Solid sorbents for CO2 capture for high efficiency carbon capture and storage applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture and storage (CCS) has evolved significantly over the past decades as a critical technology for mitigating climate change. The journey began in the 1970s with initial research into carbon capture methods, primarily focused on liquid solvents like monoethanolamine (MEA). These early approaches, while functional, suffered from high energy penalties and operational costs that limited widespread adoption.
The 1990s marked a turning point with the recognition of climate change as a global challenge, catalyzing increased research investment in CCS technologies. This period saw the first large-scale demonstrations of carbon capture at power plants, establishing technical feasibility while highlighting the need for more efficient capture methods.
By the early 2000s, solid sorbents emerged as promising alternatives to liquid-based systems. Materials such as activated carbons, zeolites, and metal-organic frameworks (MOFs) demonstrated potential for reduced energy requirements and operational flexibility. The development trajectory has since focused on enhancing sorption capacity, selectivity, and stability while reducing regeneration energy.
Current technological objectives center on addressing several key challenges. Primary among these is reducing the energy penalty associated with CO2 capture, which currently ranges from 20-30% of a power plant's output. Research aims to develop sorbents capable of achieving capture costs below $40 per ton of CO2, compared to current costs of $60-80 per ton with conventional technologies.
Another critical objective is improving sorbent durability under real-world conditions. Industrial flue gases contain impurities that can degrade sorbent performance over time, necessitating materials that maintain capacity through thousands of adsorption-desorption cycles. Researchers target sorbents with operational lifespans exceeding five years to ensure economic viability.
Scale-up represents a significant technological hurdle, as laboratory-proven materials must function effectively in industrial settings processing thousands of tons of CO2 daily. This requires advances in manufacturing techniques to produce high-quality sorbents at commercially relevant scales and costs.
The integration of solid sorbent systems with existing industrial infrastructure presents another objective, requiring designs compatible with diverse industrial processes from power generation to cement production. Ultimately, the field aims to develop "drop-in" capture solutions that minimize disruption to existing operations while maximizing carbon capture efficiency.
The 1990s marked a turning point with the recognition of climate change as a global challenge, catalyzing increased research investment in CCS technologies. This period saw the first large-scale demonstrations of carbon capture at power plants, establishing technical feasibility while highlighting the need for more efficient capture methods.
By the early 2000s, solid sorbents emerged as promising alternatives to liquid-based systems. Materials such as activated carbons, zeolites, and metal-organic frameworks (MOFs) demonstrated potential for reduced energy requirements and operational flexibility. The development trajectory has since focused on enhancing sorption capacity, selectivity, and stability while reducing regeneration energy.
Current technological objectives center on addressing several key challenges. Primary among these is reducing the energy penalty associated with CO2 capture, which currently ranges from 20-30% of a power plant's output. Research aims to develop sorbents capable of achieving capture costs below $40 per ton of CO2, compared to current costs of $60-80 per ton with conventional technologies.
Another critical objective is improving sorbent durability under real-world conditions. Industrial flue gases contain impurities that can degrade sorbent performance over time, necessitating materials that maintain capacity through thousands of adsorption-desorption cycles. Researchers target sorbents with operational lifespans exceeding five years to ensure economic viability.
Scale-up represents a significant technological hurdle, as laboratory-proven materials must function effectively in industrial settings processing thousands of tons of CO2 daily. This requires advances in manufacturing techniques to produce high-quality sorbents at commercially relevant scales and costs.
The integration of solid sorbent systems with existing industrial infrastructure presents another objective, requiring designs compatible with diverse industrial processes from power generation to cement production. Ultimately, the field aims to develop "drop-in" capture solutions that minimize disruption to existing operations while maximizing carbon capture efficiency.
Market Analysis for Carbon Capture Solutions
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental concerns and stringent regulations aimed at reducing greenhouse gas emissions. As of 2023, the market for carbon capture solutions is valued at approximately $7.5 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.9 billion by the end of the decade.
Solid sorbents for CO2 capture represent a rapidly expanding segment within this market, currently accounting for about 27% of the total carbon capture technology market share. This segment is expected to grow at an accelerated rate of 23.5% annually, outpacing other capture technologies due to its efficiency advantages and lower energy requirements.
Geographically, North America leads the market with approximately 38% share, followed by Europe at 31% and Asia-Pacific at 24%. The remaining 7% is distributed across other regions. The United States, Canada, and the European Union have established substantial funding mechanisms specifically targeting advanced carbon capture technologies, with solid sorbents receiving particular attention in research grants and industrial partnerships.
Key market drivers include increasingly stringent carbon pricing mechanisms, with carbon prices in the EU Emissions Trading System reaching €80-90 per tonne in 2023. Additionally, government incentives such as the U.S. 45Q tax credit, which now offers up to $85 per tonne for captured and sequestered CO2, are significantly improving the economic viability of CCS projects utilizing solid sorbent technologies.
Industry-specific demand shows that power generation currently represents the largest application sector (42%), followed by cement production (18%), chemical manufacturing (15%), and steel production (12%). The remaining 13% encompasses various industrial applications including hydrogen production and direct air capture initiatives.
Customer requirements are evolving toward solutions offering lower energy penalties, with the market increasingly favoring technologies that can achieve capture costs below $40 per tonne of CO2. Solid sorbents are particularly attractive in this context, as advanced materials have demonstrated potential to reduce capture costs by 30-45% compared to conventional amine scrubbing technologies.
Market barriers include high initial capital expenditure requirements, with typical industrial-scale solid sorbent installations costing between $400-700 million, and technological maturity concerns among conservative industrial adopters. However, these barriers are gradually diminishing as demonstration projects prove the technology's reliability and as economies of scale begin to reduce implementation costs.
Solid sorbents for CO2 capture represent a rapidly expanding segment within this market, currently accounting for about 27% of the total carbon capture technology market share. This segment is expected to grow at an accelerated rate of 23.5% annually, outpacing other capture technologies due to its efficiency advantages and lower energy requirements.
Geographically, North America leads the market with approximately 38% share, followed by Europe at 31% and Asia-Pacific at 24%. The remaining 7% is distributed across other regions. The United States, Canada, and the European Union have established substantial funding mechanisms specifically targeting advanced carbon capture technologies, with solid sorbents receiving particular attention in research grants and industrial partnerships.
Key market drivers include increasingly stringent carbon pricing mechanisms, with carbon prices in the EU Emissions Trading System reaching €80-90 per tonne in 2023. Additionally, government incentives such as the U.S. 45Q tax credit, which now offers up to $85 per tonne for captured and sequestered CO2, are significantly improving the economic viability of CCS projects utilizing solid sorbent technologies.
Industry-specific demand shows that power generation currently represents the largest application sector (42%), followed by cement production (18%), chemical manufacturing (15%), and steel production (12%). The remaining 13% encompasses various industrial applications including hydrogen production and direct air capture initiatives.
Customer requirements are evolving toward solutions offering lower energy penalties, with the market increasingly favoring technologies that can achieve capture costs below $40 per tonne of CO2. Solid sorbents are particularly attractive in this context, as advanced materials have demonstrated potential to reduce capture costs by 30-45% compared to conventional amine scrubbing technologies.
Market barriers include high initial capital expenditure requirements, with typical industrial-scale solid sorbent installations costing between $400-700 million, and technological maturity concerns among conservative industrial adopters. However, these barriers are gradually diminishing as demonstration projects prove the technology's reliability and as economies of scale begin to reduce implementation costs.
Solid Sorbents: Current Status and Technical Barriers
Solid sorbents have emerged as promising materials for CO2 capture in carbon capture and storage (CCS) applications due to their potential for lower energy requirements compared to conventional liquid amine scrubbing. Currently, several classes of solid sorbents are being investigated globally, including activated carbons, zeolites, metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcites.
Activated carbons offer advantages of low cost and high thermal stability, but suffer from relatively low CO2 selectivity and capacity under practical conditions. Their performance significantly decreases in humid conditions, which is a major limitation for flue gas applications. Recent developments have focused on surface modification to enhance selectivity, but breakthrough innovations are still needed.
Zeolites, particularly those with high cation content like 13X and 5A, demonstrate excellent CO2 adsorption capacity at moderate temperatures and low partial pressures. However, they exhibit severe performance degradation in the presence of water and require significant energy for regeneration. Current research efforts are directed toward developing water-resistant zeolites and optimizing the regeneration process.
Metal-organic frameworks represent one of the most rapidly developing classes of sorbents, with exceptional surface areas exceeding 6000 m²/g and highly tunable pore structures. Despite their promising laboratory performance, MOFs face significant challenges in stability under cycling conditions, manufacturing scalability, and production costs. The gap between laboratory synthesis and industrial-scale production remains substantial.
Amine-functionalized silicas combine the physical adsorption properties of porous supports with the chemical reactivity of amines toward CO2. While they show promising CO2 capacity and selectivity even in humid conditions, they face challenges related to amine leaching during cycling, oxidative degradation at elevated temperatures, and high material costs. Current research focuses on improving their long-term stability and reducing production costs.
A critical technical barrier across all solid sorbent technologies is the trade-off between adsorption capacity and regeneration energy. Sorbents with high binding energy for CO2 typically require more energy for regeneration, reducing the overall process efficiency. Additionally, the mechanical stability of sorbents under pressure and temperature swings remains problematic, with many materials showing significant attrition and capacity loss over multiple cycles.
Heat management represents another significant challenge, as the exothermic nature of CO2 adsorption can lead to temperature increases that reduce working capacity. Conversely, the endothermic desorption process requires efficient heat transfer systems that are difficult to implement in solid sorbent beds.
The gap between laboratory performance and real-world operation under industrial conditions with actual flue gas compositions (containing SOx, NOx, and other contaminants) continues to be a major hurdle for commercial deployment of solid sorbent technologies in CCS applications.
Activated carbons offer advantages of low cost and high thermal stability, but suffer from relatively low CO2 selectivity and capacity under practical conditions. Their performance significantly decreases in humid conditions, which is a major limitation for flue gas applications. Recent developments have focused on surface modification to enhance selectivity, but breakthrough innovations are still needed.
Zeolites, particularly those with high cation content like 13X and 5A, demonstrate excellent CO2 adsorption capacity at moderate temperatures and low partial pressures. However, they exhibit severe performance degradation in the presence of water and require significant energy for regeneration. Current research efforts are directed toward developing water-resistant zeolites and optimizing the regeneration process.
Metal-organic frameworks represent one of the most rapidly developing classes of sorbents, with exceptional surface areas exceeding 6000 m²/g and highly tunable pore structures. Despite their promising laboratory performance, MOFs face significant challenges in stability under cycling conditions, manufacturing scalability, and production costs. The gap between laboratory synthesis and industrial-scale production remains substantial.
Amine-functionalized silicas combine the physical adsorption properties of porous supports with the chemical reactivity of amines toward CO2. While they show promising CO2 capacity and selectivity even in humid conditions, they face challenges related to amine leaching during cycling, oxidative degradation at elevated temperatures, and high material costs. Current research focuses on improving their long-term stability and reducing production costs.
A critical technical barrier across all solid sorbent technologies is the trade-off between adsorption capacity and regeneration energy. Sorbents with high binding energy for CO2 typically require more energy for regeneration, reducing the overall process efficiency. Additionally, the mechanical stability of sorbents under pressure and temperature swings remains problematic, with many materials showing significant attrition and capacity loss over multiple cycles.
Heat management represents another significant challenge, as the exothermic nature of CO2 adsorption can lead to temperature increases that reduce working capacity. Conversely, the endothermic desorption process requires efficient heat transfer systems that are difficult to implement in solid sorbent beds.
The gap between laboratory performance and real-world operation under industrial conditions with actual flue gas compositions (containing SOx, NOx, and other contaminants) continues to be a major hurdle for commercial deployment of solid sorbent technologies in CCS applications.
Current Solid Sorbent Solutions and Implementation
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands. They have emerged as promising solid sorbents for CO2 capture due to their high surface area, tunable pore size, and chemical functionality. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and capacity. Their modular nature allows for customization to optimize CO2 adsorption performance under various conditions, making them effective for post-combustion carbon capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. These materials have shown exceptional CO2 capture efficiency due to their high surface area, tunable pore size, and chemical functionality. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and adsorption capacity. Their modular nature allows for customization to optimize CO2 capture performance under various conditions, making them promising candidates for industrial carbon capture applications.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These sorbents incorporate amine groups onto various support structures such as silica, polymers, or carbon-based materials. The amine groups form chemical bonds with CO2 through carbamate formation, enabling high selectivity and capacity. These materials can be designed with different amine types (primary, secondary, tertiary) and loadings to optimize capture efficiency. Their regeneration typically requires less energy compared to traditional liquid amine scrubbing, making them more energy-efficient for carbon capture applications.
- Zeolites and molecular sieves for selective CO2 adsorption: Zeolites and molecular sieves are crystalline aluminosilicate materials with well-defined pore structures that enable selective adsorption of CO2. These materials function through physical adsorption mechanisms, utilizing their uniform pore sizes to separate CO2 from other gases. Their high thermal stability allows for repeated adsorption-desorption cycles without significant degradation. Various types of zeolites can be synthesized or modified to enhance CO2 selectivity and capacity. The adsorption properties can be tuned by adjusting the silicon-to-aluminum ratio, cation type, and pore architecture to optimize capture efficiency under specific operating conditions.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, offer promising solutions for CO2 capture. These materials provide high surface area and porosity for physical adsorption of CO2 molecules. They can be further enhanced through chemical functionalization to increase CO2 binding affinity. Carbon-based sorbents are advantageous due to their relatively low cost, abundance, and environmental compatibility. Their performance can be optimized by controlling pore size distribution, surface chemistry, and incorporating additional functional groups to enhance selectivity and capacity for CO2 capture applications.
- Regeneration methods and energy efficiency in CO2 capture systems: Efficient regeneration of solid sorbents is crucial for the economic viability of CO2 capture systems. Various regeneration methods have been developed, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and combinations thereof. These approaches aim to minimize energy requirements while maintaining sorbent integrity over multiple cycles. Advanced system designs incorporate heat integration, novel heating methods, and optimized process configurations to reduce the energy penalty associated with sorbent regeneration. Improvements in regeneration efficiency directly impact the overall performance and cost-effectiveness of carbon capture technologies.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine a porous support structure with amine groups that chemically bind to CO2 through acid-base interactions. The amine functionality can be incorporated through impregnation, grafting, or polymerization methods. These sorbents demonstrate high CO2 selectivity even at low concentrations, good working capacity, and can operate effectively at moderate temperatures. Their regeneration typically requires less energy compared to traditional liquid amine scrubbing processes.Expand Specific Solutions03 Zeolite-based CO2 capture systems
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that make them effective for CO2 adsorption. Their high thermal stability, mechanical strength, and relatively low cost make them attractive for industrial applications. Zeolites capture CO2 primarily through physisorption mechanisms, with adsorption capacity influenced by the silicon-to-aluminum ratio, cation type, and pore architecture. Modified zeolites with enhanced hydrophobicity or incorporated functional groups can improve selectivity for CO2 over other gases, particularly in humid conditions where traditional zeolites may suffer performance degradation.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbons, carbon molecular sieves, graphene-based materials, and carbon nanotubes, offer versatile platforms for CO2 capture. These materials feature high surface areas, tunable pore structures, and surface chemistry that can be modified to enhance CO2 adsorption. Nitrogen-doped carbon materials have shown particularly promising results due to the creation of basic sites that interact favorably with acidic CO2 molecules. Carbon-based sorbents generally demonstrate good stability over multiple adsorption-desorption cycles and can be produced from renewable or waste resources, potentially reducing overall costs.Expand Specific Solutions05 Hybrid and composite sorbents for enhanced CO2 capture efficiency
Hybrid and composite sorbents combine different materials to leverage complementary properties and overcome limitations of single-component systems. These may include combinations of organic-inorganic materials, polymer-supported amines, or layered structures with distinct functional components. Such hybrid approaches can simultaneously address multiple challenges in CO2 capture, including capacity, selectivity, stability, and regeneration energy requirements. For example, combining the high surface area of porous supports with the chemical reactivity of amine groups creates materials with both physical and chemical adsorption mechanisms, enhancing overall performance across a range of operating conditions.Expand Specific Solutions
Leading Organizations in Solid Sorbent Development
The solid sorbents for CO2 capture market is in a growth phase, driven by increasing global focus on carbon reduction technologies. The market size is expanding rapidly, projected to reach several billion dollars by 2030 as carbon capture becomes essential for meeting climate goals. Technologically, the field shows varying maturity levels, with companies like Sinopec, BASF, and Susteon leading commercial development while Korean power companies (KEPCO and subsidiaries) focus on implementation. Academic institutions including Rice University, Tsinghua University, and NTNU are advancing fundamental research. Specialized firms like Carboncapture and Corning are developing innovative materials and systems, indicating a competitive landscape spanning from basic research to commercial deployment across multiple geographic regions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced metal-organic frameworks (MOFs) and amine-functionalized mesoporous silica materials for CO2 capture. Their proprietary technology combines high surface area MOFs with optimized pore structures that demonstrate CO2 adsorption capacities exceeding 4.5 mmol/g under flue gas conditions. Sinopec's solid sorbents feature rapid adsorption-desorption kinetics with complete regeneration achieved at temperatures below 120°C, significantly reducing energy penalties compared to conventional amine scrubbing. Their integrated system incorporates temperature swing adsorption (TSA) with waste heat recovery mechanisms, achieving capture costs below $40/ton CO2 in pilot demonstrations. Sinopec has successfully scaled this technology from laboratory to 100,000 tons/year demonstration projects at their refineries, showing stable performance over 1000+ cycles with minimal degradation.
Strengths: Extensive industrial infrastructure for rapid deployment and scaling; integrated approach combining material development with process engineering; access to numerous point sources for implementation. Weaknesses: Higher regeneration energy requirements compared to some emerging materials; potential for amine leaching in humid conditions requiring additional purification steps.
Carboncapture, Inc.
Technical Solution: Carboncapture, Inc. has developed a modular direct air capture (DAC) system utilizing zeolite-based solid sorbents with proprietary surface modifications that enhance CO2 selectivity and water tolerance. Their technology employs a rapid temperature-vacuum swing adsorption process that reduces regeneration energy requirements by approximately 40% compared to conventional approaches. The company's innovative "CarbonCollector" units feature a stackable, containerized design that enables distributed deployment with minimal site preparation. Each module can capture 100-1000 tons CO2/year depending on configuration, with the captured CO2 compressed for geological sequestration or utilization. Their sorbent materials demonstrate exceptional stability with <5% capacity degradation after 10,000 adsorption-desorption cycles under real-world conditions. Carboncapture's system integrates renewable energy sources and waste heat recovery to minimize operational carbon footprint, achieving net carbon-negative performance even when accounting for full lifecycle emissions.
Strengths: Purpose-built technology specifically optimized for direct air capture applications; modular approach enabling rapid scaling and deployment flexibility; integrated business model covering capture through sequestration. Weaknesses: Higher costs per ton CO2 compared to point-source capture technologies; geographic limitations based on renewable energy availability and sequestration site access.
Key Patents and Research in Advanced Sorbent Materials
Layered Solid Sorbents For Carbon Dioxide Capture
PatentActiveUS20140127104A1
Innovation
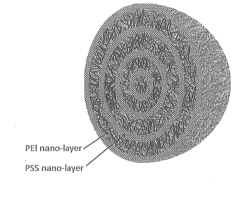
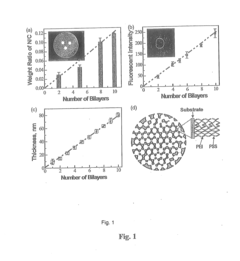
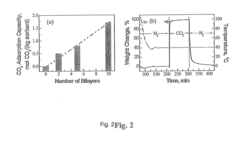
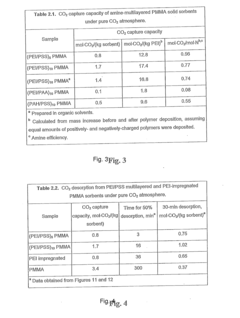
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where CO2-adsorbing polymers like polyethylenimine and oppositely charged polyelectrolytes are alternately deposited on porous substrates, creating bilayers that enhance CO2 capture and transport efficiency.
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
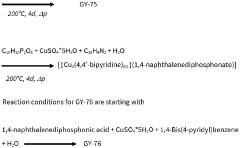
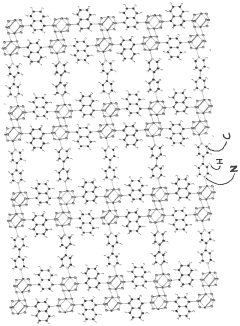
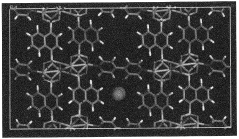
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact assessment of solid sorbent technologies for CO2 capture reveals both advantages and challenges compared to traditional amine-based liquid absorption systems. Solid sorbents typically demonstrate lower energy requirements for regeneration, with materials like metal-organic frameworks (MOFs) and zeolites requiring 30-40% less energy than conventional amine solutions. This energy efficiency translates directly to reduced greenhouse gas emissions from the capture process itself, enhancing the net carbon reduction benefit.
Water consumption represents another significant environmental advantage of solid sorbent systems. While liquid amine systems can consume 1-2 tons of water per ton of CO2 captured due to evaporative losses and degradation processes, solid sorbents operate with minimal water requirements, potentially reducing water footprint by up to 80% in arid regions where water scarcity is a concern.
The production and disposal of solid sorbents must be carefully evaluated within a life cycle assessment framework. Current research indicates that the embodied carbon in manufacturing advanced sorbents like MOFs can be substantial, with estimates ranging from 0.5-2 kg CO2 equivalent per kg of sorbent produced. However, their extended operational lifespan (typically 3-5 years compared to 1-2 years for liquid amines) often compensates for this initial environmental investment.
Land use considerations also favor solid sorbent technologies, as their higher volumetric efficiency typically results in 20-30% smaller facility footprints compared to liquid systems. This reduced spatial requirement minimizes habitat disruption and allows for more flexible installation options, including retrofitting existing industrial facilities.
Waste generation and management present mixed environmental implications. While solid sorbents produce less hazardous liquid waste than amine systems, their eventual disposal or recycling pathways remain underdeveloped. Current research focuses on sorbent regeneration techniques and material recovery processes to minimize end-of-life environmental impacts.
Air quality impacts beyond CO2 capture must also be considered. Solid sorbents typically emit fewer volatile organic compounds and have reduced potential for ammonia slip compared to amine systems. Studies indicate potential reductions of 60-90% in secondary air pollutants, though specific emissions profiles vary significantly between sorbent types.
The environmental assessment must ultimately consider regional factors, as the net benefit of solid sorbent technologies depends heavily on local energy sources, water availability, and waste management infrastructure. Comprehensive life cycle analyses suggest that solid sorbents offer environmental advantages in most deployment scenarios, though continued research is needed to optimize material sustainability and end-of-life management.
Water consumption represents another significant environmental advantage of solid sorbent systems. While liquid amine systems can consume 1-2 tons of water per ton of CO2 captured due to evaporative losses and degradation processes, solid sorbents operate with minimal water requirements, potentially reducing water footprint by up to 80% in arid regions where water scarcity is a concern.
The production and disposal of solid sorbents must be carefully evaluated within a life cycle assessment framework. Current research indicates that the embodied carbon in manufacturing advanced sorbents like MOFs can be substantial, with estimates ranging from 0.5-2 kg CO2 equivalent per kg of sorbent produced. However, their extended operational lifespan (typically 3-5 years compared to 1-2 years for liquid amines) often compensates for this initial environmental investment.
Land use considerations also favor solid sorbent technologies, as their higher volumetric efficiency typically results in 20-30% smaller facility footprints compared to liquid systems. This reduced spatial requirement minimizes habitat disruption and allows for more flexible installation options, including retrofitting existing industrial facilities.
Waste generation and management present mixed environmental implications. While solid sorbents produce less hazardous liquid waste than amine systems, their eventual disposal or recycling pathways remain underdeveloped. Current research focuses on sorbent regeneration techniques and material recovery processes to minimize end-of-life environmental impacts.
Air quality impacts beyond CO2 capture must also be considered. Solid sorbents typically emit fewer volatile organic compounds and have reduced potential for ammonia slip compared to amine systems. Studies indicate potential reductions of 60-90% in secondary air pollutants, though specific emissions profiles vary significantly between sorbent types.
The environmental assessment must ultimately consider regional factors, as the net benefit of solid sorbent technologies depends heavily on local energy sources, water availability, and waste management infrastructure. Comprehensive life cycle analyses suggest that solid sorbents offer environmental advantages in most deployment scenarios, though continued research is needed to optimize material sustainability and end-of-life management.
Techno-Economic Analysis of Solid Sorbent Systems
The techno-economic analysis of solid sorbent systems for carbon capture requires a comprehensive evaluation of both technical performance and economic viability. This analysis integrates capital expenditures (CAPEX), operational expenditures (OPEX), and overall system efficiency to determine the feasibility of implementing solid sorbent technologies at commercial scale.
Initial cost assessments indicate that solid sorbent systems may offer significant economic advantages over traditional amine-based liquid absorption processes, with potential cost reductions of 15-30% in energy requirements. The primary economic drivers include lower regeneration energy, reduced equipment corrosion, and minimized solvent degradation issues that plague liquid systems.
Capital costs for solid sorbent systems encompass sorbent manufacturing, containment vessels, heat exchangers, and auxiliary equipment. Current estimates place CAPEX requirements at approximately $600-900 per kW for retrofitted power plants, though these figures vary considerably based on system configuration and scale. The sorbent material itself represents 20-35% of total capital costs, highlighting the importance of developing materials with extended operational lifespans.
Operational expenditures are dominated by energy consumption for sorbent regeneration, sorbent replacement costs due to degradation, and maintenance requirements. Analysis shows that advanced metal-organic frameworks (MOFs) and functionalized porous polymers can achieve regeneration energy requirements as low as 2.0-2.5 GJ/tonne CO₂, compared to 3.5-4.0 GJ/tonne for conventional amine scrubbing.
Sensitivity analysis reveals that sorbent lifetime critically impacts economic viability. Materials demonstrating stability over 1,000+ cycles can reduce the levelized cost of carbon capture by up to 40% compared to sorbents requiring frequent replacement. This underscores the importance of developing degradation-resistant materials that maintain performance under industrial conditions.
The economic assessment must also consider process integration opportunities, particularly waste heat utilization for sorbent regeneration. Systems designed to leverage existing thermal resources can achieve carbon capture costs of $40-60 per tonne CO₂, approaching the economic threshold needed for widespread adoption without significant policy incentives.
Scale-up considerations present additional economic challenges, as laboratory-proven materials must be manufactured at industrial volumes while maintaining performance characteristics. Current production pathways for advanced sorbents like covalent organic frameworks remain prohibitively expensive for large-scale deployment, necessitating innovation in synthesis methods to reduce material costs by an order of magnitude.
Initial cost assessments indicate that solid sorbent systems may offer significant economic advantages over traditional amine-based liquid absorption processes, with potential cost reductions of 15-30% in energy requirements. The primary economic drivers include lower regeneration energy, reduced equipment corrosion, and minimized solvent degradation issues that plague liquid systems.
Capital costs for solid sorbent systems encompass sorbent manufacturing, containment vessels, heat exchangers, and auxiliary equipment. Current estimates place CAPEX requirements at approximately $600-900 per kW for retrofitted power plants, though these figures vary considerably based on system configuration and scale. The sorbent material itself represents 20-35% of total capital costs, highlighting the importance of developing materials with extended operational lifespans.
Operational expenditures are dominated by energy consumption for sorbent regeneration, sorbent replacement costs due to degradation, and maintenance requirements. Analysis shows that advanced metal-organic frameworks (MOFs) and functionalized porous polymers can achieve regeneration energy requirements as low as 2.0-2.5 GJ/tonne CO₂, compared to 3.5-4.0 GJ/tonne for conventional amine scrubbing.
Sensitivity analysis reveals that sorbent lifetime critically impacts economic viability. Materials demonstrating stability over 1,000+ cycles can reduce the levelized cost of carbon capture by up to 40% compared to sorbents requiring frequent replacement. This underscores the importance of developing degradation-resistant materials that maintain performance under industrial conditions.
The economic assessment must also consider process integration opportunities, particularly waste heat utilization for sorbent regeneration. Systems designed to leverage existing thermal resources can achieve carbon capture costs of $40-60 per tonne CO₂, approaching the economic threshold needed for widespread adoption without significant policy incentives.
Scale-up considerations present additional economic challenges, as laboratory-proven materials must be manufactured at industrial volumes while maintaining performance characteristics. Current production pathways for advanced sorbents like covalent organic frameworks remain prohibitively expensive for large-scale deployment, necessitating innovation in synthesis methods to reduce material costs by an order of magnitude.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!