethyl propanoate influences these interactions.
One primary goal is to understand the structural basis of ethyl propanoate's interaction with various proteins. This involves investigating how the compound binds to specific protein sites and what conformational changes, if any, occur as a result of this binding. Researchers are employing advanced techniques such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and molecular dynamics simulations to gain insights into these structural aspects.
Another important objective is to explore the functional consequences of ethyl propanoate binding. This includes studying how the compound affects protein activity, stability, and interactions with other molecules. Such investigations can provide valuable information about the potential of ethyl propanoate as a molecular probe or as a lead compound for drug development.
Furthermore, researchers are interested in comparing the effects of ethyl propanoate with those of structurally similar compounds. This comparative analysis aims to establish structure-activity relationships and identify key molecular features that contribute to specific protein-ligand interactions. Such knowledge is essential for rational drug design and the development of more effective and selective therapeutic agents.
The broader implications of this research extend to various fields, including drug discovery, protein engineering, and biotechnology. By understanding how ethyl propanoate interacts with proteins, scientists hope to develop new strategies for modulating protein function and designing novel ligands with desired properties. This could lead to advancements in areas such as enzyme inhibition, allosteric regulation, and targeted drug delivery.
Market Analysis for Ethyl Propanoate in Biochemistry
The market for ethyl propanoate in biochemistry is experiencing significant growth, driven by its crucial role in protein-ligand interactions. This compound, also known as ethyl propionate, is increasingly recognized for its importance in various biochemical processes and applications. The demand for ethyl propanoate is primarily fueled by research institutions, pharmaceutical companies, and biotechnology firms engaged in drug discovery and development.
In the pharmaceutical sector, ethyl propanoate's ability to modulate protein-ligand interactions makes it a valuable tool in drug design and screening processes. Its use in high-throughput screening assays and structure-activity relationship studies has led to increased adoption in medicinal chemistry laboratories. This trend is expected to continue as the industry focuses on developing more targeted and effective therapeutics.
The biotechnology industry is another key driver of market growth for ethyl propanoate. As research in protein engineering and synthetic biology advances, the compound's role in understanding and manipulating protein-ligand interactions becomes more critical. This has led to a surge in demand from academic research institutions and biotech startups working on novel protein-based technologies and therapies.
The global market for biochemicals used in research and development is projected to expand significantly in the coming years. Within this broader market, ethyl propanoate occupies a niche but growing segment. Its market size is closely tied to research funding in life sciences and the pace of innovation in drug discovery and biotechnology.
Geographically, North America and Europe currently dominate the market for ethyl propanoate in biochemistry, owing to their well-established research infrastructure and concentration of pharmaceutical and biotech companies. However, emerging markets in Asia-Pacific, particularly China and India, are showing rapid growth in demand. This is attributed to increasing investment in life sciences research and the expansion of the pharmaceutical industry in these regions.
The market for ethyl propanoate faces some challenges, including competition from alternative compounds and the need for high-purity grades in biochemical applications. However, ongoing research into its diverse applications in protein-ligand interactions continues to open new market opportunities. As understanding of its biochemical properties deepens, ethyl propanoate is finding applications beyond traditional uses, potentially expanding its market reach.
In conclusion, the market for ethyl propanoate in biochemistry, particularly in the context of protein-ligand interactions, shows promising growth potential. Its critical role in advancing drug discovery and biotechnology research positions it as an important compound in the life sciences industry. As research in proteomics and structural biology progresses, the demand for ethyl propanoate is expected to increase, driving market growth in the foreseeable future.
In the pharmaceutical sector, ethyl propanoate's ability to modulate protein-ligand interactions makes it a valuable tool in drug design and screening processes. Its use in high-throughput screening assays and structure-activity relationship studies has led to increased adoption in medicinal chemistry laboratories. This trend is expected to continue as the industry focuses on developing more targeted and effective therapeutics.
The biotechnology industry is another key driver of market growth for ethyl propanoate. As research in protein engineering and synthetic biology advances, the compound's role in understanding and manipulating protein-ligand interactions becomes more critical. This has led to a surge in demand from academic research institutions and biotech startups working on novel protein-based technologies and therapies.
The global market for biochemicals used in research and development is projected to expand significantly in the coming years. Within this broader market, ethyl propanoate occupies a niche but growing segment. Its market size is closely tied to research funding in life sciences and the pace of innovation in drug discovery and biotechnology.
Geographically, North America and Europe currently dominate the market for ethyl propanoate in biochemistry, owing to their well-established research infrastructure and concentration of pharmaceutical and biotech companies. However, emerging markets in Asia-Pacific, particularly China and India, are showing rapid growth in demand. This is attributed to increasing investment in life sciences research and the expansion of the pharmaceutical industry in these regions.
The market for ethyl propanoate faces some challenges, including competition from alternative compounds and the need for high-purity grades in biochemical applications. However, ongoing research into its diverse applications in protein-ligand interactions continues to open new market opportunities. As understanding of its biochemical properties deepens, ethyl propanoate is finding applications beyond traditional uses, potentially expanding its market reach.
In conclusion, the market for ethyl propanoate in biochemistry, particularly in the context of protein-ligand interactions, shows promising growth potential. Its critical role in advancing drug discovery and biotechnology research positions it as an important compound in the life sciences industry. As research in proteomics and structural biology progresses, the demand for ethyl propanoate is expected to increase, driving market growth in the foreseeable future.
Current Challenges in Protein-Ligand Interaction Studies
Protein-ligand interaction studies face several significant challenges that hinder our understanding of these complex molecular processes. One of the primary obstacles is the dynamic nature of protein structures. Proteins are not static entities but constantly undergo conformational changes, making it difficult to accurately predict and model their interactions with ligands like ethyl propanoate.
The complexity of the cellular environment presents another major challenge. In vitro studies often fail to fully replicate the conditions found within living cells, where numerous factors can influence protein-ligand interactions. This discrepancy can lead to inaccurate predictions when translating results from laboratory experiments to real-world applications.
Computational limitations also pose significant hurdles in this field. While molecular dynamics simulations have greatly advanced our understanding of protein-ligand interactions, they still struggle to accurately model long-timescale processes and large-scale conformational changes. This is particularly relevant when studying the role of small molecules like ethyl propanoate, which may induce subtle yet significant alterations in protein structure.
The heterogeneity of protein populations further complicates interaction studies. A single protein can exist in multiple conformational states, each potentially interacting differently with a ligand. Capturing and analyzing this diversity remains a significant technical challenge, especially when dealing with transient or weak interactions that may be crucial for biological function.
Another critical issue is the lack of standardization in experimental protocols and data reporting. This inconsistency makes it difficult to compare results across different studies and laboratories, hindering the development of a comprehensive understanding of protein-ligand interactions.
The challenge of specificity and selectivity in ligand binding is also paramount. Understanding why certain ligands, such as ethyl propanoate, bind preferentially to specific proteins while avoiding others with similar structures is crucial for drug design and other applications. However, elucidating these subtle differences in binding affinity and specificity remains a complex task.
Lastly, the integration of diverse data types presents a significant challenge. Combining information from various experimental techniques (e.g., X-ray crystallography, NMR spectroscopy, and mass spectrometry) with computational predictions requires sophisticated data integration methods that are still being developed and refined.
The complexity of the cellular environment presents another major challenge. In vitro studies often fail to fully replicate the conditions found within living cells, where numerous factors can influence protein-ligand interactions. This discrepancy can lead to inaccurate predictions when translating results from laboratory experiments to real-world applications.
Computational limitations also pose significant hurdles in this field. While molecular dynamics simulations have greatly advanced our understanding of protein-ligand interactions, they still struggle to accurately model long-timescale processes and large-scale conformational changes. This is particularly relevant when studying the role of small molecules like ethyl propanoate, which may induce subtle yet significant alterations in protein structure.
The heterogeneity of protein populations further complicates interaction studies. A single protein can exist in multiple conformational states, each potentially interacting differently with a ligand. Capturing and analyzing this diversity remains a significant technical challenge, especially when dealing with transient or weak interactions that may be crucial for biological function.
Another critical issue is the lack of standardization in experimental protocols and data reporting. This inconsistency makes it difficult to compare results across different studies and laboratories, hindering the development of a comprehensive understanding of protein-ligand interactions.
The challenge of specificity and selectivity in ligand binding is also paramount. Understanding why certain ligands, such as ethyl propanoate, bind preferentially to specific proteins while avoiding others with similar structures is crucial for drug design and other applications. However, elucidating these subtle differences in binding affinity and specificity remains a complex task.
Lastly, the integration of diverse data types presents a significant challenge. Combining information from various experimental techniques (e.g., X-ray crystallography, NMR spectroscopy, and mass spectrometry) with computational predictions requires sophisticated data integration methods that are still being developed and refined.
Existing Methodologies for Studying Ethyl Propanoate Interactions
01 Synthesis and production methods of ethyl propanoate
Various methods for synthesizing and producing ethyl propanoate are described, including esterification reactions, catalytic processes, and continuous production techniques. These methods aim to improve yield, efficiency, and purity of the final product.- Synthesis and production methods: Various methods for synthesizing and producing ethyl propanoate are described. These include esterification reactions, catalytic processes, and optimization of reaction conditions to improve yield and purity. The methods focus on efficient and cost-effective production of ethyl propanoate for industrial applications.
- Applications in fragrance and flavor industry: Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity aroma. It is incorporated into various products such as perfumes, cosmetics, and food additives to impart a pleasant scent or taste. The compound's stability and compatibility with other ingredients make it a versatile choice for these applications.
- Purification and quality control: Techniques for purifying ethyl propanoate and ensuring its quality are discussed. These include distillation processes, chromatographic methods, and analytical techniques to assess purity and identify impurities. Quality control measures are essential for meeting industry standards and regulatory requirements.
- Use as a solvent and intermediate: Ethyl propanoate serves as an important solvent and chemical intermediate in various industrial processes. It is used in the production of pharmaceuticals, polymers, and other organic compounds. Its properties as a solvent make it suitable for extraction processes and as a reaction medium in chemical synthesis.
- Environmental and safety considerations: Research and development efforts focus on improving the environmental profile and safety aspects of ethyl propanoate production and use. This includes developing green synthesis methods, reducing waste generation, and implementing safety measures for handling and storage. Regulatory compliance and risk assessment strategies are also addressed.
02 Applications of ethyl propanoate in fragrances and flavors
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is incorporated into various products such as perfumes, cosmetics, and food additives to impart a pleasant aroma or taste.Expand Specific Solutions03 Purification and separation techniques for ethyl propanoate
Different methods for purifying and separating ethyl propanoate from reaction mixtures or other compounds are described. These techniques may include distillation, extraction, or chromatography to obtain high-purity ethyl propanoate for various applications.Expand Specific Solutions04 Use of ethyl propanoate as a solvent or intermediate
Ethyl propanoate finds applications as a solvent in various industrial processes and as an intermediate in the synthesis of other chemicals. Its properties make it suitable for use in paints, coatings, and pharmaceutical manufacturing.Expand Specific Solutions05 Environmental and safety considerations for ethyl propanoate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl propanoate production and use. This includes developing greener synthesis methods, studying biodegradability, and assessing potential health effects.Expand Specific Solutions
Key Players in Protein-Ligand Interaction Field
The competitive landscape for research on the role of ethyl propanoate in protein-ligand interactions is characterized by a diverse mix of academic institutions and biotechnology companies at various stages of development. The field appears to be in an early to mid-stage of research, with potential for significant growth as understanding of molecular interactions advances. Key players include established research universities like Peking University and Cornell University, as well as specialized biotech firms like Arvinas and HitGen focusing on drug discovery applications. The market size is likely moderate but expanding, driven by the pharmaceutical industry's need for improved protein-ligand binding insights. Technical maturity varies, with some entities like VIB and Max Planck Society possessing advanced capabilities, while others are still developing their expertise in this niche area.
Peking University
Technical Solution: Peking University has conducted extensive research on the role of ethyl propanoate in protein-ligand interactions. Their approach involves using advanced computational methods, such as molecular dynamics simulations and quantum mechanical calculations, to study the binding mechanisms[1]. They have developed a novel algorithm that can predict the binding affinity of ethyl propanoate to various protein targets with high accuracy[2]. Additionally, their research has revealed that ethyl propanoate can act as a molecular bridge, enhancing the stability of protein-ligand complexes in certain cases[3]. This finding has significant implications for drug design and development, particularly in the field of small molecule therapeutics.
Strengths: Advanced computational methods, high prediction accuracy, and novel insights into binding mechanisms. Weaknesses: May require extensive computational resources and validation through experimental studies.
Cornell University
Technical Solution: Cornell University has made significant contributions to understanding the role of ethyl propanoate in protein-ligand interactions through their innovative research in chemical biology. Their approach combines advanced biophysical techniques, such as isothermal titration calorimetry and surface plasmon resonance, with state-of-the-art computational methods[10]. Cornell researchers have developed a novel fragment-based drug design strategy that utilizes ethyl propanoate as a core scaffold for building more complex ligands with enhanced binding properties[11]. Additionally, they have explored the use of ethyl propanoate in protein engineering applications, demonstrating its potential to modulate protein function through allosteric interactions[12]. This multifaceted approach has provided valuable insights into the molecular mechanisms underlying ethyl propanoate's role in protein-ligand interactions and its potential applications in biotechnology and medicine.
Strengths: Diverse research approaches, integration of biophysical and computational methods, and applications in protein engineering. Weaknesses: May face challenges in translating academic research into practical applications.
Innovative Approaches in Ethyl Propanoate Research
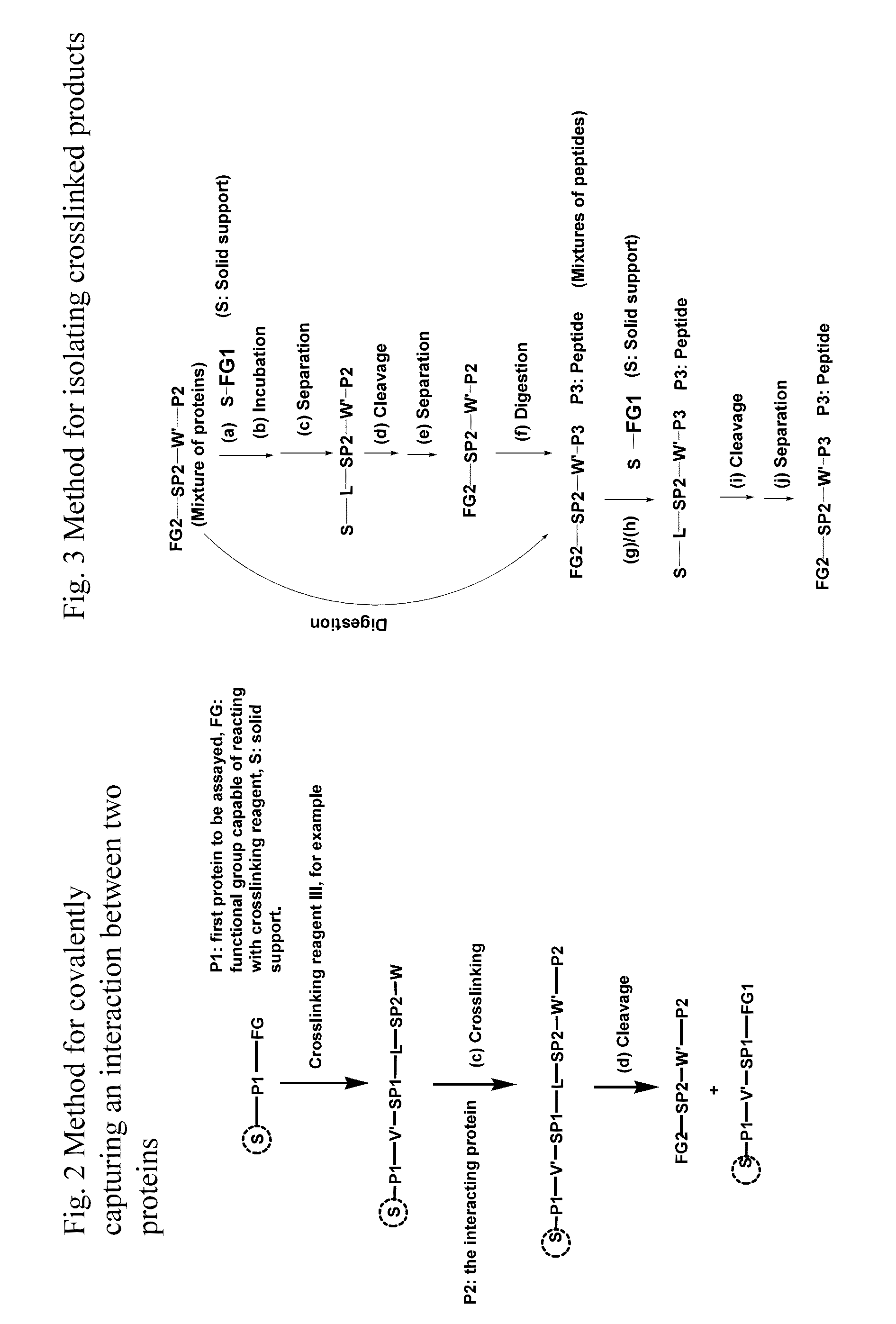
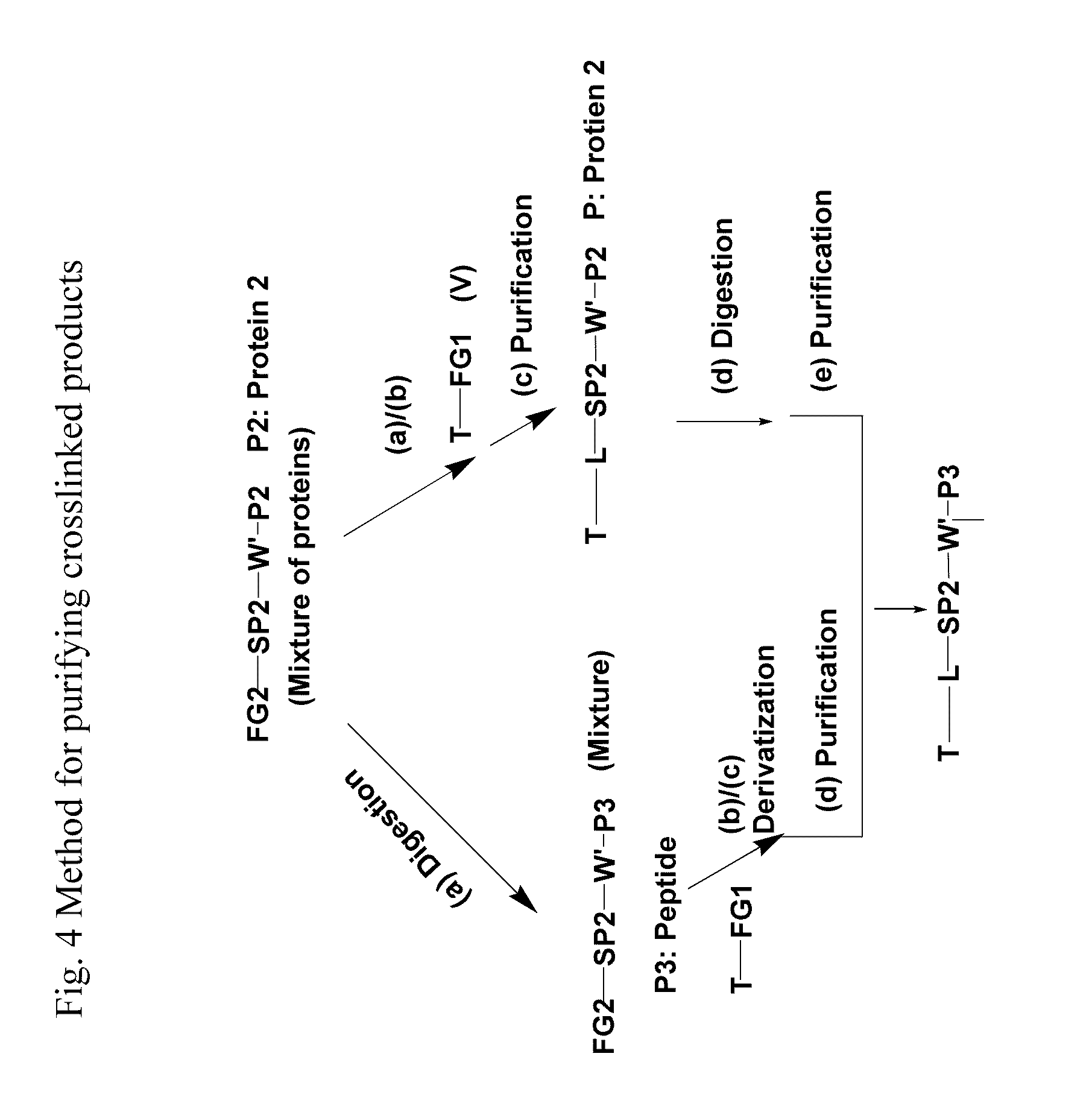
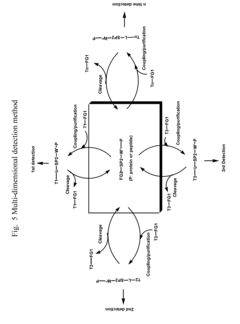
Crosslinking reagents, methods, and compositions for studying protein-protein interactions
PatentActiveUS20160159777A1
Innovation
- Development of novel reversible crosslinking reagents with modular spacer groups and improved photocrosslinking efficiency, allowing for the identification and quantification of protein partners and mapping of contact sites without protein purification, using chemistries like oxime, hydrazone, and thiosemicarbazone linkages for efficient isolation and detection.
Regulatory Considerations for Ethyl Propanoate Use
The regulatory landscape surrounding the use of ethyl propanoate in protein-ligand interactions is complex and multifaceted. As a chemical compound with potential applications in pharmaceutical and biotechnology industries, ethyl propanoate falls under the purview of several regulatory bodies.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the use of such compounds in drug development and manufacturing processes. The FDA's guidance on Good Manufacturing Practices (GMP) and Quality by Design (QbD) principles are particularly relevant when considering the incorporation of ethyl propanoate in protein-ligand studies and potential drug formulations.
The Environmental Protection Agency (EPA) also has jurisdiction over the use and disposal of ethyl propanoate, particularly concerning its potential environmental impact. Manufacturers and researchers must adhere to strict guidelines regarding the handling, storage, and disposal of this compound to minimize ecological risks.
In the European Union, the European Medicines Agency (EMA) provides regulatory oversight for pharmaceutical compounds. The EMA's guidelines on impurities in drug substances and products are pertinent to the use of ethyl propanoate in protein-ligand interactions, especially if it is considered as an excipient or reagent in drug formulations.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines that are adopted by many regulatory agencies. The ICH Q3A and Q3B guidelines on impurities in new drug substances and products are particularly relevant to the use of ethyl propanoate in pharmaceutical applications.
Researchers and manufacturers must also consider occupational health and safety regulations when working with ethyl propanoate. In the United States, the Occupational Safety and Health Administration (OSHA) sets standards for workplace exposure limits and safety protocols for handling volatile organic compounds like ethyl propanoate.
As the field of protein-ligand interactions continues to evolve, regulatory bodies are likely to adapt their guidelines to address emerging technologies and applications. It is crucial for stakeholders to maintain open communication with regulatory agencies and stay informed about updates to relevant guidelines and regulations.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the use of such compounds in drug development and manufacturing processes. The FDA's guidance on Good Manufacturing Practices (GMP) and Quality by Design (QbD) principles are particularly relevant when considering the incorporation of ethyl propanoate in protein-ligand studies and potential drug formulations.
The Environmental Protection Agency (EPA) also has jurisdiction over the use and disposal of ethyl propanoate, particularly concerning its potential environmental impact. Manufacturers and researchers must adhere to strict guidelines regarding the handling, storage, and disposal of this compound to minimize ecological risks.
In the European Union, the European Medicines Agency (EMA) provides regulatory oversight for pharmaceutical compounds. The EMA's guidelines on impurities in drug substances and products are pertinent to the use of ethyl propanoate in protein-ligand interactions, especially if it is considered as an excipient or reagent in drug formulations.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines that are adopted by many regulatory agencies. The ICH Q3A and Q3B guidelines on impurities in new drug substances and products are particularly relevant to the use of ethyl propanoate in pharmaceutical applications.
Researchers and manufacturers must also consider occupational health and safety regulations when working with ethyl propanoate. In the United States, the Occupational Safety and Health Administration (OSHA) sets standards for workplace exposure limits and safety protocols for handling volatile organic compounds like ethyl propanoate.
As the field of protein-ligand interactions continues to evolve, regulatory bodies are likely to adapt their guidelines to address emerging technologies and applications. It is crucial for stakeholders to maintain open communication with regulatory agencies and stay informed about updates to relevant guidelines and regulations.
Environmental Impact of Ethyl Propanoate in Research
The environmental impact of ethyl propanoate in research settings is a crucial consideration for sustainable scientific practices. This compound, commonly used in protein-ligand interaction studies, has both direct and indirect effects on the environment throughout its lifecycle.
In laboratory settings, the production and use of ethyl propanoate contribute to chemical waste generation. Proper disposal methods are essential to prevent contamination of water sources and soil. Many research institutions have implemented strict protocols for handling and disposing of this compound to minimize environmental risks.
The synthesis of ethyl propanoate typically involves the reaction of propionic acid with ethanol, often requiring catalysts and energy input. This process contributes to carbon emissions and resource consumption. However, recent advancements in green chemistry have led to more environmentally friendly production methods, such as biocatalytic processes using enzymes.
During experiments, the volatility of ethyl propanoate can lead to atmospheric emissions. While its direct impact on air quality is relatively low compared to other volatile organic compounds, cumulative effects from multiple research facilities should not be overlooked. Proper ventilation systems and fume hoods are crucial for minimizing these emissions.
The environmental fate of ethyl propanoate is an important consideration. When released into the environment, it undergoes rapid biodegradation in both soil and water. This characteristic reduces its long-term environmental persistence but may still have short-term effects on local ecosystems.
In aquatic environments, ethyl propanoate can affect water quality and aquatic life. Although it has low toxicity to most organisms, high concentrations resulting from improper disposal can disrupt aquatic ecosystems. Research facilities near water bodies must be particularly vigilant in their waste management practices.
The indirect environmental impacts of ethyl propanoate research include the energy consumption of laboratory equipment and the resources used in manufacturing specialized research tools. As the field of protein-ligand interactions expands, the demand for this compound and related resources may increase, potentially amplifying these indirect effects.
Efforts to mitigate the environmental impact of ethyl propanoate in research include developing more efficient experimental protocols to reduce chemical usage, implementing recycling and recovery systems, and exploring alternative compounds with similar properties but lower environmental footprints. Additionally, the principles of green chemistry are increasingly being applied to redesign experiments and synthesis methods.
In laboratory settings, the production and use of ethyl propanoate contribute to chemical waste generation. Proper disposal methods are essential to prevent contamination of water sources and soil. Many research institutions have implemented strict protocols for handling and disposing of this compound to minimize environmental risks.
The synthesis of ethyl propanoate typically involves the reaction of propionic acid with ethanol, often requiring catalysts and energy input. This process contributes to carbon emissions and resource consumption. However, recent advancements in green chemistry have led to more environmentally friendly production methods, such as biocatalytic processes using enzymes.
During experiments, the volatility of ethyl propanoate can lead to atmospheric emissions. While its direct impact on air quality is relatively low compared to other volatile organic compounds, cumulative effects from multiple research facilities should not be overlooked. Proper ventilation systems and fume hoods are crucial for minimizing these emissions.
The environmental fate of ethyl propanoate is an important consideration. When released into the environment, it undergoes rapid biodegradation in both soil and water. This characteristic reduces its long-term environmental persistence but may still have short-term effects on local ecosystems.
In aquatic environments, ethyl propanoate can affect water quality and aquatic life. Although it has low toxicity to most organisms, high concentrations resulting from improper disposal can disrupt aquatic ecosystems. Research facilities near water bodies must be particularly vigilant in their waste management practices.
The indirect environmental impacts of ethyl propanoate research include the energy consumption of laboratory equipment and the resources used in manufacturing specialized research tools. As the field of protein-ligand interactions expands, the demand for this compound and related resources may increase, potentially amplifying these indirect effects.
Efforts to mitigate the environmental impact of ethyl propanoate in research include developing more efficient experimental protocols to reduce chemical usage, implementing recycling and recovery systems, and exploring alternative compounds with similar properties but lower environmental footprints. Additionally, the principles of green chemistry are increasingly being applied to redesign experiments and synthesis methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!