Use FTIR to Assess Chemical Reactions Kinetics
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
FTIR Spectroscopy Background and Research Objectives
Fourier Transform Infrared (FTIR) spectroscopy has evolved significantly since its inception in the mid-20th century, becoming an indispensable analytical tool across various scientific disciplines. The technique leverages the interaction between infrared radiation and molecular bonds to generate spectral fingerprints unique to specific chemical compounds. This non-destructive analytical method has transformed from simple qualitative identification to sophisticated quantitative analysis of complex chemical systems.
The historical development of FTIR technology shows remarkable progress in instrumentation precision, computational capabilities, and methodological approaches. Early infrared spectroscopy relied on dispersive instruments with limited resolution and sensitivity. The introduction of Fourier transform mathematics and interferometry in the 1960s revolutionized the field, enabling faster data acquisition and significantly improved signal-to-noise ratios through the Fellgett advantage.
Recent technological advancements have further enhanced FTIR capabilities, particularly in time-resolved measurements critical for kinetic studies. Modern FTIR systems feature rapid-scan capabilities, step-scan techniques, and time-resolved spectroscopy options that allow researchers to monitor chemical reactions in real-time with millisecond or even microsecond resolution. These developments have positioned FTIR as a powerful tool for studying reaction kinetics across diverse chemical processes.
The primary objective of this technical research is to comprehensively evaluate the application of FTIR spectroscopy in assessing chemical reaction kinetics. We aim to explore how FTIR can provide insights into reaction mechanisms, rate constants, activation energies, and intermediate species formation. The research seeks to identify optimal methodological approaches for different reaction types, from simple solution-phase transformations to complex heterogeneous catalytic processes.
Additionally, this investigation intends to assess emerging FTIR technologies that may further enhance kinetic measurements, including microfluidic FTIR systems, ultrafast spectroscopy techniques, and advanced chemometric data analysis methods. By examining these innovations, we aim to establish a forward-looking perspective on how FTIR capabilities for kinetic studies may evolve in the near future.
The research also aims to identify current limitations and challenges in applying FTIR to kinetic studies, such as temporal resolution constraints, spectral interference issues, and data interpretation complexities. Understanding these barriers is essential for developing improved methodologies and instrumentation that can overcome existing technical hurdles.
Through this comprehensive technical assessment, we expect to provide valuable insights for both academic research and industrial applications where understanding reaction kinetics is crucial for process optimization, catalyst development, pharmaceutical formulation, and materials science innovation.
The historical development of FTIR technology shows remarkable progress in instrumentation precision, computational capabilities, and methodological approaches. Early infrared spectroscopy relied on dispersive instruments with limited resolution and sensitivity. The introduction of Fourier transform mathematics and interferometry in the 1960s revolutionized the field, enabling faster data acquisition and significantly improved signal-to-noise ratios through the Fellgett advantage.
Recent technological advancements have further enhanced FTIR capabilities, particularly in time-resolved measurements critical for kinetic studies. Modern FTIR systems feature rapid-scan capabilities, step-scan techniques, and time-resolved spectroscopy options that allow researchers to monitor chemical reactions in real-time with millisecond or even microsecond resolution. These developments have positioned FTIR as a powerful tool for studying reaction kinetics across diverse chemical processes.
The primary objective of this technical research is to comprehensively evaluate the application of FTIR spectroscopy in assessing chemical reaction kinetics. We aim to explore how FTIR can provide insights into reaction mechanisms, rate constants, activation energies, and intermediate species formation. The research seeks to identify optimal methodological approaches for different reaction types, from simple solution-phase transformations to complex heterogeneous catalytic processes.
Additionally, this investigation intends to assess emerging FTIR technologies that may further enhance kinetic measurements, including microfluidic FTIR systems, ultrafast spectroscopy techniques, and advanced chemometric data analysis methods. By examining these innovations, we aim to establish a forward-looking perspective on how FTIR capabilities for kinetic studies may evolve in the near future.
The research also aims to identify current limitations and challenges in applying FTIR to kinetic studies, such as temporal resolution constraints, spectral interference issues, and data interpretation complexities. Understanding these barriers is essential for developing improved methodologies and instrumentation that can overcome existing technical hurdles.
Through this comprehensive technical assessment, we expect to provide valuable insights for both academic research and industrial applications where understanding reaction kinetics is crucial for process optimization, catalyst development, pharmaceutical formulation, and materials science innovation.
Market Applications for Reaction Kinetics Analysis
The application of FTIR for reaction kinetics analysis spans numerous industries, creating substantial market opportunities. In the pharmaceutical sector, FTIR kinetics analysis enables real-time monitoring of drug synthesis reactions, significantly reducing development timelines and ensuring batch-to-batch consistency. This capability is particularly valuable for complex API manufacturing processes where reaction pathway understanding directly impacts product quality and regulatory compliance.
The petrochemical industry represents another major market, where FTIR kinetics analysis facilitates optimization of catalytic processes in refineries and chemical plants. By providing immediate feedback on reaction progression, companies can fine-tune operating conditions to maximize yield and minimize byproduct formation, translating to substantial cost savings in large-scale operations.
Polymer manufacturing benefits extensively from FTIR kinetics analysis, as it allows precise monitoring of polymerization reactions. This enables manufacturers to develop materials with tailored properties by controlling reaction rates and termination points. The growing demand for specialty polymers in electronics, automotive, and medical applications has further amplified the need for sophisticated reaction monitoring technologies.
The food and beverage industry utilizes FTIR kinetics analysis for quality control and process optimization. Applications include monitoring fermentation processes, studying lipid oxidation kinetics, and analyzing enzymatic reactions critical to food production. These capabilities support the development of improved preservation methods and novel food processing techniques.
Environmental monitoring represents an emerging application area, where FTIR kinetics analysis helps track degradation rates of pollutants and assess remediation strategies. This information is crucial for developing effective environmental management protocols and evaluating the persistence of contaminants in various ecosystems.
Academic and research institutions constitute a significant market segment, utilizing FTIR kinetics analysis for fundamental research across chemistry, materials science, and biology. The technique's versatility makes it valuable for investigating reaction mechanisms, catalyst performance, and biomolecular interactions.
The global market for analytical instruments used in reaction kinetics studies, including FTIR systems, continues to expand as industries increasingly prioritize process optimization and quality control. This growth is further driven by regulatory requirements demanding more thorough characterization of chemical processes and products, particularly in pharmaceutical and food industries where consumer safety is paramount.
The petrochemical industry represents another major market, where FTIR kinetics analysis facilitates optimization of catalytic processes in refineries and chemical plants. By providing immediate feedback on reaction progression, companies can fine-tune operating conditions to maximize yield and minimize byproduct formation, translating to substantial cost savings in large-scale operations.
Polymer manufacturing benefits extensively from FTIR kinetics analysis, as it allows precise monitoring of polymerization reactions. This enables manufacturers to develop materials with tailored properties by controlling reaction rates and termination points. The growing demand for specialty polymers in electronics, automotive, and medical applications has further amplified the need for sophisticated reaction monitoring technologies.
The food and beverage industry utilizes FTIR kinetics analysis for quality control and process optimization. Applications include monitoring fermentation processes, studying lipid oxidation kinetics, and analyzing enzymatic reactions critical to food production. These capabilities support the development of improved preservation methods and novel food processing techniques.
Environmental monitoring represents an emerging application area, where FTIR kinetics analysis helps track degradation rates of pollutants and assess remediation strategies. This information is crucial for developing effective environmental management protocols and evaluating the persistence of contaminants in various ecosystems.
Academic and research institutions constitute a significant market segment, utilizing FTIR kinetics analysis for fundamental research across chemistry, materials science, and biology. The technique's versatility makes it valuable for investigating reaction mechanisms, catalyst performance, and biomolecular interactions.
The global market for analytical instruments used in reaction kinetics studies, including FTIR systems, continues to expand as industries increasingly prioritize process optimization and quality control. This growth is further driven by regulatory requirements demanding more thorough characterization of chemical processes and products, particularly in pharmaceutical and food industries where consumer safety is paramount.
Current Challenges in FTIR Kinetic Measurements
Despite significant advancements in FTIR technology for kinetic measurements, several substantial challenges persist that limit the full potential of this analytical technique. The temporal resolution of conventional FTIR spectrometers remains a significant constraint when studying rapid chemical reactions. Most commercial instruments operate at scan rates of 20-100 scans per second, which proves inadequate for capturing ultrafast reaction intermediates that form and disappear within milliseconds or microseconds.
Signal-to-noise ratio (SNR) presents another critical challenge, particularly when monitoring low concentration species or weak absorption bands. The inherent trade-off between temporal resolution and SNR forces researchers to compromise between faster acquisition rates and data quality. This limitation becomes particularly problematic when studying complex reaction mechanisms where minor intermediates play crucial roles.
Sample handling and environmental control during kinetic measurements introduce additional complications. Maintaining precise temperature, pressure, and mixing conditions while simultaneously collecting spectral data requires sophisticated experimental setups. Any fluctuations in these parameters can significantly affect reaction rates and introduce artifacts in the kinetic data, compromising the reliability of the results.
Data processing and interpretation challenges are equally significant. The multivariate nature of FTIR spectra, with overlapping bands and baseline shifts, makes it difficult to extract accurate kinetic information. Current chemometric methods often struggle with complex reaction systems involving multiple parallel or consecutive reactions, leading to ambiguities in mechanistic interpretations.
Calibration issues further complicate quantitative kinetic analysis. The Beer-Lambert relationship between absorbance and concentration can deviate under certain conditions, particularly at high concentrations or when molecular interactions affect absorption coefficients. This non-linearity introduces systematic errors in rate constant determinations.
Integration with other analytical techniques remains challenging but necessary for comprehensive reaction monitoring. While FTIR provides valuable molecular structure information, complementary techniques are often required for complete mechanistic understanding. However, synchronizing data acquisition and correlating results across different analytical platforms presents significant technical hurdles.
Miniaturization and in-situ applications represent emerging challenges as researchers increasingly seek to monitor reactions in real-world environments rather than laboratory settings. Developing robust, portable FTIR systems that maintain high performance under field conditions requires overcoming substantial engineering obstacles related to optical alignment stability, vibration resistance, and environmental interference.
Signal-to-noise ratio (SNR) presents another critical challenge, particularly when monitoring low concentration species or weak absorption bands. The inherent trade-off between temporal resolution and SNR forces researchers to compromise between faster acquisition rates and data quality. This limitation becomes particularly problematic when studying complex reaction mechanisms where minor intermediates play crucial roles.
Sample handling and environmental control during kinetic measurements introduce additional complications. Maintaining precise temperature, pressure, and mixing conditions while simultaneously collecting spectral data requires sophisticated experimental setups. Any fluctuations in these parameters can significantly affect reaction rates and introduce artifacts in the kinetic data, compromising the reliability of the results.
Data processing and interpretation challenges are equally significant. The multivariate nature of FTIR spectra, with overlapping bands and baseline shifts, makes it difficult to extract accurate kinetic information. Current chemometric methods often struggle with complex reaction systems involving multiple parallel or consecutive reactions, leading to ambiguities in mechanistic interpretations.
Calibration issues further complicate quantitative kinetic analysis. The Beer-Lambert relationship between absorbance and concentration can deviate under certain conditions, particularly at high concentrations or when molecular interactions affect absorption coefficients. This non-linearity introduces systematic errors in rate constant determinations.
Integration with other analytical techniques remains challenging but necessary for comprehensive reaction monitoring. While FTIR provides valuable molecular structure information, complementary techniques are often required for complete mechanistic understanding. However, synchronizing data acquisition and correlating results across different analytical platforms presents significant technical hurdles.
Miniaturization and in-situ applications represent emerging challenges as researchers increasingly seek to monitor reactions in real-world environments rather than laboratory settings. Developing robust, portable FTIR systems that maintain high performance under field conditions requires overcoming substantial engineering obstacles related to optical alignment stability, vibration resistance, and environmental interference.
Established Methodologies for FTIR Kinetic Analysis
01 Real-time monitoring of reaction kinetics using FTIR
FTIR spectroscopy can be used for real-time monitoring of chemical reactions, allowing researchers to track changes in molecular structures as reactions progress. This technique provides valuable insights into reaction mechanisms, rates, and intermediates by measuring the absorption of infrared radiation by molecules at different stages of the reaction. Real-time monitoring enables the determination of reaction kinetics parameters such as rate constants and activation energies, which are essential for understanding and optimizing chemical processes.- Real-time monitoring of reaction kinetics using FTIR: FTIR spectroscopy can be used for real-time monitoring of chemical reactions to study reaction kinetics. This approach allows researchers to track changes in functional groups during reactions, providing insights into reaction rates, mechanisms, and intermediates. The technique enables continuous data collection without interrupting the reaction process, making it valuable for understanding reaction progress and optimizing reaction conditions.
- FTIR instrumentation for kinetic studies: Specialized FTIR instrumentation has been developed specifically for reaction kinetics studies. These systems often include temperature-controlled reaction cells, flow systems, and rapid-scanning capabilities to capture fast reactions. Advanced equipment may incorporate automated sampling, multiple detection channels, and integration with other analytical techniques to provide comprehensive data on reaction kinetics under various conditions.
- Quantitative analysis methods for reaction kinetics using FTIR: Mathematical and computational methods have been developed to extract quantitative kinetic parameters from FTIR spectral data. These include multivariate analysis, chemometric techniques, and specialized algorithms for processing time-resolved spectra. By analyzing peak intensities, areas, and shifts over time, researchers can determine reaction orders, rate constants, activation energies, and other kinetic parameters essential for understanding reaction mechanisms.
- In-situ FTIR for heterogeneous and catalytic reaction kinetics: In-situ FTIR techniques have been developed to study reaction kinetics at interfaces and in heterogeneous systems, including catalytic reactions. These methods allow for monitoring reactions directly on catalyst surfaces, in multiphase systems, or under extreme conditions. Special sampling accessories and methodologies enable researchers to observe surface-bound intermediates and elucidate catalytic mechanisms that would be difficult to study using conventional techniques.
- Integration of FTIR with other techniques for comprehensive kinetic analysis: FTIR spectroscopy can be integrated with other analytical techniques to provide more comprehensive information about reaction kinetics. These hybrid approaches combine FTIR with techniques such as mass spectrometry, chromatography, or thermal analysis to simultaneously monitor multiple aspects of reactions. Such integrated systems offer complementary data that enhances understanding of complex reaction mechanisms and kinetics across different time scales and reaction conditions.
02 FTIR coupled with microfluidic systems for reaction kinetics
The integration of FTIR spectroscopy with microfluidic systems creates powerful platforms for studying reaction kinetics at microscale. These systems allow for precise control of reaction conditions, reduced reagent consumption, and enhanced mixing efficiency. Microfluidic FTIR setups enable continuous flow analysis of reactions, providing high temporal resolution data on reaction progress. This approach is particularly valuable for fast reactions or those requiring controlled environments, offering advantages in terms of reproducibility and throughput compared to conventional batch methods.Expand Specific Solutions03 Temperature-controlled FTIR for kinetic studies
Temperature-controlled FTIR systems are essential tools for investigating the temperature dependence of reaction kinetics. These setups incorporate heating or cooling elements that maintain precise temperature conditions during spectroscopic measurements. By conducting FTIR analyses at different temperatures, researchers can determine thermodynamic parameters such as activation energy and entropy changes. This approach is particularly valuable for studying reaction mechanisms and for optimizing process conditions in industrial applications where temperature effects are significant.Expand Specific Solutions04 Chemometric analysis of FTIR data for reaction kinetics
Advanced chemometric methods are applied to FTIR spectral data to extract detailed kinetic information from complex reaction systems. These mathematical and statistical techniques, including principal component analysis, partial least squares regression, and multivariate curve resolution, help separate overlapping spectral features and quantify multiple reaction components simultaneously. Chemometric approaches enable the determination of reaction orders, rate constants, and mechanistic pathways even in systems with multiple parallel or consecutive reactions. This methodology enhances the information content obtained from FTIR measurements and improves the accuracy of kinetic models.Expand Specific Solutions05 In-situ FTIR for heterogeneous catalysis kinetics
In-situ FTIR spectroscopy is a powerful technique for studying reaction kinetics in heterogeneous catalytic systems. This approach allows for the direct observation of surface species and intermediates on catalyst surfaces under reaction conditions. By monitoring the formation and consumption of surface-bound species in real-time, researchers can elucidate catalytic mechanisms and identify rate-determining steps. In-situ FTIR provides insights into catalyst activation, deactivation, and regeneration processes, which are crucial for developing more efficient and selective catalytic materials for industrial applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The FTIR-based chemical reaction kinetics analysis market is currently in a growth phase, with increasing adoption across pharmaceutical, petrochemical, and materials science sectors. The market size is estimated to be expanding at 5-8% annually, driven by demand for real-time reaction monitoring capabilities. Technologically, the field shows varying maturity levels, with established players like ExxonMobil Technology & Engineering and Saudi Arabian Oil Co. leading industrial applications, while research institutions such as Heriot-Watt University and Tianjin University advance fundamental methodologies. Companies including Schlumberger Technologies and Battelle Memorial Institute are developing specialized applications for energy sectors, while QuantaRed Technologies and Spectra Analysis Instruments focus on innovative instrumentation solutions. The integration of AI and automation with FTIR kinetics analysis represents the emerging competitive frontier, with collaborative ecosystems forming between academic and industrial players.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced FTIR spectroscopy systems for real-time monitoring of chemical reaction kinetics in petroleum processing. Their technology utilizes attenuated total reflection (ATR) FTIR coupled with multivariate analysis to track reaction progression in complex hydrocarbon mixtures. The company has implemented in-situ FTIR monitoring systems that operate under high temperature and pressure conditions typical in industrial catalytic processes. ExxonMobil's approach incorporates chemometric modeling to deconvolute overlapping spectral features, allowing for quantitative determination of multiple reaction components simultaneously. Their systems include specialized flow cells designed to withstand harsh chemical environments while maintaining optical integrity for accurate spectroscopic measurements. The technology enables millisecond time resolution for capturing fast reaction intermediates, providing crucial insights into reaction mechanisms and pathways.
Strengths: Superior capability for analyzing complex hydrocarbon mixtures under industrial conditions; robust systems designed for harsh environments; excellent integration with existing refinery processes. Weaknesses: Proprietary systems with limited compatibility with third-party equipment; high implementation costs; requires specialized expertise for data interpretation.
Battelle Memorial Institute
Technical Solution: Battelle has pioneered innovative FTIR methodologies for chemical kinetics analysis, particularly in environmental and defense applications. Their approach combines time-resolved FTIR spectroscopy with automated sampling systems to monitor reaction progression with minimal human intervention. Battelle's technology incorporates specialized algorithms for baseline correction and spectral deconvolution that enhance sensitivity for detecting trace reaction intermediates. Their systems feature temperature-controlled reaction chambers with multiple sampling ports allowing for simultaneous monitoring at different reaction stages. Battelle has developed portable FTIR systems for field deployment that maintain laboratory-grade precision while operating in non-ideal conditions. The institute has also created custom software packages that integrate kinetic modeling with spectral analysis, enabling real-time reaction parameter extraction and predictive capabilities for reaction outcomes based on initial conditions and environmental factors.
Strengths: Exceptional versatility across diverse application domains; strong integration of hardware and software solutions; excellent portability for field applications. Weaknesses: Systems sometimes prioritize versatility over sensitivity for specific applications; higher maintenance requirements compared to dedicated single-purpose systems.
Key Innovations in Time-Resolved FTIR Techniques
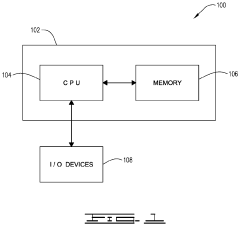
Apparatus and method for dark voltage removal for enhancing dynamic range, bandwidth, and quality of step-scan Fourier transform infrared (FTIR) spectroscopy data
PatentInactiveUS11047794B2
Innovation
- A signal conditioning module that removes dark current interferogram offset voltages during nanosecond step-scan measurements, enhancing the detection of small-scale signals and providing low noise, high gain, and improved resolution, allowing for continuous data collection and access to previously unobtainable IR spectral information.
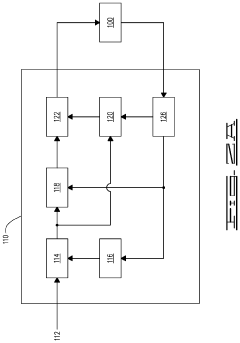
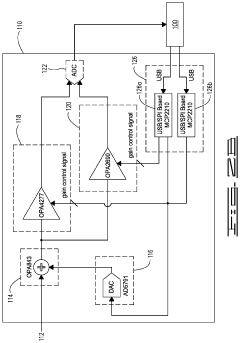
Fourier transform infrared spectroscopy instrument
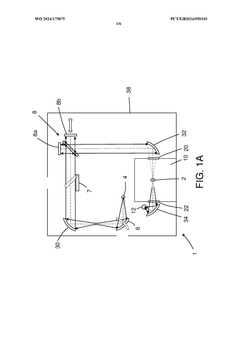
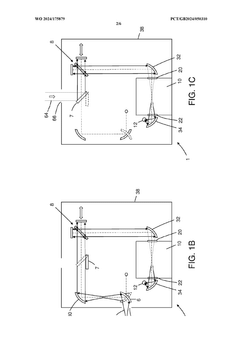
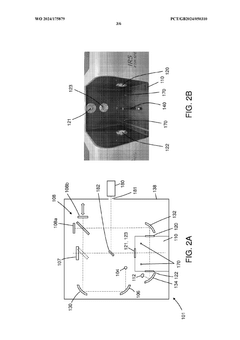
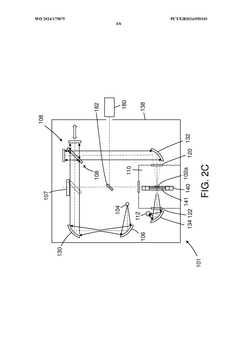
PatentWO2024175879A1
Innovation
- A reconfigurable FTIR spectroscopy instrument with a sample chamber that can accommodate different sample holder accessories for conventional and luminescence spectroscopy configurations, utilizing a rotatable mirror as an optical switch to seamlessly switch between configurations, allowing for easy transition between transmission, reflection, ATR, PL, and Raman spectroscopy measurements.
Data Processing and Computational Methods
The processing of FTIR spectral data for kinetic analysis requires sophisticated computational approaches to extract meaningful reaction parameters. Raw FTIR data typically contains noise, baseline drift, and overlapping spectral features that must be addressed through preprocessing techniques. Common preprocessing methods include baseline correction algorithms such as asymmetric least squares, Savitzky-Golay derivatives, and wavelet transforms that effectively separate signal from background interference. Spectral resolution enhancement techniques like deconvolution and second derivative analysis are particularly valuable for resolving overlapping bands that represent different chemical species involved in reaction mechanisms.
Multivariate statistical methods have revolutionized FTIR kinetic data analysis. Principal Component Analysis (PCA) reduces data dimensionality while preserving essential spectral variations, enabling the identification of reaction intermediates that might otherwise remain undetected. Partial Least Squares (PLS) regression establishes quantitative relationships between spectral changes and concentration profiles, facilitating the determination of reaction orders and rate constants.
Chemometric approaches such as Multivariate Curve Resolution (MCR) and Evolving Factor Analysis (EFA) have proven particularly powerful for resolving complex reaction systems. These methods can extract pure component spectra and concentration profiles from mixed spectral data without prior knowledge of the chemical system, offering insights into reaction pathways that traditional univariate methods might miss.
Kinetic modeling software packages specifically designed for spectroscopic data have emerged as essential tools. These platforms implement various kinetic models, from simple zero, first, and second-order reactions to complex consecutive and parallel reaction schemes. Advanced packages incorporate global analysis capabilities that simultaneously fit multiple datasets across different experimental conditions, significantly improving the reliability of extracted kinetic parameters.
Machine learning algorithms represent the cutting edge in FTIR kinetic analysis. Neural networks and support vector machines can identify subtle spectral patterns associated with reaction progress, while genetic algorithms optimize kinetic model parameters by mimicking evolutionary processes. These approaches are particularly valuable for systems with complex reaction mechanisms that defy traditional kinetic analysis.
Real-time data processing algorithms enable in-situ monitoring of reactions, providing immediate feedback for process control applications. These methods typically employ dimensionality reduction and feature extraction techniques optimized for computational efficiency, allowing for rapid decision-making in industrial settings.
Multivariate statistical methods have revolutionized FTIR kinetic data analysis. Principal Component Analysis (PCA) reduces data dimensionality while preserving essential spectral variations, enabling the identification of reaction intermediates that might otherwise remain undetected. Partial Least Squares (PLS) regression establishes quantitative relationships between spectral changes and concentration profiles, facilitating the determination of reaction orders and rate constants.
Chemometric approaches such as Multivariate Curve Resolution (MCR) and Evolving Factor Analysis (EFA) have proven particularly powerful for resolving complex reaction systems. These methods can extract pure component spectra and concentration profiles from mixed spectral data without prior knowledge of the chemical system, offering insights into reaction pathways that traditional univariate methods might miss.
Kinetic modeling software packages specifically designed for spectroscopic data have emerged as essential tools. These platforms implement various kinetic models, from simple zero, first, and second-order reactions to complex consecutive and parallel reaction schemes. Advanced packages incorporate global analysis capabilities that simultaneously fit multiple datasets across different experimental conditions, significantly improving the reliability of extracted kinetic parameters.
Machine learning algorithms represent the cutting edge in FTIR kinetic analysis. Neural networks and support vector machines can identify subtle spectral patterns associated with reaction progress, while genetic algorithms optimize kinetic model parameters by mimicking evolutionary processes. These approaches are particularly valuable for systems with complex reaction mechanisms that defy traditional kinetic analysis.
Real-time data processing algorithms enable in-situ monitoring of reactions, providing immediate feedback for process control applications. These methods typically employ dimensionality reduction and feature extraction techniques optimized for computational efficiency, allowing for rapid decision-making in industrial settings.
Instrument Calibration and Validation Protocols
Instrument calibration and validation protocols are critical components when using FTIR spectroscopy for chemical reaction kinetics studies. Proper calibration ensures measurement accuracy, while validation confirms the reliability of analytical methods. For kinetics applications, where precise temporal changes in chemical composition are monitored, these protocols become even more essential.
The calibration of FTIR instruments for kinetic studies should follow a systematic approach beginning with wavelength calibration using certified reference materials such as polystyrene films or NIST traceable standards. This establishes the accuracy of the x-axis (wavenumber) measurements. Intensity calibration follows, utilizing reference compounds with known absorption coefficients to ensure quantitative measurements are accurate across the spectral range of interest.
Temperature calibration deserves special attention in kinetics studies, as reaction rates are highly temperature-dependent. Calibration cells with precise temperature control and monitoring capabilities should be employed, with verification using materials that exhibit well-characterized phase transitions or spectral changes at specific temperatures.
For time-resolved measurements, temporal calibration is essential. This involves verifying the instrument's timing mechanisms using standard reactions with well-established kinetics or electronic timing references. The temporal resolution must be appropriate for the reaction timescale being studied, ranging from milliseconds to hours depending on the application.
Validation protocols should include assessments of linearity, precision, accuracy, specificity, and robustness. Linearity testing confirms that absorbance measurements follow Beer's Law across the concentration ranges relevant to the kinetic study. Precision testing evaluates both intra-day and inter-day variability through repeated measurements of standard samples.
Method specificity validation ensures that spectral features used for kinetic analysis are uniquely attributable to the chemical species of interest, with minimal interference from other reaction components. This often involves deconvolution techniques and multivariate analysis to isolate specific spectral contributions.
Robustness testing examines the method's stability under varying experimental conditions, such as slight changes in temperature, sample preparation, or instrument parameters. This is particularly important for kinetics studies that may run for extended periods.
A comprehensive validation protocol should also include system suitability tests performed before each kinetic experiment, verifying that the instrument is operating within specified parameters. These typically include signal-to-noise ratio checks, resolution tests, and baseline stability assessments.
Documentation of all calibration and validation procedures is essential, including certificates for reference materials, calibration curves, statistical analyses of validation data, and maintenance records. This documentation supports data integrity and facilitates regulatory compliance when applicable.
The calibration of FTIR instruments for kinetic studies should follow a systematic approach beginning with wavelength calibration using certified reference materials such as polystyrene films or NIST traceable standards. This establishes the accuracy of the x-axis (wavenumber) measurements. Intensity calibration follows, utilizing reference compounds with known absorption coefficients to ensure quantitative measurements are accurate across the spectral range of interest.
Temperature calibration deserves special attention in kinetics studies, as reaction rates are highly temperature-dependent. Calibration cells with precise temperature control and monitoring capabilities should be employed, with verification using materials that exhibit well-characterized phase transitions or spectral changes at specific temperatures.
For time-resolved measurements, temporal calibration is essential. This involves verifying the instrument's timing mechanisms using standard reactions with well-established kinetics or electronic timing references. The temporal resolution must be appropriate for the reaction timescale being studied, ranging from milliseconds to hours depending on the application.
Validation protocols should include assessments of linearity, precision, accuracy, specificity, and robustness. Linearity testing confirms that absorbance measurements follow Beer's Law across the concentration ranges relevant to the kinetic study. Precision testing evaluates both intra-day and inter-day variability through repeated measurements of standard samples.
Method specificity validation ensures that spectral features used for kinetic analysis are uniquely attributable to the chemical species of interest, with minimal interference from other reaction components. This often involves deconvolution techniques and multivariate analysis to isolate specific spectral contributions.
Robustness testing examines the method's stability under varying experimental conditions, such as slight changes in temperature, sample preparation, or instrument parameters. This is particularly important for kinetics studies that may run for extended periods.
A comprehensive validation protocol should also include system suitability tests performed before each kinetic experiment, verifying that the instrument is operating within specified parameters. These typically include signal-to-noise ratio checks, resolution tests, and baseline stability assessments.
Documentation of all calibration and validation procedures is essential, including certificates for reference materials, calibration curves, statistical analyses of validation data, and maintenance records. This documentation supports data integrity and facilitates regulatory compliance when applicable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!