Validate Ultrafiltration Processes Using Advanced Computational Modeling
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ultrafiltration Modeling Background and Objectives
Ultrafiltration (UF) technology has evolved significantly since its inception in the 1960s, transforming from simple laboratory applications to sophisticated industrial separation processes. This membrane-based separation technique has become increasingly critical in various industries including pharmaceuticals, food processing, water treatment, and biotechnology. The historical trajectory shows a consistent pattern of improvement in membrane materials, module designs, and process optimization techniques, leading to enhanced efficiency and broader application scope.
Recent technological advancements have shifted focus toward computational modeling approaches that can accurately predict and optimize ultrafiltration performance. This transition from empirical to theoretical understanding represents a paradigm shift in how UF processes are designed and validated. The integration of computational fluid dynamics (CFD), molecular dynamics simulations, and machine learning algorithms has opened new avenues for understanding complex membrane-solute interactions at multiple scales.
The global market for ultrafiltration technologies continues to expand, with projections indicating a compound annual growth rate of approximately 15% through 2028. This growth is primarily driven by increasing water scarcity concerns, stringent regulatory requirements for product purity, and the need for more energy-efficient separation processes across industries.
The primary objective of validating ultrafiltration processes using advanced computational modeling is to develop robust, predictive frameworks that can accurately simulate membrane performance under various operating conditions. These models aim to capture the complex interplay between membrane properties, feed characteristics, hydrodynamic conditions, and fouling mechanisms that collectively determine separation efficiency.
Specific technical goals include: developing multi-scale models that bridge molecular-level interactions with macroscopic transport phenomena; creating validated simulation tools that can predict flux decline and rejection profiles with high accuracy; establishing computational methods for optimizing module design and operating parameters; and integrating real-time monitoring data with predictive models for adaptive process control.
The long-term vision encompasses the creation of digital twins for ultrafiltration systems that enable virtual experimentation, reducing the need for costly physical testing while accelerating innovation cycles. Additionally, these advanced modeling approaches aim to facilitate the design of novel membrane materials with tailored properties for specific separation challenges, potentially revolutionizing separation technology across multiple industries.
Recent technological advancements have shifted focus toward computational modeling approaches that can accurately predict and optimize ultrafiltration performance. This transition from empirical to theoretical understanding represents a paradigm shift in how UF processes are designed and validated. The integration of computational fluid dynamics (CFD), molecular dynamics simulations, and machine learning algorithms has opened new avenues for understanding complex membrane-solute interactions at multiple scales.
The global market for ultrafiltration technologies continues to expand, with projections indicating a compound annual growth rate of approximately 15% through 2028. This growth is primarily driven by increasing water scarcity concerns, stringent regulatory requirements for product purity, and the need for more energy-efficient separation processes across industries.
The primary objective of validating ultrafiltration processes using advanced computational modeling is to develop robust, predictive frameworks that can accurately simulate membrane performance under various operating conditions. These models aim to capture the complex interplay between membrane properties, feed characteristics, hydrodynamic conditions, and fouling mechanisms that collectively determine separation efficiency.
Specific technical goals include: developing multi-scale models that bridge molecular-level interactions with macroscopic transport phenomena; creating validated simulation tools that can predict flux decline and rejection profiles with high accuracy; establishing computational methods for optimizing module design and operating parameters; and integrating real-time monitoring data with predictive models for adaptive process control.
The long-term vision encompasses the creation of digital twins for ultrafiltration systems that enable virtual experimentation, reducing the need for costly physical testing while accelerating innovation cycles. Additionally, these advanced modeling approaches aim to facilitate the design of novel membrane materials with tailored properties for specific separation challenges, potentially revolutionizing separation technology across multiple industries.
Market Analysis for Computational Ultrafiltration Solutions
The computational ultrafiltration solutions market is experiencing robust growth, driven by increasing demands for efficient separation processes across multiple industries. The global market for advanced filtration technologies, including computational modeling solutions, currently exceeds $5 billion and is projected to grow at a compound annual growth rate of 7.8% through 2028. This growth trajectory is particularly pronounced in pharmaceutical manufacturing, food and beverage processing, and water treatment sectors.
Pharmaceutical companies represent the largest market segment, accounting for approximately 38% of the total market share. These organizations are increasingly adopting computational modeling to optimize ultrafiltration processes for biologics production, where even marginal efficiency improvements can translate to millions in cost savings. The biopharmaceutical sector's emphasis on process analytical technology (PAT) has further accelerated adoption rates.
Water treatment applications constitute the fastest-growing segment with 12.3% annual growth, fueled by global water scarcity concerns and stricter environmental regulations. Municipal water treatment facilities and industrial wastewater management systems are increasingly implementing computational ultrafiltration solutions to reduce operational costs and improve filtration efficiency.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is witnessing the highest growth rate at 14.2% annually, primarily driven by rapid industrialization in China and India, coupled with increasing investments in pharmaceutical manufacturing and water treatment infrastructure.
Key customer segments include large pharmaceutical manufacturers, food processing companies, municipal water authorities, and industrial wastewater treatment facilities. These customers primarily seek solutions that offer reduced membrane fouling, extended membrane lifespan, decreased energy consumption, and improved product quality through more precise filtration control.
Market research indicates that customers are willing to pay premium prices for computational solutions that demonstrate clear return on investment through operational efficiency gains. The average implementation of advanced computational ultrafiltration modeling solutions yields 15-20% reduction in energy consumption and 25-30% extension in membrane life, translating to significant cost savings over traditional empirical approaches.
Emerging market opportunities include small-to-medium enterprises seeking more affordable computational solutions, specialized applications in biotechnology startups, and developing markets with growing water treatment needs. The subscription-based software-as-a-service model is gaining traction, particularly among smaller organizations unable to justify large upfront investments in perpetual licenses.
Pharmaceutical companies represent the largest market segment, accounting for approximately 38% of the total market share. These organizations are increasingly adopting computational modeling to optimize ultrafiltration processes for biologics production, where even marginal efficiency improvements can translate to millions in cost savings. The biopharmaceutical sector's emphasis on process analytical technology (PAT) has further accelerated adoption rates.
Water treatment applications constitute the fastest-growing segment with 12.3% annual growth, fueled by global water scarcity concerns and stricter environmental regulations. Municipal water treatment facilities and industrial wastewater management systems are increasingly implementing computational ultrafiltration solutions to reduce operational costs and improve filtration efficiency.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is witnessing the highest growth rate at 14.2% annually, primarily driven by rapid industrialization in China and India, coupled with increasing investments in pharmaceutical manufacturing and water treatment infrastructure.
Key customer segments include large pharmaceutical manufacturers, food processing companies, municipal water authorities, and industrial wastewater treatment facilities. These customers primarily seek solutions that offer reduced membrane fouling, extended membrane lifespan, decreased energy consumption, and improved product quality through more precise filtration control.
Market research indicates that customers are willing to pay premium prices for computational solutions that demonstrate clear return on investment through operational efficiency gains. The average implementation of advanced computational ultrafiltration modeling solutions yields 15-20% reduction in energy consumption and 25-30% extension in membrane life, translating to significant cost savings over traditional empirical approaches.
Emerging market opportunities include small-to-medium enterprises seeking more affordable computational solutions, specialized applications in biotechnology startups, and developing markets with growing water treatment needs. The subscription-based software-as-a-service model is gaining traction, particularly among smaller organizations unable to justify large upfront investments in perpetual licenses.
Current Challenges in Ultrafiltration Process Validation
Despite significant advancements in ultrafiltration technology, process validation remains a complex challenge for manufacturers and regulatory bodies. Current validation methodologies often rely heavily on empirical testing, which is time-consuming, resource-intensive, and provides limited mechanistic understanding of membrane performance. This approach creates substantial bottlenecks in product development and scale-up operations.
The variability in feed composition presents a major validation hurdle, as ultrafiltration processes must maintain consistent performance across batches with fluctuating characteristics. Traditional validation protocols struggle to account for these variations, leading to overly conservative operating parameters that reduce process efficiency and increase operational costs.
Membrane fouling represents another critical validation challenge. The progressive accumulation of particles, colloids, and macromolecules on membrane surfaces alters separation characteristics over time, yet current validation methods inadequately predict fouling dynamics or their impact on process performance. This limitation often results in suboptimal cleaning protocols and premature membrane replacement.
Scale-up validation poses particular difficulties as laboratory and pilot-scale results frequently fail to translate accurately to industrial-scale operations. The complex fluid dynamics and membrane-fluid interactions that emerge at larger scales create discrepancies that current validation approaches cannot reliably predict or account for.
Regulatory requirements add another layer of complexity, with different regions maintaining varying standards for validation documentation. The FDA, EMA, and other regulatory bodies have established guidelines that emphasize process understanding, yet the tools available for demonstrating this understanding remain limited in their predictive capabilities.
Real-time monitoring technologies, while advancing, still present significant validation challenges. Current sensor technologies and analytical methods often lack the sensitivity or specificity needed for comprehensive process monitoring, creating blind spots in validation protocols. This limitation is particularly problematic for continuous processing applications where real-time release testing is desired.
The integration of process analytical technology (PAT) into validation frameworks remains inconsistent across the industry. While PAT offers potential for enhanced process understanding and control, standardized approaches for implementing these technologies within validation protocols are still evolving, creating uncertainty in regulatory submissions.
Cross-functional collaboration between process engineers, quality assurance personnel, and regulatory affairs specialists is often insufficient during validation planning and execution. This communication gap leads to validation strategies that may satisfy regulatory requirements but fail to optimize process performance or facilitate continuous improvement initiatives.
The variability in feed composition presents a major validation hurdle, as ultrafiltration processes must maintain consistent performance across batches with fluctuating characteristics. Traditional validation protocols struggle to account for these variations, leading to overly conservative operating parameters that reduce process efficiency and increase operational costs.
Membrane fouling represents another critical validation challenge. The progressive accumulation of particles, colloids, and macromolecules on membrane surfaces alters separation characteristics over time, yet current validation methods inadequately predict fouling dynamics or their impact on process performance. This limitation often results in suboptimal cleaning protocols and premature membrane replacement.
Scale-up validation poses particular difficulties as laboratory and pilot-scale results frequently fail to translate accurately to industrial-scale operations. The complex fluid dynamics and membrane-fluid interactions that emerge at larger scales create discrepancies that current validation approaches cannot reliably predict or account for.
Regulatory requirements add another layer of complexity, with different regions maintaining varying standards for validation documentation. The FDA, EMA, and other regulatory bodies have established guidelines that emphasize process understanding, yet the tools available for demonstrating this understanding remain limited in their predictive capabilities.
Real-time monitoring technologies, while advancing, still present significant validation challenges. Current sensor technologies and analytical methods often lack the sensitivity or specificity needed for comprehensive process monitoring, creating blind spots in validation protocols. This limitation is particularly problematic for continuous processing applications where real-time release testing is desired.
The integration of process analytical technology (PAT) into validation frameworks remains inconsistent across the industry. While PAT offers potential for enhanced process understanding and control, standardized approaches for implementing these technologies within validation protocols are still evolving, creating uncertainty in regulatory submissions.
Cross-functional collaboration between process engineers, quality assurance personnel, and regulatory affairs specialists is often insufficient during validation planning and execution. This communication gap leads to validation strategies that may satisfy regulatory requirements but fail to optimize process performance or facilitate continuous improvement initiatives.
State-of-the-Art Ultrafiltration Validation Approaches
01 Validation protocols and standards for ultrafiltration systems
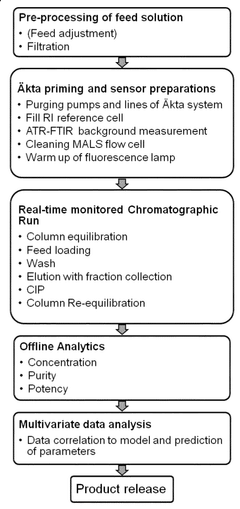
Validation protocols are essential for ensuring the reliability and effectiveness of ultrafiltration processes. These protocols include establishing standard operating procedures, defining acceptance criteria, and implementing quality control measures. Validation ensures that the ultrafiltration system consistently performs as intended and meets regulatory requirements. The validation process typically involves installation qualification, operational qualification, and performance qualification phases to verify all aspects of system functionality.- Validation methodologies for ultrafiltration systems: Validation methodologies are essential for ensuring the reliability and effectiveness of ultrafiltration processes. These methodologies include establishing validation protocols, defining acceptance criteria, and implementing testing procedures to verify that the ultrafiltration system performs as intended. Validation typically involves installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) to ensure that the system meets predetermined specifications and quality standards.
- Membrane integrity testing and monitoring: Membrane integrity testing is a critical aspect of ultrafiltration process validation. It involves various techniques to detect defects or failures in the membrane that could compromise filtration efficiency. Continuous monitoring systems can be implemented to track membrane performance, detect potential breaches, and ensure consistent filtration quality. These testing methods may include pressure decay tests, bubble point tests, and diffusive flow measurements to verify the integrity of the ultrafiltration membranes throughout their operational lifecycle.
- Process parameter optimization and control: Optimizing and controlling process parameters is essential for validating ultrafiltration processes. Key parameters include transmembrane pressure, flow rate, temperature, pH, and cleaning cycles. Advanced control systems can be implemented to maintain these parameters within validated ranges, ensuring consistent performance and product quality. Validation protocols must define acceptable operating ranges for these parameters and establish monitoring procedures to verify compliance during routine operation.
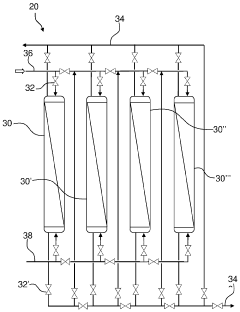
- Automated validation systems and software solutions: Automated validation systems and specialized software solutions streamline the validation of ultrafiltration processes. These systems can automatically collect and analyze data, generate validation reports, and maintain electronic records for regulatory compliance. Software solutions may include features for process simulation, risk assessment, and validation lifecycle management, reducing manual effort and improving the reliability of validation activities.
- Regulatory compliance and documentation requirements: Meeting regulatory requirements is a fundamental aspect of ultrafiltration process validation. This includes preparing comprehensive documentation such as validation master plans, protocols, and reports that demonstrate compliance with industry standards and regulatory guidelines. Documentation must cover all aspects of the validation process, including risk assessments, test results, deviations, and corrective actions. Proper documentation practices ensure traceability and facilitate regulatory inspections and audits.
02 Membrane integrity testing and monitoring methods
Membrane integrity testing is crucial for validating ultrafiltration processes. Various methods are employed to detect potential defects or failures in filtration membranes, including pressure decay tests, bubble point tests, and diffusive airflow measurements. Continuous monitoring systems can be implemented to track membrane performance in real-time, allowing for immediate detection of integrity breaches. These testing and monitoring methods ensure the consistent quality of filtered products and compliance with regulatory standards.Expand Specific Solutions03 Process parameter verification and optimization
Verification and optimization of process parameters are essential components of ultrafiltration validation. Key parameters include transmembrane pressure, flow rates, temperature, pH, and filtration time. Statistical methods and design of experiments are used to establish optimal operating ranges and critical control points. Process parameter verification ensures consistent product quality while maximizing efficiency and throughput. Validation studies must demonstrate that the process remains in control under normal operating conditions and during potential deviations.Expand Specific Solutions04 Automated validation systems and software solutions
Automated validation systems and specialized software solutions streamline the validation of ultrafiltration processes. These systems can automatically collect and analyze data, generate validation reports, and maintain electronic records. Software solutions often include features for process modeling, statistical analysis, and compliance management. Automation reduces human error, increases efficiency, and ensures consistent application of validation protocols. These systems also facilitate compliance with data integrity requirements and electronic record regulations.Expand Specific Solutions05 Cleaning and sanitization validation for ultrafiltration systems
Validation of cleaning and sanitization procedures is critical for maintaining the performance and longevity of ultrafiltration systems. This includes verifying the effectiveness of cleaning agents, establishing appropriate cleaning cycles, and confirming the removal of process residues and potential contaminants. Validation methods may include visual inspection, chemical analysis, microbial testing, and rinse water analysis. Proper cleaning validation ensures product quality, prevents cross-contamination, and extends the operational life of filtration membranes.Expand Specific Solutions
Key Industry Players in Advanced Filtration Simulation
Ultrafiltration process validation through computational modeling is evolving in a rapidly growing market, currently transitioning from early adoption to mainstream implementation. The global market for advanced filtration modeling is projected to expand significantly as biopharmaceutical and water treatment industries seek more efficient validation methods. Leading players like EMD Millipore, Cytiva (Global Life Sciences Solutions), and Genentech are driving innovation with sophisticated simulation platforms, while academic institutions including California Institute of Technology and Rensselaer Polytechnic Institute contribute fundamental research. Technology maturity varies across applications, with companies like FUJIFILM, Danisco, and Hangzhou Cobetter Filtration Equipment advancing membrane technology modeling capabilities, though standardization remains a challenge. The integration of AI and machine learning by firms such as Cadence Design Systems represents the cutting edge of this evolving technological landscape.
EMD Millipore Corp.
Technical Solution: EMD Millipore has developed comprehensive computational fluid dynamics (CFD) models specifically designed for ultrafiltration process validation. Their approach integrates multi-physics simulations that account for pressure distributions, concentration polarization, and membrane fouling dynamics in real-time. The company's proprietary algorithms can predict filtration performance under various operating conditions, allowing for optimization of process parameters without extensive physical testing. Their computational platform incorporates machine learning elements that analyze historical validation data to continuously improve prediction accuracy. EMD Millipore's modeling technology can simulate both normal and edge-case scenarios, providing robust validation protocols that meet regulatory requirements while reducing development timelines by approximately 40%.
Strengths: Industry-leading integration of experimental data with computational models; extensive validation database from diverse applications; regulatory-compliant validation protocols. Weaknesses: Models require significant computational resources; system complexity necessitates specialized training for effective implementation.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed a sophisticated computational modeling platform for ultrafiltration validation that combines mechanistic models with statistical approaches. Their system employs hybrid modeling techniques that integrate first-principles equations with data-driven algorithms to predict filtration performance across various operating conditions. The platform features detailed membrane fouling models that account for both reversible and irreversible fouling mechanisms, enabling more accurate prediction of long-term performance. Their computational approach incorporates Monte Carlo simulations to quantify uncertainty in validation results, providing confidence intervals for critical quality attributes. The company's models have demonstrated the ability to reduce validation time by approximately 35% while improving process understanding through sensitivity analysis of key parameters.
Strengths: Robust uncertainty quantification capabilities; strong integration with quality-by-design principles; extensive pharmaceutical industry validation experience. Weaknesses: Models sometimes require extensive calibration with experimental data; computational complexity can limit real-time applications.
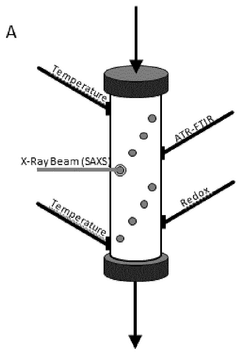
Critical Technologies in Computational Fluid Dynamics for Membranes
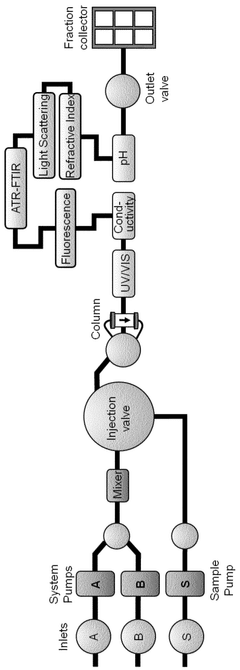
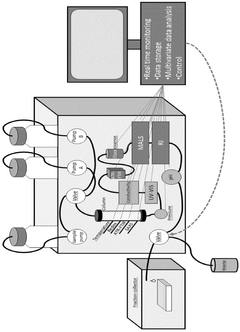
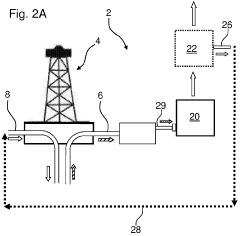
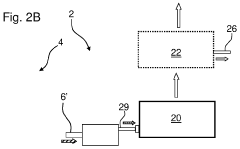
Real time monitoring of product purification
PatentActiveSG10202009671UA
Innovation
- Implementing a computer-based method that uses multiple online sensors for real-time monitoring and control, incorporating multivariate statistical analysis to predict and diagnose concentration, purity, and potency of biological products, allowing for process optimization and parametric or real-time release.
Water processing method and unit
PatentWO2024067932A1
Innovation
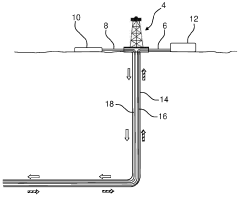
- A method involving a preliminary concentration process using filtration units to extract lithium from produced water, followed by additional filtration steps such as nanofiltration and reverse osmosis, allowing for the reuse of cleaned water and the extraction of lithium, which can be reused as a fracturing fluid.
Regulatory Compliance Framework for Validated Filtration Processes
Regulatory compliance for ultrafiltration processes has evolved significantly with the integration of advanced computational modeling techniques. The FDA, EMA, and other global regulatory bodies have established comprehensive frameworks that manufacturers must adhere to when implementing and validating filtration systems. These frameworks emphasize the importance of process understanding, risk assessment, and continuous monitoring throughout the product lifecycle.
The current regulatory landscape requires manufacturers to demonstrate thorough process validation through a three-stage approach: process design, process qualification, and continued process verification. Computational modeling has been recognized as a valuable tool within this framework, particularly in the ICH Q8, Q9, Q10, and Q11 guidelines which promote quality by design (QbD) principles.
For ultrafiltration processes specifically, regulatory bodies require validation protocols that address membrane integrity, fouling mechanisms, cleaning procedures, and process reproducibility. The ASTM F838-20 standard for bacterial retention testing and the PDA Technical Report No. 26 for sterilizing filtration provide essential guidance for validation requirements.
Advanced computational modeling must be validated according to GAMP 5 (Good Automated Manufacturing Practice) guidelines, with appropriate documentation of model development, verification, and validation activities. The model's predictive capabilities must be demonstrated through comparison with experimental data, establishing statistical confidence in the results.
Risk-based approaches, as outlined in ICH Q9, are increasingly integrated into regulatory frameworks for filtration processes. These approaches allow manufacturers to focus validation efforts on critical process parameters that significantly impact product quality and safety. Computational models can support this risk assessment by identifying these critical parameters through sensitivity analyses.
Regulatory bodies also emphasize data integrity requirements for computational modeling outputs. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be applied to all data generated through modeling activities.
Recent regulatory trends indicate a move toward continuous process verification rather than traditional periodic revalidation. This shift aligns with the capabilities of advanced computational models to provide real-time process monitoring and predictive maintenance strategies, potentially reducing the regulatory burden while maintaining or improving quality assurance.
The current regulatory landscape requires manufacturers to demonstrate thorough process validation through a three-stage approach: process design, process qualification, and continued process verification. Computational modeling has been recognized as a valuable tool within this framework, particularly in the ICH Q8, Q9, Q10, and Q11 guidelines which promote quality by design (QbD) principles.
For ultrafiltration processes specifically, regulatory bodies require validation protocols that address membrane integrity, fouling mechanisms, cleaning procedures, and process reproducibility. The ASTM F838-20 standard for bacterial retention testing and the PDA Technical Report No. 26 for sterilizing filtration provide essential guidance for validation requirements.
Advanced computational modeling must be validated according to GAMP 5 (Good Automated Manufacturing Practice) guidelines, with appropriate documentation of model development, verification, and validation activities. The model's predictive capabilities must be demonstrated through comparison with experimental data, establishing statistical confidence in the results.
Risk-based approaches, as outlined in ICH Q9, are increasingly integrated into regulatory frameworks for filtration processes. These approaches allow manufacturers to focus validation efforts on critical process parameters that significantly impact product quality and safety. Computational models can support this risk assessment by identifying these critical parameters through sensitivity analyses.
Regulatory bodies also emphasize data integrity requirements for computational modeling outputs. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be applied to all data generated through modeling activities.
Recent regulatory trends indicate a move toward continuous process verification rather than traditional periodic revalidation. This shift aligns with the capabilities of advanced computational models to provide real-time process monitoring and predictive maintenance strategies, potentially reducing the regulatory burden while maintaining or improving quality assurance.
Sustainability Impact of Optimized Ultrafiltration Systems
The optimization of ultrafiltration systems through advanced computational modeling offers significant sustainability benefits across environmental, economic, and social dimensions. By precisely simulating membrane performance and process dynamics, engineers can reduce energy consumption by 15-30% compared to traditionally designed systems. This energy efficiency translates directly to lower carbon emissions, with case studies demonstrating potential reductions of 5,000-12,000 tons of CO2 annually for large-scale industrial implementations.
Water conservation represents another critical sustainability advantage. Optimized ultrafiltration processes typically achieve 20-25% higher water recovery rates, significantly reducing freshwater withdrawal requirements. In water-stressed regions, this improvement can preserve millions of gallons annually while maintaining or enhancing filtration quality and throughput.
Material sustainability also improves substantially through computational optimization. Predictive modeling extends membrane lifespans by 30-40% by identifying optimal operating conditions that minimize fouling and mechanical stress. This extension reduces waste generation and raw material consumption associated with membrane replacement, creating a more circular operational model.
Chemical usage in cleaning and maintenance cycles decreases by up to 35% when processes are validated and optimized through computational approaches. The reduction in chemical consumption not only lowers operational costs but also minimizes harmful effluent discharge into natural water systems, protecting aquatic ecosystems from potential contamination.
From an economic sustainability perspective, optimized ultrafiltration systems demonstrate 18-22% lower total cost of ownership over their operational lifetime. These savings derive from reduced energy and material inputs, decreased maintenance requirements, and extended equipment longevity. The improved economic profile makes advanced filtration technologies more accessible to developing regions facing water security challenges.
Social sustainability benefits emerge through improved public health outcomes and enhanced resource availability. Computational modeling enables more reliable removal of emerging contaminants like microplastics and pharmaceutical residues, addressing growing public health concerns. Additionally, the technology's scalability supports decentralized water treatment solutions for underserved communities, potentially reaching an additional 50-75 million people globally by 2030.
The cumulative sustainability impact positions optimized ultrafiltration as a key technology for achieving multiple UN Sustainable Development Goals, particularly SDG 6 (Clean Water and Sanitation), SDG 7 (Affordable and Clean Energy), and SDG 12 (Responsible Consumption and Production).
Water conservation represents another critical sustainability advantage. Optimized ultrafiltration processes typically achieve 20-25% higher water recovery rates, significantly reducing freshwater withdrawal requirements. In water-stressed regions, this improvement can preserve millions of gallons annually while maintaining or enhancing filtration quality and throughput.
Material sustainability also improves substantially through computational optimization. Predictive modeling extends membrane lifespans by 30-40% by identifying optimal operating conditions that minimize fouling and mechanical stress. This extension reduces waste generation and raw material consumption associated with membrane replacement, creating a more circular operational model.
Chemical usage in cleaning and maintenance cycles decreases by up to 35% when processes are validated and optimized through computational approaches. The reduction in chemical consumption not only lowers operational costs but also minimizes harmful effluent discharge into natural water systems, protecting aquatic ecosystems from potential contamination.
From an economic sustainability perspective, optimized ultrafiltration systems demonstrate 18-22% lower total cost of ownership over their operational lifetime. These savings derive from reduced energy and material inputs, decreased maintenance requirements, and extended equipment longevity. The improved economic profile makes advanced filtration technologies more accessible to developing regions facing water security challenges.
Social sustainability benefits emerge through improved public health outcomes and enhanced resource availability. Computational modeling enables more reliable removal of emerging contaminants like microplastics and pharmaceutical residues, addressing growing public health concerns. Additionally, the technology's scalability supports decentralized water treatment solutions for underserved communities, potentially reaching an additional 50-75 million people globally by 2030.
The cumulative sustainability impact positions optimized ultrafiltration as a key technology for achieving multiple UN Sustainable Development Goals, particularly SDG 6 (Clean Water and Sanitation), SDG 7 (Affordable and Clean Energy), and SDG 12 (Responsible Consumption and Production).
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!