Validate Thermopile Accuracy for Medical Thermography
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermopile Technology Background and Objectives
Thermopile sensors have evolved significantly since their inception in the early 20th century, transitioning from basic temperature measurement tools to sophisticated components in modern medical thermography systems. The fundamental operating principle of thermopiles—converting thermal energy into electrical signals through the Seebeck effect—has remained consistent, while the implementation technology has advanced dramatically in precision, miniaturization, and integration capabilities.
The medical thermography field has witnessed accelerated development over the past decade, with thermopile sensors becoming increasingly critical for non-contact temperature measurement applications. This growth has been driven by the healthcare industry's demand for reliable, accurate, and non-invasive temperature monitoring solutions, particularly evident during global health crises such as the COVID-19 pandemic, which highlighted the necessity for rapid temperature screening technologies.
Current thermopile technology in medical applications operates within specific parameters, typically offering temperature measurement ranges from 20°C to 40°C with resolution capabilities approaching 0.1°C under optimal conditions. However, the validation of measurement accuracy remains a significant challenge, particularly when considering the variability of environmental conditions and the physiological diversity of patients.
The primary technical objective in thermopile validation for medical thermography centers on establishing robust methodologies to verify and ensure measurement accuracy across diverse clinical scenarios. This includes developing standardized calibration protocols that account for ambient temperature fluctuations, emissivity variations of different skin types, and the influence of distance and angle on measurement precision.
Secondary objectives include enhancing the signal-to-noise ratio of thermopile sensors to improve detection sensitivity, reducing power consumption for portable medical devices, and integrating advanced signal processing algorithms to compensate for environmental interferences. These improvements aim to elevate thermopile technology from screening tools to diagnostic-grade instruments capable of supporting clinical decision-making processes.
The long-term technological trajectory for thermopile sensors in medical applications points toward multi-spectral sensing capabilities, allowing for simultaneous measurement of temperature and other physiological parameters. This evolution aligns with the broader trend of sensor fusion in healthcare monitoring, where multiple data streams combine to provide comprehensive patient assessment.
Understanding the current limitations and future potential of thermopile technology is essential for establishing realistic validation frameworks that can evolve alongside technological advancements. The ultimate goal remains consistent: to develop thermopile-based medical thermography systems that deliver laboratory-grade accuracy in real-world clinical environments, thereby expanding their utility beyond basic screening to more sophisticated diagnostic applications.
The medical thermography field has witnessed accelerated development over the past decade, with thermopile sensors becoming increasingly critical for non-contact temperature measurement applications. This growth has been driven by the healthcare industry's demand for reliable, accurate, and non-invasive temperature monitoring solutions, particularly evident during global health crises such as the COVID-19 pandemic, which highlighted the necessity for rapid temperature screening technologies.
Current thermopile technology in medical applications operates within specific parameters, typically offering temperature measurement ranges from 20°C to 40°C with resolution capabilities approaching 0.1°C under optimal conditions. However, the validation of measurement accuracy remains a significant challenge, particularly when considering the variability of environmental conditions and the physiological diversity of patients.
The primary technical objective in thermopile validation for medical thermography centers on establishing robust methodologies to verify and ensure measurement accuracy across diverse clinical scenarios. This includes developing standardized calibration protocols that account for ambient temperature fluctuations, emissivity variations of different skin types, and the influence of distance and angle on measurement precision.
Secondary objectives include enhancing the signal-to-noise ratio of thermopile sensors to improve detection sensitivity, reducing power consumption for portable medical devices, and integrating advanced signal processing algorithms to compensate for environmental interferences. These improvements aim to elevate thermopile technology from screening tools to diagnostic-grade instruments capable of supporting clinical decision-making processes.
The long-term technological trajectory for thermopile sensors in medical applications points toward multi-spectral sensing capabilities, allowing for simultaneous measurement of temperature and other physiological parameters. This evolution aligns with the broader trend of sensor fusion in healthcare monitoring, where multiple data streams combine to provide comprehensive patient assessment.
Understanding the current limitations and future potential of thermopile technology is essential for establishing realistic validation frameworks that can evolve alongside technological advancements. The ultimate goal remains consistent: to develop thermopile-based medical thermography systems that deliver laboratory-grade accuracy in real-world clinical environments, thereby expanding their utility beyond basic screening to more sophisticated diagnostic applications.
Market Analysis for Medical Thermography Applications
The medical thermography market is experiencing significant growth, driven by increasing demand for non-invasive diagnostic tools and the rising prevalence of chronic diseases. The global medical thermography market was valued at approximately $2.1 billion in 2022 and is projected to reach $3.7 billion by 2028, growing at a CAGR of 9.8% during the forecast period. This growth trajectory underscores the expanding clinical acceptance and integration of thermographic technologies in healthcare settings.
North America currently dominates the market, accounting for nearly 40% of global revenue, followed by Europe and Asia-Pacific. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to improving healthcare infrastructure and increasing healthcare expenditure. The market penetration in developing economies remains relatively low, presenting substantial growth opportunities for manufacturers and service providers.
The medical thermography market can be segmented by application into oncology, neurology, cardiovascular diseases, inflammatory diseases, and others. Oncology represents the largest application segment, particularly for breast cancer screening, where thermography serves as a complementary diagnostic tool. The cardiovascular segment is projected to grow at the highest rate due to the increasing prevalence of cardiovascular diseases globally.
By end-user, hospitals and diagnostic centers constitute the largest market segment, followed by research laboratories and homecare settings. The integration of thermography in telemedicine platforms is creating new market opportunities, especially in remote patient monitoring applications. This trend has been accelerated by the COVID-19 pandemic, which highlighted the importance of contactless diagnostic tools.
Key market drivers include technological advancements in sensor technology, increasing awareness about early disease detection, and growing preference for non-invasive diagnostic procedures. The development of portable and smartphone-compatible thermographic devices is expanding the market reach to point-of-care settings and home healthcare applications.
Challenges facing market growth include limited reimbursement policies, lack of standardized protocols for thermographic imaging interpretation, and competition from established diagnostic modalities. Additionally, concerns regarding the accuracy and reliability of thermopile sensors in varying environmental conditions present technical barriers that manufacturers must address to enhance market acceptance.
The competitive landscape is characterized by a mix of established medical device manufacturers and emerging technology companies. Strategic collaborations between technology providers and healthcare institutions are becoming increasingly common, aimed at validating thermographic applications in clinical settings and developing specialized solutions for specific medical conditions.
North America currently dominates the market, accounting for nearly 40% of global revenue, followed by Europe and Asia-Pacific. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to improving healthcare infrastructure and increasing healthcare expenditure. The market penetration in developing economies remains relatively low, presenting substantial growth opportunities for manufacturers and service providers.
The medical thermography market can be segmented by application into oncology, neurology, cardiovascular diseases, inflammatory diseases, and others. Oncology represents the largest application segment, particularly for breast cancer screening, where thermography serves as a complementary diagnostic tool. The cardiovascular segment is projected to grow at the highest rate due to the increasing prevalence of cardiovascular diseases globally.
By end-user, hospitals and diagnostic centers constitute the largest market segment, followed by research laboratories and homecare settings. The integration of thermography in telemedicine platforms is creating new market opportunities, especially in remote patient monitoring applications. This trend has been accelerated by the COVID-19 pandemic, which highlighted the importance of contactless diagnostic tools.
Key market drivers include technological advancements in sensor technology, increasing awareness about early disease detection, and growing preference for non-invasive diagnostic procedures. The development of portable and smartphone-compatible thermographic devices is expanding the market reach to point-of-care settings and home healthcare applications.
Challenges facing market growth include limited reimbursement policies, lack of standardized protocols for thermographic imaging interpretation, and competition from established diagnostic modalities. Additionally, concerns regarding the accuracy and reliability of thermopile sensors in varying environmental conditions present technical barriers that manufacturers must address to enhance market acceptance.
The competitive landscape is characterized by a mix of established medical device manufacturers and emerging technology companies. Strategic collaborations between technology providers and healthcare institutions are becoming increasingly common, aimed at validating thermographic applications in clinical settings and developing specialized solutions for specific medical conditions.
Current Challenges in Thermopile Accuracy Validation
Despite significant advancements in thermopile sensor technology for medical thermography applications, several critical challenges persist in validating their accuracy for clinical use. The primary obstacle remains the inherent thermal drift characteristics of thermopile sensors, which can introduce measurement errors of up to ±0.3°C under varying ambient conditions. This drift significantly impacts reliability in medical settings where precision within ±0.1°C is often required for diagnostic confidence.
Calibration complexity presents another substantial challenge. Current calibration protocols typically employ blackbody reference sources in controlled laboratory environments, which poorly represent the dynamic conditions encountered in clinical settings. The translation of laboratory calibration to real-world medical environments introduces validation gaps that compromise measurement consistency across different operational scenarios.
Environmental interference factors substantially complicate accuracy validation efforts. Ambient temperature fluctuations, air currents, humidity variations, and electromagnetic interference from nearby medical equipment all contribute to measurement uncertainties that are difficult to quantify and compensate for systematically. These variables create a complex validation matrix that current testing methodologies struggle to address comprehensively.
The biological variability of human subjects introduces additional validation complexities. Skin emissivity variations across different demographic groups (estimated between 0.94-0.99), perspiration levels, and subcutaneous blood flow patterns create reference point inconsistencies that challenge the establishment of universal accuracy standards for thermopile-based medical devices.
Regulatory frameworks present procedural challenges in validation methodology. Different regions maintain varying standards for thermographic device accuracy, with the FDA requiring validation against traceable temperature standards with uncertainties below ±0.1°C, while other jurisdictions may accept ±0.2°C tolerances. This regulatory fragmentation complicates the development of globally accepted validation protocols.
Technical limitations in reference instrumentation also impede validation progress. Current gold-standard contact thermometry methods used for comparison studies introduce their own measurement uncertainties and potential for measurement artifacts through contact-induced local temperature changes. Meanwhile, high-precision non-contact reference systems remain prohibitively expensive for widespread validation implementation.
Data processing algorithms represent a final significant challenge area. The signal processing techniques employed to extract temperature readings from raw thermopile outputs vary considerably between manufacturers, creating a "black box" effect that complicates independent validation efforts. The proprietary nature of these algorithms limits transparency in understanding how measurement uncertainties propagate through the system.
Calibration complexity presents another substantial challenge. Current calibration protocols typically employ blackbody reference sources in controlled laboratory environments, which poorly represent the dynamic conditions encountered in clinical settings. The translation of laboratory calibration to real-world medical environments introduces validation gaps that compromise measurement consistency across different operational scenarios.
Environmental interference factors substantially complicate accuracy validation efforts. Ambient temperature fluctuations, air currents, humidity variations, and electromagnetic interference from nearby medical equipment all contribute to measurement uncertainties that are difficult to quantify and compensate for systematically. These variables create a complex validation matrix that current testing methodologies struggle to address comprehensively.
The biological variability of human subjects introduces additional validation complexities. Skin emissivity variations across different demographic groups (estimated between 0.94-0.99), perspiration levels, and subcutaneous blood flow patterns create reference point inconsistencies that challenge the establishment of universal accuracy standards for thermopile-based medical devices.
Regulatory frameworks present procedural challenges in validation methodology. Different regions maintain varying standards for thermographic device accuracy, with the FDA requiring validation against traceable temperature standards with uncertainties below ±0.1°C, while other jurisdictions may accept ±0.2°C tolerances. This regulatory fragmentation complicates the development of globally accepted validation protocols.
Technical limitations in reference instrumentation also impede validation progress. Current gold-standard contact thermometry methods used for comparison studies introduce their own measurement uncertainties and potential for measurement artifacts through contact-induced local temperature changes. Meanwhile, high-precision non-contact reference systems remain prohibitively expensive for widespread validation implementation.
Data processing algorithms represent a final significant challenge area. The signal processing techniques employed to extract temperature readings from raw thermopile outputs vary considerably between manufacturers, creating a "black box" effect that complicates independent validation efforts. The proprietary nature of these algorithms limits transparency in understanding how measurement uncertainties propagate through the system.
Current Validation Methodologies for Thermopile Sensors
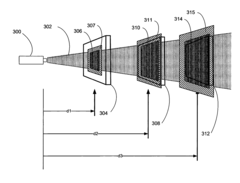
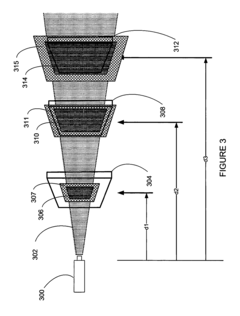
01 Thermopile design improvements for accuracy enhancement
Various design improvements can enhance thermopile accuracy, including optimized thermocouple junction arrangements, improved thermal isolation techniques, and advanced semiconductor materials. These design elements minimize thermal noise and drift while maximizing temperature sensitivity. Specific improvements include specialized junction geometries, thermal barrier structures, and precision manufacturing techniques that reduce measurement errors and increase signal-to-noise ratio.- Calibration techniques for thermopile accuracy: Various calibration techniques can be employed to enhance thermopile accuracy. These include temperature compensation methods, reference temperature measurements, and algorithmic corrections that account for environmental variations. Calibration processes may involve comparing thermopile outputs against known temperature standards or using multiple measurement points to create correction curves. These techniques help minimize systematic errors and improve the overall accuracy of thermopile-based temperature measurements.
- Structural design improvements for thermopiles: The physical design and construction of thermopiles significantly impact their measurement accuracy. Innovations include optimized thermocouple junction arrangements, improved thermal isolation structures, and specialized substrate materials that reduce thermal noise. Advanced manufacturing techniques allow for more precise placement of thermocouples and better control of thermal gradients across the device. These structural improvements help to enhance sensitivity while reducing measurement errors in thermopile sensors.
- Signal processing methods for enhanced accuracy: Advanced signal processing techniques can significantly improve thermopile accuracy. These include digital filtering algorithms, noise reduction methods, and signal amplification with error correction. Some approaches involve real-time data processing to compensate for drift and environmental factors. Machine learning algorithms may also be employed to recognize and correct systematic measurement errors, resulting in more accurate temperature readings from thermopile sensors.
- Thermal isolation and environmental compensation: Maintaining accurate thermopile measurements requires effective thermal isolation and environmental compensation. Techniques include specialized housing designs that shield the sensor from external thermal influences, active temperature control systems, and materials with specific thermal properties. Some designs incorporate reference sensors to detect and compensate for ambient temperature changes. These approaches help minimize the impact of environmental factors on measurement accuracy.
- Multi-junction and array configurations: Using multiple thermocouples in specific arrangements can improve measurement accuracy. These configurations include series-connected thermopiles for increased sensitivity, differential measurement setups, and two-dimensional arrays that provide spatial temperature distribution data. Some designs incorporate redundant sensing elements for error detection and correction. By combining multiple measurement points, these approaches can reduce random errors and improve the overall accuracy of temperature measurements.
02 Calibration methods for thermopile accuracy
Calibration techniques significantly improve thermopile accuracy by compensating for inherent device variations and environmental factors. These methods include multi-point temperature calibration, reference temperature comparison, and algorithmic correction of measurement errors. Advanced calibration approaches utilize machine learning and statistical models to account for non-linear responses across different operating conditions, substantially reducing measurement uncertainty and improving long-term stability.Expand Specific Solutions03 Signal processing techniques for improved thermopile readings
Advanced signal processing techniques enhance thermopile accuracy by filtering noise, amplifying weak signals, and compensating for environmental variations. These include digital filtering algorithms, differential amplification circuits, and temperature compensation software. Implementation of specialized integrated circuits and microprocessors enables real-time signal correction, drift compensation, and adaptive filtering that significantly improves measurement precision under varying conditions.Expand Specific Solutions04 Environmental compensation methods for thermopiles
Environmental factors significantly impact thermopile accuracy, necessitating compensation methods for ambient temperature variations, humidity effects, and electromagnetic interference. These methods include thermal shielding designs, humidity-resistant coatings, and electromagnetic isolation techniques. Additional approaches involve ambient temperature monitoring with feedback correction algorithms and specialized housing designs that maintain stable operating conditions for the thermopile sensors.Expand Specific Solutions05 Material innovations for high-accuracy thermopiles
Advanced materials significantly improve thermopile accuracy through enhanced thermoelectric properties and thermal stability. Novel semiconductor compounds, nanomaterials, and specialized metal alloys increase the Seebeck coefficient while reducing thermal conductivity. These materials enable higher sensitivity, better temperature linearity, and reduced aging effects. Specialized coating materials and junction treatments further improve long-term stability and reduce degradation from environmental exposure.Expand Specific Solutions
Key Industry Players in Medical Thermography
Medical thermography using thermopile technology is currently in a growth phase, with increasing market adoption driven by the demand for non-contact temperature measurement solutions. The global market for medical thermography is expanding at a CAGR of approximately 6-8%, fueled by applications in fever screening, inflammation detection, and chronic condition monitoring. Leading players like Philips, OMRON, and Maxim Integrated are advancing thermopile sensor accuracy through proprietary calibration algorithms and reference designs, while companies such as Konica Minolta and BOE Technology are integrating these sensors into comprehensive medical imaging systems. The technology has reached moderate maturity with ±0.1-0.3°C accuracy levels, though challenges remain in ambient temperature compensation and clinical validation across diverse patient populations.
Koninklijke Philips NV
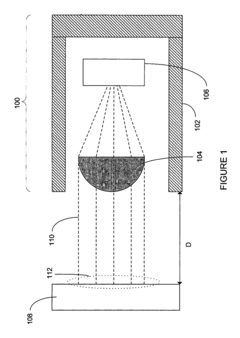
Technical Solution: Philips has developed advanced thermopile-based medical thermography systems that utilize multi-point sensing arrays with proprietary calibration algorithms. Their technology incorporates self-referencing temperature compensation mechanisms that continuously adjust for ambient temperature variations, significantly improving measurement stability. The system employs a dual-sensor approach where one thermopile measures the target while another monitors the ambient reference, allowing for dynamic differential analysis. Philips' solution includes specialized optical filters that isolate the 8-14μm wavelength band most relevant for medical diagnostics, enhancing signal-to-noise ratio. Their validation protocol involves comparison against blackbody reference sources across multiple temperature points (32-42°C) with statistical analysis of measurement variance to ensure clinical-grade accuracy of ±0.1°C for core body temperature estimation.
Strengths: Superior accuracy in varying ambient conditions due to advanced compensation algorithms; extensive clinical validation data supporting medical-grade measurements. Weaknesses: Higher cost compared to simpler thermopile implementations; requires more complex calibration procedures during manufacturing.
Maxim Integrated Products LLC
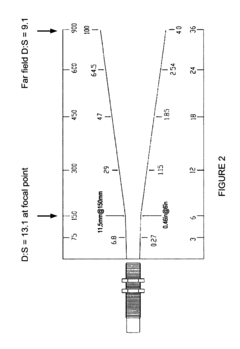
Technical Solution: Maxim Integrated has pioneered high-precision thermopile solutions specifically designed for medical thermography applications. Their MAX30205 family integrates thermopile sensors with high-resolution ADCs (up to 16-bit) and specialized signal processing circuits on a single chip. The technology employs factory calibration against medical-grade reference standards across the critical 35-42°C range with accuracy validation to within ±0.1°C. Maxim's approach includes proprietary digital filtering algorithms that reduce measurement noise while maintaining fast response times (typically <1 second). Their validation methodology incorporates temperature-controlled chambers with precision reference thermometers traceable to national standards institutes. The integrated solution also features automatic emissivity compensation based on target surface characteristics, addressing a key challenge in non-contact temperature measurement for medical applications.
Strengths: Highly integrated single-chip solution reduces system complexity; excellent accuracy in the critical clinical temperature range; fast response time suitable for medical screening. Weaknesses: Limited field of view compared to imaging arrays; requires careful positioning relative to the measurement site.
Critical Patents and Research in Thermopile Accuracy
System and method for determining accuracy of an infrared thermometer measurement
PatentActiveUS20100292952A1
Innovation
- The use of a sensor array in an infrared thermometer, where each detector element provides independent temperature measurements, with a quality control mechanism to analyze output signals and notify the user if the object does not sufficiently fill the field of view, allowing for positioning corrections to ensure accurate readings.
Thermopile sensor and temperature measuring method by infrared rays
PatentWO2001050102A1
Innovation
- Incorporating a resistor with a self-regulating positive temperature coefficient characteristic in the thermopile sensor, which maintains the cold junction at a constant bias temperature, synchronizing the thermal response speed with the thermopile output, and using a semiconductor heating element and thermistor for precise temperature measurement.
Regulatory Standards for Medical Thermography Devices
Medical thermography devices are subject to stringent regulatory frameworks that ensure their safety, efficacy, and reliability in clinical settings. The International Electrotechnical Commission (IEC) has established IEC 80601-2-59 as the primary standard for medical electrical equipment used in thermography for human fever screening. This standard specifies particular requirements for basic safety and essential performance, including accuracy parameters for thermopile sensors.
The U.S. Food and Drug Administration (FDA) classifies medical thermography devices as Class II medical devices, requiring premarket notification (510(k)) before commercialization. For thermopile-based systems, the FDA mandates demonstration of measurement accuracy within ±0.3°C (±0.5°F) in the clinically relevant temperature range of 35-42°C. Additionally, manufacturers must validate their devices against traceable temperature reference standards and conduct clinical trials comparing performance against predicate devices.
In the European Union, medical thermography devices fall under the Medical Device Regulation (MDR 2017/745), which replaced the Medical Device Directive (MDD) with a transition period ending in May 2021. The MDR requires more rigorous clinical evidence and post-market surveillance for all medical devices, including thermographic systems. Specifically for thermopiles used in medical applications, conformity with EN ISO 80601-2-56 is mandatory, which addresses the particular requirements for clinical thermometers for body temperature measurement.
The International Organization for Standardization (ISO) provides additional guidance through ISO/TR 13154:2017, which outlines screening procedures using thermographs for human febrile temperature detection. This technical report includes recommendations for environmental conditions, subject preparation, and measurement protocols that directly impact thermopile accuracy validation.
China's National Medical Products Administration (NMPA) has implemented YY/T 1151-2009 standard specifically for infrared thermometers, including those utilizing thermopile technology. This standard requires accuracy verification through comparative testing against blackbody radiators at multiple temperature points across the clinical range.
Regulatory bodies also mandate regular calibration and verification procedures for medical thermography devices. ASTM E1965-98 provides standardized test methods for infrared thermometers for intermittent determination of patient temperature, including protocols for validating thermopile sensor accuracy against reference temperature sources.
For manufacturers seeking global market access, harmonization of these various standards becomes crucial. The International Medical Device Regulators Forum (IMDRF) works to align regulatory approaches across different jurisdictions, though significant regional variations in requirements for thermopile accuracy validation persist, necessitating tailored validation protocols for different markets.
The U.S. Food and Drug Administration (FDA) classifies medical thermography devices as Class II medical devices, requiring premarket notification (510(k)) before commercialization. For thermopile-based systems, the FDA mandates demonstration of measurement accuracy within ±0.3°C (±0.5°F) in the clinically relevant temperature range of 35-42°C. Additionally, manufacturers must validate their devices against traceable temperature reference standards and conduct clinical trials comparing performance against predicate devices.
In the European Union, medical thermography devices fall under the Medical Device Regulation (MDR 2017/745), which replaced the Medical Device Directive (MDD) with a transition period ending in May 2021. The MDR requires more rigorous clinical evidence and post-market surveillance for all medical devices, including thermographic systems. Specifically for thermopiles used in medical applications, conformity with EN ISO 80601-2-56 is mandatory, which addresses the particular requirements for clinical thermometers for body temperature measurement.
The International Organization for Standardization (ISO) provides additional guidance through ISO/TR 13154:2017, which outlines screening procedures using thermographs for human febrile temperature detection. This technical report includes recommendations for environmental conditions, subject preparation, and measurement protocols that directly impact thermopile accuracy validation.
China's National Medical Products Administration (NMPA) has implemented YY/T 1151-2009 standard specifically for infrared thermometers, including those utilizing thermopile technology. This standard requires accuracy verification through comparative testing against blackbody radiators at multiple temperature points across the clinical range.
Regulatory bodies also mandate regular calibration and verification procedures for medical thermography devices. ASTM E1965-98 provides standardized test methods for infrared thermometers for intermittent determination of patient temperature, including protocols for validating thermopile sensor accuracy against reference temperature sources.
For manufacturers seeking global market access, harmonization of these various standards becomes crucial. The International Medical Device Regulators Forum (IMDRF) works to align regulatory approaches across different jurisdictions, though significant regional variations in requirements for thermopile accuracy validation persist, necessitating tailored validation protocols for different markets.
Clinical Validation Protocols and Best Practices
Clinical validation of thermopile sensors for medical thermography applications requires rigorous protocols to ensure accuracy, reliability, and clinical relevance. These protocols must adhere to international standards such as ISO 80601-2-56 for clinical thermometers and ASTM E1965 for infrared thermometers, which establish minimum performance requirements for medical-grade thermal imaging devices.
The validation process typically begins with laboratory calibration against blackbody reference sources at multiple temperature points within the clinically relevant range (typically 30-42°C). This calibration should be performed under controlled environmental conditions with ambient temperature maintained at 23±2°C and relative humidity between 30-70%. Statistical analysis of measurement deviations should demonstrate accuracy within ±0.2°C for core temperature measurements and ±0.3°C for surface temperature applications.
Following laboratory validation, clinical trials must be conducted in phases. Phase I involves testing on healthy volunteers to establish baseline performance metrics and identify potential confounding factors. Phase II expands to include patients with various medical conditions to assess performance across different clinical scenarios. These trials should include diverse demographic groups to account for variations in skin properties that may affect thermal readings.
Cross-validation against gold standard measurement techniques is essential. For core temperature assessment, comparison with invasive methods such as pulmonary artery catheters provides definitive reference data. For surface temperature mapping, contact thermometry or high-resolution reference thermal cameras serve as benchmarks. Bland-Altman analysis should be employed to quantify agreement between measurement methods.
Environmental factors significantly impact thermopile performance in clinical settings. Validation protocols must include testing under various ambient conditions, including temperature extremes (15-35°C), varying humidity levels, and air flow conditions that simulate real-world clinical environments. Thermal drift compensation algorithms should be evaluated for their effectiveness in maintaining accuracy during environmental fluctuations.
Best practices for clinical validation include implementing blinded assessment methodologies where operators are unaware of reference temperature values. Repeatability testing should involve multiple operators taking measurements on the same subjects to quantify inter-operator variability. Long-term stability assessment is crucial, requiring periodic recalibration and validation over months to ensure sustained accuracy throughout the device's intended service life.
Documentation of validation results must be comprehensive, including detailed statistical analyses with confidence intervals, measurement uncertainty calculations, and clear reporting of all environmental conditions during testing. This documentation forms the foundation for regulatory submissions and clinical adoption of thermopile-based medical thermography systems.
The validation process typically begins with laboratory calibration against blackbody reference sources at multiple temperature points within the clinically relevant range (typically 30-42°C). This calibration should be performed under controlled environmental conditions with ambient temperature maintained at 23±2°C and relative humidity between 30-70%. Statistical analysis of measurement deviations should demonstrate accuracy within ±0.2°C for core temperature measurements and ±0.3°C for surface temperature applications.
Following laboratory validation, clinical trials must be conducted in phases. Phase I involves testing on healthy volunteers to establish baseline performance metrics and identify potential confounding factors. Phase II expands to include patients with various medical conditions to assess performance across different clinical scenarios. These trials should include diverse demographic groups to account for variations in skin properties that may affect thermal readings.
Cross-validation against gold standard measurement techniques is essential. For core temperature assessment, comparison with invasive methods such as pulmonary artery catheters provides definitive reference data. For surface temperature mapping, contact thermometry or high-resolution reference thermal cameras serve as benchmarks. Bland-Altman analysis should be employed to quantify agreement between measurement methods.
Environmental factors significantly impact thermopile performance in clinical settings. Validation protocols must include testing under various ambient conditions, including temperature extremes (15-35°C), varying humidity levels, and air flow conditions that simulate real-world clinical environments. Thermal drift compensation algorithms should be evaluated for their effectiveness in maintaining accuracy during environmental fluctuations.
Best practices for clinical validation include implementing blinded assessment methodologies where operators are unaware of reference temperature values. Repeatability testing should involve multiple operators taking measurements on the same subjects to quantify inter-operator variability. Long-term stability assessment is crucial, requiring periodic recalibration and validation over months to ensure sustained accuracy throughout the device's intended service life.
Documentation of validation results must be comprehensive, including detailed statistical analyses with confidence intervals, measurement uncertainty calculations, and clear reporting of all environmental conditions during testing. This documentation forms the foundation for regulatory submissions and clinical adoption of thermopile-based medical thermography systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!