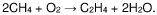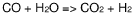Synthesis of Ethyl Propanoate in Continuous Flow Reactors
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Continuous Flow Synthesis Background and Objectives
Continuous flow synthesis represents a paradigm shift in chemical manufacturing, offering significant advantages over traditional batch processes. This approach has gained considerable attention in recent years due to its potential to revolutionize the production of various chemicals, including ethyl propanoate. The evolution of continuous flow technology can be traced back to the early 2000s when researchers began exploring its applications in organic synthesis.
The primary objective of implementing continuous flow synthesis for ethyl propanoate production is to enhance efficiency, safety, and scalability. By transitioning from batch to continuous processes, manufacturers aim to achieve better control over reaction parameters, improved heat and mass transfer, and reduced reaction times. These benefits are particularly relevant for the synthesis of ethyl propanoate, which involves the esterification of propionic acid with ethanol.
Continuous flow reactors offer several advantages for this synthesis, including precise temperature control, efficient mixing, and the ability to handle potentially hazardous intermediates safely. The technology also allows for real-time monitoring and adjustment of reaction conditions, leading to improved product quality and consistency. Furthermore, the compact nature of continuous flow systems can result in a smaller environmental footprint compared to traditional batch processes.
The development of continuous flow synthesis for ethyl propanoate aligns with broader industry trends towards process intensification and green chemistry. By minimizing waste generation, reducing energy consumption, and improving atom economy, this approach addresses key sustainability challenges faced by the chemical industry. Additionally, the potential for modular and scalable production systems opens up new possibilities for on-demand manufacturing and decentralized production.
As research in this field progresses, the focus is increasingly shifting towards overcoming technical challenges such as catalyst immobilization, handling of multiphase systems, and integration of downstream processing. The ultimate goal is to develop a fully integrated, continuous manufacturing process for ethyl propanoate that can be easily scaled and adapted to meet varying production demands.
In conclusion, the background and objectives of continuous flow synthesis for ethyl propanoate production reflect a broader technological trend in the chemical industry. By addressing key limitations of batch processes and offering new opportunities for process optimization, this approach has the potential to transform the production of not only ethyl propanoate but also a wide range of other chemical compounds.
The primary objective of implementing continuous flow synthesis for ethyl propanoate production is to enhance efficiency, safety, and scalability. By transitioning from batch to continuous processes, manufacturers aim to achieve better control over reaction parameters, improved heat and mass transfer, and reduced reaction times. These benefits are particularly relevant for the synthesis of ethyl propanoate, which involves the esterification of propionic acid with ethanol.
Continuous flow reactors offer several advantages for this synthesis, including precise temperature control, efficient mixing, and the ability to handle potentially hazardous intermediates safely. The technology also allows for real-time monitoring and adjustment of reaction conditions, leading to improved product quality and consistency. Furthermore, the compact nature of continuous flow systems can result in a smaller environmental footprint compared to traditional batch processes.
The development of continuous flow synthesis for ethyl propanoate aligns with broader industry trends towards process intensification and green chemistry. By minimizing waste generation, reducing energy consumption, and improving atom economy, this approach addresses key sustainability challenges faced by the chemical industry. Additionally, the potential for modular and scalable production systems opens up new possibilities for on-demand manufacturing and decentralized production.
As research in this field progresses, the focus is increasingly shifting towards overcoming technical challenges such as catalyst immobilization, handling of multiphase systems, and integration of downstream processing. The ultimate goal is to develop a fully integrated, continuous manufacturing process for ethyl propanoate that can be easily scaled and adapted to meet varying production demands.
In conclusion, the background and objectives of continuous flow synthesis for ethyl propanoate production reflect a broader technological trend in the chemical industry. By addressing key limitations of batch processes and offering new opportunities for process optimization, this approach has the potential to transform the production of not only ethyl propanoate but also a wide range of other chemical compounds.
Market Analysis for Ethyl Propanoate
The global market for ethyl propanoate has been experiencing steady growth, driven by its versatile applications across various industries. This ester compound, also known as ethyl propionate, finds extensive use in flavors and fragrances, solvents, and as an intermediate in pharmaceutical and agrochemical production. The increasing demand for natural and synthetic flavors in the food and beverage industry has been a significant factor contributing to the market expansion of ethyl propanoate.
In the flavor and fragrance sector, ethyl propanoate is valued for its fruity, rum-like aroma, making it a popular choice in the formulation of fruit-flavored products, particularly those with apple, pear, or pineapple notes. The growing consumer preference for natural and organic products has further boosted the demand for ethyl propanoate derived from natural sources, aligning with the clean label trend in the food industry.
The pharmaceutical industry represents another key market for ethyl propanoate, where it serves as a crucial intermediate in the synthesis of various active pharmaceutical ingredients (APIs). The compound's role in drug manufacturing processes has ensured a consistent demand from pharmaceutical companies worldwide, with the market benefiting from the overall growth of the global pharmaceutical sector.
In the agrochemical industry, ethyl propanoate finds application in the production of pesticides and herbicides, contributing to the market's growth as agricultural practices intensify globally to meet the food demands of a growing population. The compound's effectiveness as a solvent in various industrial processes has also expanded its market reach across multiple sectors.
Geographically, North America and Europe have traditionally been the largest markets for ethyl propanoate, owing to their well-established flavor and fragrance industries, as well as robust pharmaceutical sectors. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, rising disposable incomes, and changing consumer preferences in countries like China and India.
The market for ethyl propanoate is characterized by a mix of large multinational corporations and smaller, specialized chemical manufacturers. Key players in the market have been focusing on expanding their production capacities and improving their synthesis processes to meet the growing demand while maintaining competitive pricing. The adoption of continuous flow reactors for the synthesis of ethyl propanoate represents a significant technological advancement in this regard, offering potential improvements in efficiency, product quality, and environmental sustainability.
In the flavor and fragrance sector, ethyl propanoate is valued for its fruity, rum-like aroma, making it a popular choice in the formulation of fruit-flavored products, particularly those with apple, pear, or pineapple notes. The growing consumer preference for natural and organic products has further boosted the demand for ethyl propanoate derived from natural sources, aligning with the clean label trend in the food industry.
The pharmaceutical industry represents another key market for ethyl propanoate, where it serves as a crucial intermediate in the synthesis of various active pharmaceutical ingredients (APIs). The compound's role in drug manufacturing processes has ensured a consistent demand from pharmaceutical companies worldwide, with the market benefiting from the overall growth of the global pharmaceutical sector.
In the agrochemical industry, ethyl propanoate finds application in the production of pesticides and herbicides, contributing to the market's growth as agricultural practices intensify globally to meet the food demands of a growing population. The compound's effectiveness as a solvent in various industrial processes has also expanded its market reach across multiple sectors.
Geographically, North America and Europe have traditionally been the largest markets for ethyl propanoate, owing to their well-established flavor and fragrance industries, as well as robust pharmaceutical sectors. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, rising disposable incomes, and changing consumer preferences in countries like China and India.
The market for ethyl propanoate is characterized by a mix of large multinational corporations and smaller, specialized chemical manufacturers. Key players in the market have been focusing on expanding their production capacities and improving their synthesis processes to meet the growing demand while maintaining competitive pricing. The adoption of continuous flow reactors for the synthesis of ethyl propanoate represents a significant technological advancement in this regard, offering potential improvements in efficiency, product quality, and environmental sustainability.
Current Challenges in Continuous Flow Reactors
Continuous flow reactors have revolutionized the synthesis of ethyl propanoate, offering numerous advantages over traditional batch processes. However, several challenges persist in this field, hindering the full realization of their potential. One of the primary obstacles is achieving optimal mixing and heat transfer within the reactor. The confined space and rapid flow rates can lead to incomplete mixing, resulting in non-uniform product quality and reduced yields.
Temperature control presents another significant challenge. The exothermic nature of the esterification reaction between propionic acid and ethanol requires precise temperature management to prevent hot spots and ensure reaction efficiency. Conventional cooling methods may struggle to dissipate heat quickly enough, particularly in larger-scale operations.
Catalyst deactivation and fouling pose ongoing issues in continuous flow reactors. The high surface area-to-volume ratio of these systems can lead to increased catalyst-wall interactions, potentially causing catalyst degradation or deposition. This not only reduces reaction efficiency but also necessitates frequent reactor cleaning and maintenance, impacting overall process economics.
Scaling up continuous flow processes for industrial production remains a complex task. While lab-scale reactors demonstrate promising results, translating these to commercial-scale operations often reveals unforeseen challenges. Maintaining consistent flow patterns, pressure drops, and residence times across larger reactor volumes requires sophisticated engineering solutions.
The handling of multiphase systems in continuous flow reactors presents additional complications. Gas-liquid or liquid-liquid reactions, common in ethyl propanoate synthesis, can lead to flow instabilities and phase separation issues. Ensuring adequate contact between reactants in different phases while maintaining stable flow conditions is crucial for reaction efficiency.
Material compatibility is another critical consideration. The corrosive nature of propionic acid and the potential for side reactions necessitate careful selection of reactor materials. Finding cost-effective materials that can withstand the chemical environment while providing optimal heat transfer properties remains an ongoing challenge.
Process control and monitoring in continuous flow systems require advanced instrumentation and real-time analytics. Developing robust sensors and control systems capable of rapidly detecting and responding to process variations is essential for maintaining product quality and process safety. The integration of these systems with existing manufacturing infrastructure presents both technical and logistical challenges.
Temperature control presents another significant challenge. The exothermic nature of the esterification reaction between propionic acid and ethanol requires precise temperature management to prevent hot spots and ensure reaction efficiency. Conventional cooling methods may struggle to dissipate heat quickly enough, particularly in larger-scale operations.
Catalyst deactivation and fouling pose ongoing issues in continuous flow reactors. The high surface area-to-volume ratio of these systems can lead to increased catalyst-wall interactions, potentially causing catalyst degradation or deposition. This not only reduces reaction efficiency but also necessitates frequent reactor cleaning and maintenance, impacting overall process economics.
Scaling up continuous flow processes for industrial production remains a complex task. While lab-scale reactors demonstrate promising results, translating these to commercial-scale operations often reveals unforeseen challenges. Maintaining consistent flow patterns, pressure drops, and residence times across larger reactor volumes requires sophisticated engineering solutions.
The handling of multiphase systems in continuous flow reactors presents additional complications. Gas-liquid or liquid-liquid reactions, common in ethyl propanoate synthesis, can lead to flow instabilities and phase separation issues. Ensuring adequate contact between reactants in different phases while maintaining stable flow conditions is crucial for reaction efficiency.
Material compatibility is another critical consideration. The corrosive nature of propionic acid and the potential for side reactions necessitate careful selection of reactor materials. Finding cost-effective materials that can withstand the chemical environment while providing optimal heat transfer properties remains an ongoing challenge.
Process control and monitoring in continuous flow systems require advanced instrumentation and real-time analytics. Developing robust sensors and control systems capable of rapidly detecting and responding to process variations is essential for maintaining product quality and process safety. The integration of these systems with existing manufacturing infrastructure presents both technical and logistical challenges.
Existing Continuous Flow Synthesis Methods
01 Synthesis methods for ethyl propanoate
Various methods for synthesizing ethyl propanoate are described, including esterification reactions between propionic acid and ethanol, as well as catalytic processes using different catalysts and reaction conditions. These methods aim to improve yield, selectivity, and efficiency in the production of ethyl propanoate.- Synthesis methods for ethyl propanoate: Various methods for synthesizing ethyl propanoate are described, including esterification of propionic acid with ethanol, catalytic processes, and continuous flow reactions. These methods aim to improve yield, reduce reaction time, and enhance product purity.
- Applications in fragrance and flavor industry: Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is incorporated into various products such as perfumes, cosmetics, and food additives to impart a pleasant aroma and taste.
- Purification and separation techniques: Different methods for purifying and separating ethyl propanoate from reaction mixtures or other compounds are described. These techniques include distillation, extraction, and chromatography, aimed at obtaining high-purity ethyl propanoate for various applications.
- Use as a solvent and intermediate: Ethyl propanoate finds applications as a solvent in various industrial processes and as an intermediate in the synthesis of other chemicals. Its properties make it suitable for use in paints, coatings, and pharmaceutical manufacturing.
- Environmental and safety considerations: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl propanoate production and use. This includes developing green synthesis methods, reducing waste, and enhancing handling procedures to minimize risks associated with its flammability and potential health effects.
02 Applications in fragrance and flavor industry
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is incorporated into various products such as perfumes, cosmetics, and food flavorings to impart a pleasant aroma and taste. The compound's low toxicity and stability make it suitable for these applications.Expand Specific Solutions03 Use as a solvent and intermediate
Ethyl propanoate serves as an important solvent in various industrial processes, particularly in the production of paints, coatings, and adhesives. It is also used as a chemical intermediate in the synthesis of other compounds, including pharmaceuticals and agrochemicals.Expand Specific Solutions04 Purification and quality control
Various methods for purifying ethyl propanoate and ensuring its quality are described. These include distillation techniques, chromatographic separation, and analytical methods for determining purity and identifying impurities. Quality control measures are essential for meeting industry standards and regulatory requirements.Expand Specific Solutions05 Environmental and safety considerations
Research and development efforts focus on improving the environmental profile and safety aspects of ethyl propanoate production and use. This includes developing green synthesis methods, reducing waste generation, and implementing safer handling procedures. Additionally, studies on the compound's biodegradability and potential environmental impacts are conducted.Expand Specific Solutions
Key Players in Flow Chemistry Industry
The synthesis of ethyl propanoate in continuous flow reactors represents an evolving field in chemical engineering, currently in a transitional phase between early development and commercial application. The market size for this technology is growing, driven by increasing demand for more efficient and sustainable production methods in the pharmaceutical and fine chemical industries. While the technology is advancing, it has not yet reached full maturity, with ongoing research and development efforts focused on optimizing process parameters and scaling up production. Key players in this space include BASF Corp., Dow Global Technologies LLC, and Evonik Operations GmbH, who are leveraging their expertise in chemical manufacturing and process engineering to develop innovative continuous flow reactor systems. Smaller, specialized companies and academic institutions are also contributing to technological advancements in this field.
BASF Corp.
Technical Solution: BASF has developed an innovative continuous flow reactor system for the synthesis of ethyl propanoate. Their approach utilizes a microreactor technology with integrated heat exchangers, allowing for precise temperature control and improved mixing[1]. The system employs a heterogeneous acid catalyst fixed in a packed bed reactor, which enables a solvent-free process and facilitates easier product separation[2]. BASF's continuous flow setup achieves high conversion rates (>95%) and selectivity (>98%) for ethyl propanoate production, with residence times of less than 10 minutes[3]. The process operates at moderate temperatures (60-80°C) and pressures (5-10 bar), optimizing energy efficiency while maintaining high product quality[4].
Strengths: High conversion and selectivity, energy-efficient process, solvent-free operation, and easy product separation. Weaknesses: Potential catalyst deactivation over time, higher initial capital investment compared to batch processes.
Dow Global Technologies LLC
Technical Solution: Dow has pioneered a continuous flow reactor design for ethyl propanoate synthesis using a novel reactive distillation approach. Their system combines reaction and separation in a single unit, significantly reducing equipment footprint and energy consumption[1]. The process utilizes a homogeneous acid catalyst in the liquid phase, with ethanol and propionic acid fed continuously into the reactor column[2]. As the reaction progresses, the ethyl propanoate product is continuously removed via distillation, driving the equilibrium towards product formation. Dow's technology achieves conversion rates of up to 98% and product purities exceeding 99.5%[3]. The system operates under mild conditions (atmospheric pressure, 70-90°C), with a total residence time of approximately 30 minutes[4].
Strengths: High conversion and product purity, integrated reaction and separation, reduced equipment and energy requirements. Weaknesses: Potential challenges in catalyst recovery and recycling, sensitivity to feed composition fluctuations.
Innovative Approaches in Ethyl Propanoate Synthesis
Process for the conversion of methane into propanal
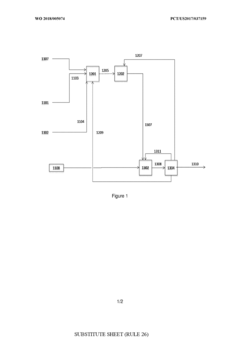
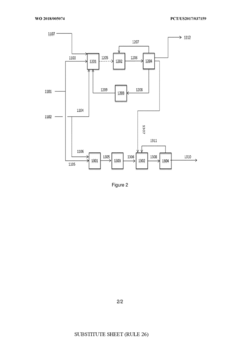
PatentWO2018005074A1
Innovation
- A process involving oxidative coupling of methane with oxygen in a gas phase reaction, followed by hydroformylation in a second reactor, where the molar ratios of syngas to ethylene and CO2 to ethane are optimized by adjusting the H2 to CO ratio using steam co-feeding and water gas shift reactions, eliminating the need for separate syngas production and separation steps.
Continuous synthesis of an ethylene and butadiene copolymer
PatentActiveUS20200131288A1
Innovation
- A continuous process involving a series of stirred polymerization reactors with controlled feeding of ethylene, butadiene, and a hydrocarbon solvent, using a metallocene catalytic system to maintain a specific mole ratio of ethylene to butadiene, ensuring a high molar fraction of ethylene units and controlled microstructure, with operating conditions optimized for productivity and homogeneity.
Green Chemistry Aspects of Continuous Flow Synthesis
The continuous flow synthesis of ethyl propanoate presents significant advantages from a green chemistry perspective. This approach aligns with several key principles of green chemistry, including waste reduction, improved energy efficiency, and enhanced process safety.
Continuous flow reactors enable precise control over reaction parameters, leading to optimized yields and reduced byproduct formation. This results in a more efficient use of raw materials and minimizes waste generation. The ability to fine-tune reaction conditions in real-time also allows for the implementation of in-line purification techniques, further reducing the need for resource-intensive downstream processing.
Energy efficiency is another crucial aspect of green chemistry that is addressed by continuous flow synthesis. The high surface area-to-volume ratio of microreactors facilitates rapid heat transfer, allowing for better temperature control and reduced energy consumption compared to batch processes. This improved heat management also enables the use of more intensive reaction conditions, potentially eliminating the need for energy-intensive catalysts or solvents.
Process safety is significantly enhanced in continuous flow systems. The small reactor volumes and continuous operation reduce the risks associated with handling large quantities of potentially hazardous materials. This inherent safety feature aligns with green chemistry principles by minimizing the potential for accidents and environmental releases.
The use of continuous flow reactors also opens up opportunities for process intensification. Multiple reaction steps can be integrated into a single, streamlined process, reducing the overall footprint of the production system. This consolidation not only saves space but also minimizes material transfer operations, further reducing energy consumption and the potential for material losses.
Furthermore, continuous flow synthesis of ethyl propanoate allows for easier scaling of production. The modular nature of flow reactors enables a more straightforward transition from laboratory scale to industrial production, potentially reducing the resources required for process development and scale-up.
In conclusion, the application of continuous flow technology to the synthesis of ethyl propanoate demonstrates significant potential for improving the sustainability and environmental impact of this chemical process. By addressing key green chemistry principles, this approach represents a promising direction for the future of sustainable chemical manufacturing.
Continuous flow reactors enable precise control over reaction parameters, leading to optimized yields and reduced byproduct formation. This results in a more efficient use of raw materials and minimizes waste generation. The ability to fine-tune reaction conditions in real-time also allows for the implementation of in-line purification techniques, further reducing the need for resource-intensive downstream processing.
Energy efficiency is another crucial aspect of green chemistry that is addressed by continuous flow synthesis. The high surface area-to-volume ratio of microreactors facilitates rapid heat transfer, allowing for better temperature control and reduced energy consumption compared to batch processes. This improved heat management also enables the use of more intensive reaction conditions, potentially eliminating the need for energy-intensive catalysts or solvents.
Process safety is significantly enhanced in continuous flow systems. The small reactor volumes and continuous operation reduce the risks associated with handling large quantities of potentially hazardous materials. This inherent safety feature aligns with green chemistry principles by minimizing the potential for accidents and environmental releases.
The use of continuous flow reactors also opens up opportunities for process intensification. Multiple reaction steps can be integrated into a single, streamlined process, reducing the overall footprint of the production system. This consolidation not only saves space but also minimizes material transfer operations, further reducing energy consumption and the potential for material losses.
Furthermore, continuous flow synthesis of ethyl propanoate allows for easier scaling of production. The modular nature of flow reactors enables a more straightforward transition from laboratory scale to industrial production, potentially reducing the resources required for process development and scale-up.
In conclusion, the application of continuous flow technology to the synthesis of ethyl propanoate demonstrates significant potential for improving the sustainability and environmental impact of this chemical process. By addressing key green chemistry principles, this approach represents a promising direction for the future of sustainable chemical manufacturing.
Scale-up Strategies for Industrial Production
Scaling up the synthesis of ethyl propanoate in continuous flow reactors for industrial production requires careful consideration of several key factors. The transition from laboratory-scale to industrial-scale production involves addressing challenges related to reactor design, process control, and optimization of operating conditions.
One of the primary strategies for scale-up is the implementation of modular reactor systems. This approach involves replicating smaller reactor units in parallel, allowing for easier scale-up while maintaining the benefits of continuous flow processing. Modular systems offer flexibility in production capacity and can be more easily adapted to varying demand. Additionally, they provide redundancy, which is crucial for maintaining consistent production in case of individual unit failures.
Process intensification techniques play a crucial role in scale-up strategies. These may include the use of advanced mixing technologies, such as static mixers or microstructured reactors, to enhance mass and heat transfer. Improved mixing efficiency can lead to better reaction kinetics and higher product yields, even at larger scales. Furthermore, the integration of in-line monitoring and process analytical technologies (PAT) enables real-time quality control and process optimization.
Heat management is a critical aspect of scaling up ethyl propanoate synthesis. As reaction volumes increase, efficient heat removal becomes more challenging. Strategies to address this include the use of heat exchangers integrated into the reactor design, as well as the implementation of temperature control zones along the reactor length. Advanced cooling systems, such as microwave-assisted cooling or the use of supercritical fluids as heat transfer media, may also be considered for large-scale production.
Material selection and reactor construction are vital considerations for industrial-scale production. Corrosion-resistant materials, such as stainless steel or specialized alloys, may be necessary to withstand the acidic conditions typically associated with ester synthesis. The reactor design should also account for pressure requirements, especially if operating under supercritical conditions or at elevated temperatures.
Continuous separation and purification strategies must be integrated into the scale-up process. This may involve the use of in-line distillation columns, membrane separators, or other continuous separation techniques to efficiently isolate the ethyl propanoate product. The implementation of recycle streams for unreacted reagents can significantly improve overall process efficiency and reduce waste generation.
Automation and process control systems are essential for maintaining consistent product quality and optimizing production efficiency at industrial scales. Advanced control algorithms, such as model predictive control, can be employed to manage complex process variables and ensure optimal operating conditions are maintained throughout the production cycle.
One of the primary strategies for scale-up is the implementation of modular reactor systems. This approach involves replicating smaller reactor units in parallel, allowing for easier scale-up while maintaining the benefits of continuous flow processing. Modular systems offer flexibility in production capacity and can be more easily adapted to varying demand. Additionally, they provide redundancy, which is crucial for maintaining consistent production in case of individual unit failures.
Process intensification techniques play a crucial role in scale-up strategies. These may include the use of advanced mixing technologies, such as static mixers or microstructured reactors, to enhance mass and heat transfer. Improved mixing efficiency can lead to better reaction kinetics and higher product yields, even at larger scales. Furthermore, the integration of in-line monitoring and process analytical technologies (PAT) enables real-time quality control and process optimization.
Heat management is a critical aspect of scaling up ethyl propanoate synthesis. As reaction volumes increase, efficient heat removal becomes more challenging. Strategies to address this include the use of heat exchangers integrated into the reactor design, as well as the implementation of temperature control zones along the reactor length. Advanced cooling systems, such as microwave-assisted cooling or the use of supercritical fluids as heat transfer media, may also be considered for large-scale production.
Material selection and reactor construction are vital considerations for industrial-scale production. Corrosion-resistant materials, such as stainless steel or specialized alloys, may be necessary to withstand the acidic conditions typically associated with ester synthesis. The reactor design should also account for pressure requirements, especially if operating under supercritical conditions or at elevated temperatures.
Continuous separation and purification strategies must be integrated into the scale-up process. This may involve the use of in-line distillation columns, membrane separators, or other continuous separation techniques to efficiently isolate the ethyl propanoate product. The implementation of recycle streams for unreacted reagents can significantly improve overall process efficiency and reduce waste generation.
Automation and process control systems are essential for maintaining consistent product quality and optimizing production efficiency at industrial scales. Advanced control algorithms, such as model predictive control, can be employed to manage complex process variables and ensure optimal operating conditions are maintained throughout the production cycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!