Advanced process control (PAT) for real-time monitoring of viral vector production quality attributes
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Viral Vector PAT Evolution and Objectives
Viral vector production has evolved significantly over the past three decades, transitioning from laboratory-scale research tools to critical components in commercial gene therapy manufacturing. The initial development phase in the 1990s focused primarily on basic viral vector design with minimal process monitoring capabilities. Quality assessment was predominantly performed through offline testing with significant time delays between production and results availability.
The early 2000s marked the beginning of the analytical revolution in viral vector production, with the introduction of basic spectroscopic techniques and early-stage process analytical technology (PAT) concepts borrowed from traditional pharmaceutical manufacturing. However, these approaches remained largely disconnected from real-time decision making, serving primarily as retrospective quality verification tools.
A paradigm shift occurred around 2010-2015 with the FDA's Quality by Design (QbD) initiative gaining traction in biologics manufacturing, establishing the foundation for modern PAT implementation in viral vector production. This period saw the emergence of specialized analytical techniques for viral vector characterization, including advanced chromatography methods, nanoparticle tracking analysis, and early applications of spectroscopic methods for in-process monitoring.
Current technological trends are focused on integrating multiple analytical platforms with automated data processing systems to enable comprehensive real-time monitoring of critical quality attributes (CQAs). The industry is rapidly moving toward closed-system manufacturing with integrated sensors that can continuously monitor parameters such as viral capsid integrity, vector genome titer, empty/full capsid ratio, and host cell protein contamination levels.
The primary objective of advanced PAT implementation in viral vector production is to establish robust real-time monitoring systems that can detect deviations in CQAs during manufacturing, enabling immediate corrective actions before batch completion. This approach aims to significantly reduce batch failures, improve consistency between production runs, and ultimately lower manufacturing costs while enhancing product quality.
Secondary objectives include developing predictive models that can anticipate quality outcomes based on early process indicators, establishing digital twins of production processes for simulation and optimization, and creating standardized PAT frameworks that can be rapidly deployed across different viral vector platforms (AAV, lentivirus, adenovirus).
The long-term vision encompasses fully autonomous viral vector manufacturing systems with self-regulating capabilities based on continuous PAT feedback loops, enabling consistent production of high-quality viral vectors with minimal human intervention, thereby addressing the scalability challenges currently facing the gene therapy industry.
The early 2000s marked the beginning of the analytical revolution in viral vector production, with the introduction of basic spectroscopic techniques and early-stage process analytical technology (PAT) concepts borrowed from traditional pharmaceutical manufacturing. However, these approaches remained largely disconnected from real-time decision making, serving primarily as retrospective quality verification tools.
A paradigm shift occurred around 2010-2015 with the FDA's Quality by Design (QbD) initiative gaining traction in biologics manufacturing, establishing the foundation for modern PAT implementation in viral vector production. This period saw the emergence of specialized analytical techniques for viral vector characterization, including advanced chromatography methods, nanoparticle tracking analysis, and early applications of spectroscopic methods for in-process monitoring.
Current technological trends are focused on integrating multiple analytical platforms with automated data processing systems to enable comprehensive real-time monitoring of critical quality attributes (CQAs). The industry is rapidly moving toward closed-system manufacturing with integrated sensors that can continuously monitor parameters such as viral capsid integrity, vector genome titer, empty/full capsid ratio, and host cell protein contamination levels.
The primary objective of advanced PAT implementation in viral vector production is to establish robust real-time monitoring systems that can detect deviations in CQAs during manufacturing, enabling immediate corrective actions before batch completion. This approach aims to significantly reduce batch failures, improve consistency between production runs, and ultimately lower manufacturing costs while enhancing product quality.
Secondary objectives include developing predictive models that can anticipate quality outcomes based on early process indicators, establishing digital twins of production processes for simulation and optimization, and creating standardized PAT frameworks that can be rapidly deployed across different viral vector platforms (AAV, lentivirus, adenovirus).
The long-term vision encompasses fully autonomous viral vector manufacturing systems with self-regulating capabilities based on continuous PAT feedback loops, enabling consistent production of high-quality viral vectors with minimal human intervention, thereby addressing the scalability challenges currently facing the gene therapy industry.
Market Demand for Real-time Viral Vector QC
The viral vector manufacturing market is experiencing unprecedented growth, driven primarily by the expanding field of gene therapy and vaccine development. Current market projections indicate that the global viral vector manufacturing market will reach approximately $2.5 billion by 2026, with a compound annual growth rate exceeding 20%. This remarkable growth trajectory underscores the urgent need for advanced real-time quality control solutions in viral vector production.
Traditional quality control methods for viral vector production rely heavily on offline testing, which introduces significant delays in the manufacturing process. These delays can extend production timelines by weeks, substantially increasing costs and reducing manufacturing efficiency. Industry reports suggest that implementing real-time monitoring technologies could reduce production timelines by up to 40% and decrease manufacturing costs by 25-30%, representing significant economic incentives for adoption.
Regulatory agencies worldwide, including the FDA and EMA, are increasingly emphasizing the importance of continuous monitoring and process analytical technology (PAT) in biopharmaceutical manufacturing. The FDA's Quality by Design (QbD) initiative specifically encourages manufacturers to implement real-time monitoring solutions to ensure consistent product quality. This regulatory push is creating additional market demand for advanced PAT solutions in viral vector production.
Biopharma companies are facing mounting pressure to accelerate time-to-market for gene therapies and vaccines, particularly evident during recent global health crises. Market research indicates that companies with robust real-time monitoring capabilities can reduce their development cycles by 30-50% compared to competitors using traditional methods, providing a significant competitive advantage in rapidly evolving therapeutic areas.
The increasing complexity of viral vector products and their critical quality attributes necessitates more sophisticated monitoring approaches. Current market surveys reveal that over 75% of viral vector manufacturers report challenges with batch-to-batch consistency and product quality assurance, directly impacting product efficacy and safety profiles. Real-time monitoring technologies address these challenges by enabling immediate process adjustments and quality assurance.
Contract development and manufacturing organizations (CDMOs), which currently handle approximately 60% of viral vector production globally, are particularly motivated to adopt advanced monitoring technologies. These organizations can leverage real-time QC capabilities as a competitive differentiator, with market research showing that CDMOs offering advanced PAT solutions command premium pricing of 15-20% over those using conventional methods.
The convergence of these market forces—regulatory requirements, economic pressures, competitive dynamics, and quality imperatives—is creating robust demand for advanced process control and real-time monitoring solutions in viral vector manufacturing, positioning this technological area for significant growth and innovation.
Traditional quality control methods for viral vector production rely heavily on offline testing, which introduces significant delays in the manufacturing process. These delays can extend production timelines by weeks, substantially increasing costs and reducing manufacturing efficiency. Industry reports suggest that implementing real-time monitoring technologies could reduce production timelines by up to 40% and decrease manufacturing costs by 25-30%, representing significant economic incentives for adoption.
Regulatory agencies worldwide, including the FDA and EMA, are increasingly emphasizing the importance of continuous monitoring and process analytical technology (PAT) in biopharmaceutical manufacturing. The FDA's Quality by Design (QbD) initiative specifically encourages manufacturers to implement real-time monitoring solutions to ensure consistent product quality. This regulatory push is creating additional market demand for advanced PAT solutions in viral vector production.
Biopharma companies are facing mounting pressure to accelerate time-to-market for gene therapies and vaccines, particularly evident during recent global health crises. Market research indicates that companies with robust real-time monitoring capabilities can reduce their development cycles by 30-50% compared to competitors using traditional methods, providing a significant competitive advantage in rapidly evolving therapeutic areas.
The increasing complexity of viral vector products and their critical quality attributes necessitates more sophisticated monitoring approaches. Current market surveys reveal that over 75% of viral vector manufacturers report challenges with batch-to-batch consistency and product quality assurance, directly impacting product efficacy and safety profiles. Real-time monitoring technologies address these challenges by enabling immediate process adjustments and quality assurance.
Contract development and manufacturing organizations (CDMOs), which currently handle approximately 60% of viral vector production globally, are particularly motivated to adopt advanced monitoring technologies. These organizations can leverage real-time QC capabilities as a competitive differentiator, with market research showing that CDMOs offering advanced PAT solutions command premium pricing of 15-20% over those using conventional methods.
The convergence of these market forces—regulatory requirements, economic pressures, competitive dynamics, and quality imperatives—is creating robust demand for advanced process control and real-time monitoring solutions in viral vector manufacturing, positioning this technological area for significant growth and innovation.
Current PAT Challenges in Viral Vector Production
Despite significant advancements in viral vector production technologies, Process Analytical Technology (PAT) implementation faces substantial challenges that hinder real-time monitoring of critical quality attributes. The complexity of viral vector production processes, involving multiple biological steps and intricate cellular interactions, creates a multifaceted monitoring environment that current PAT solutions struggle to address comprehensively.
One primary challenge is the limited availability of real-time analytical methods capable of measuring critical quality attributes during production. Traditional testing methods often require sample extraction and offline analysis, introducing delays that prevent timely process adjustments. For instance, viral titer determination typically requires time-consuming plaque assays or qPCR methods that cannot provide immediate feedback during production runs.
Sensor technology limitations represent another significant hurdle. Current in-line sensors lack the specificity and sensitivity required to detect subtle changes in viral vector quality attributes. While basic parameters like pH, temperature, and dissolved oxygen can be monitored continuously, more complex attributes such as vector potency, empty/full capsid ratio, and genomic integrity remain difficult to assess in real-time without disrupting the production process.
Data integration and interpretation challenges further complicate PAT implementation. The vast amount of data generated from multiple process parameters creates computational bottlenecks, while the lack of standardized data analysis frameworks makes it difficult to extract actionable insights promptly. Many facilities struggle with establishing clear correlations between monitored parameters and final product quality, limiting the predictive capability of current PAT systems.
Regulatory uncertainty also impedes PAT advancement in viral vector manufacturing. The evolving regulatory landscape for advanced therapy medicinal products (ATMPs) means that manufacturers often lack clear guidance on PAT implementation requirements, validation protocols, and acceptance criteria for real-time release testing.
Scale-up considerations present additional complications, as PAT solutions developed for small-scale research often fail to translate effectively to commercial manufacturing environments. Sensors and monitoring systems that function well in laboratory bioreactors may encounter interference, fouling, or sensitivity issues when deployed in larger production vessels.
Cost barriers remain significant, with specialized PAT equipment requiring substantial capital investment. Many viral vector manufacturers, particularly smaller companies and academic institutions, find it challenging to justify these expenses without clear demonstration of return on investment through improved process efficiency and product quality.
One primary challenge is the limited availability of real-time analytical methods capable of measuring critical quality attributes during production. Traditional testing methods often require sample extraction and offline analysis, introducing delays that prevent timely process adjustments. For instance, viral titer determination typically requires time-consuming plaque assays or qPCR methods that cannot provide immediate feedback during production runs.
Sensor technology limitations represent another significant hurdle. Current in-line sensors lack the specificity and sensitivity required to detect subtle changes in viral vector quality attributes. While basic parameters like pH, temperature, and dissolved oxygen can be monitored continuously, more complex attributes such as vector potency, empty/full capsid ratio, and genomic integrity remain difficult to assess in real-time without disrupting the production process.
Data integration and interpretation challenges further complicate PAT implementation. The vast amount of data generated from multiple process parameters creates computational bottlenecks, while the lack of standardized data analysis frameworks makes it difficult to extract actionable insights promptly. Many facilities struggle with establishing clear correlations between monitored parameters and final product quality, limiting the predictive capability of current PAT systems.
Regulatory uncertainty also impedes PAT advancement in viral vector manufacturing. The evolving regulatory landscape for advanced therapy medicinal products (ATMPs) means that manufacturers often lack clear guidance on PAT implementation requirements, validation protocols, and acceptance criteria for real-time release testing.
Scale-up considerations present additional complications, as PAT solutions developed for small-scale research often fail to translate effectively to commercial manufacturing environments. Sensors and monitoring systems that function well in laboratory bioreactors may encounter interference, fouling, or sensitivity issues when deployed in larger production vessels.
Cost barriers remain significant, with specialized PAT equipment requiring substantial capital investment. Many viral vector manufacturers, particularly smaller companies and academic institutions, find it challenging to justify these expenses without clear demonstration of return on investment through improved process efficiency and product quality.
Existing PAT Solutions for Viral Vector Quality Control
01 Real-time monitoring systems for pharmaceutical manufacturing
Advanced process control systems that enable real-time monitoring of critical quality attributes in pharmaceutical manufacturing processes. These systems utilize sensors and analytical tools to continuously track parameters such as temperature, pressure, and chemical composition during production, allowing for immediate adjustments to maintain product quality. The implementation of Process Analytical Technology (PAT) frameworks helps ensure consistent product quality while reducing waste and production time.- Real-time monitoring systems for pharmaceutical manufacturing: Advanced process control systems that enable real-time monitoring of critical quality attributes in pharmaceutical manufacturing processes. These systems utilize sensors and analytical tools to continuously track parameters such as temperature, pressure, and chemical composition during production, allowing for immediate adjustments to maintain product quality and consistency. The implementation of Process Analytical Technology (PAT) frameworks helps in ensuring that final product quality meets predetermined specifications.
- Spectroscopic techniques for quality attribute monitoring: Integration of spectroscopic methods such as near-infrared (NIR), Raman, and UV-visible spectroscopy for real-time monitoring of quality attributes. These non-destructive analytical techniques provide immediate data on chemical composition, physical properties, and process parameters without requiring sample preparation. The spectral data is processed using chemometric models to correlate with critical quality attributes, enabling continuous verification of product specifications during manufacturing.
- Data analytics and machine learning for PAT implementation: Advanced data analytics and machine learning algorithms that process large volumes of process data to identify patterns, predict quality outcomes, and optimize manufacturing processes. These computational approaches enable the extraction of meaningful insights from complex multivariate data generated during production. By implementing predictive models, manufacturers can anticipate quality deviations before they occur and take preventive actions, reducing waste and improving process efficiency.
- Integrated control systems for automated process adjustments: Comprehensive control systems that automatically adjust process parameters based on real-time quality attribute measurements. These closed-loop control systems utilize feedback mechanisms to maintain critical process parameters within their optimal ranges. When deviations are detected, the system can automatically implement corrective actions without human intervention, ensuring consistent product quality and reducing variability in manufacturing outcomes.
- Wireless sensor networks for distributed quality monitoring: Deployment of wireless sensor networks throughout manufacturing facilities to enable comprehensive monitoring of quality attributes across multiple process points. These networks consist of interconnected sensors that transmit data to central monitoring systems in real-time. The distributed nature of these monitoring systems allows for more complete process understanding and quality assurance, particularly in complex manufacturing environments with multiple unit operations or production lines.
02 Spectroscopic techniques for quality attribute monitoring
Integration of spectroscopic methods such as near-infrared (NIR), Raman, and UV-visible spectroscopy for non-destructive, real-time monitoring of quality attributes. These techniques allow for continuous analysis of chemical composition, blend uniformity, and other critical parameters without interrupting the manufacturing process. The spectral data is processed using advanced algorithms to provide immediate feedback on product quality, enabling timely process adjustments.Expand Specific Solutions03 Data analytics and machine learning for PAT implementation
Application of advanced data analytics and machine learning algorithms to process the large volumes of data generated by PAT systems. These computational approaches enable pattern recognition, anomaly detection, and predictive modeling of manufacturing processes. By analyzing historical and real-time data, these systems can anticipate quality issues before they occur and suggest optimal process parameters to maintain product quality attributes within specifications.Expand Specific Solutions04 Integrated control systems for automated process adjustments
Development of closed-loop control systems that automatically adjust process parameters based on real-time quality attribute measurements. These systems integrate sensors, data analysis, and control mechanisms to maintain optimal manufacturing conditions without human intervention. By continuously monitoring critical quality attributes and making immediate adjustments, these systems ensure consistent product quality while reducing operator dependency and minimizing the risk of human error.Expand Specific Solutions05 Regulatory compliance and validation of PAT methodologies
Frameworks and methodologies for validating PAT systems to ensure regulatory compliance while implementing real-time quality monitoring. These approaches include establishing appropriate sampling strategies, defining acceptance criteria for quality attributes, and developing robust calibration models for analytical instruments. The validation processes ensure that PAT implementations meet regulatory requirements while providing reliable real-time data on product quality attributes throughout the manufacturing process.Expand Specific Solutions
Key Industry Players in Viral Vector PAT
The viral vector production monitoring landscape is evolving rapidly, with the market currently in a growth phase as advanced process analytical technology (PAT) becomes essential for biopharmaceutical manufacturing. The global market size is expanding significantly, driven by increasing demand for gene therapies and vaccines. Technologically, the field shows varying maturity levels, with established pharmaceutical companies like Amgen, Takeda, Regeneron, and Merck leading implementation, while biotech specialists such as Finesse Solutions and Optimal Industrial Technologies provide specialized PAT solutions. Academic-industry partnerships with institutions like Zhejiang University and Clarkson University are accelerating innovation. Companies like WuXi Biologics and bluebird bio are integrating real-time monitoring capabilities to enhance manufacturing efficiency and quality control, positioning themselves advantageously as regulatory requirements for process monitoring become more stringent.
Janssen Biotech, Inc.
Technical Solution: Janssen Biotech has developed a comprehensive PAT platform for viral vector manufacturing that integrates multiple analytical technologies for real-time quality monitoring. Their system employs a network of specialized sensors that continuously monitor critical process parameters affecting viral vector quality, including pH, dissolved oxygen, metabolite concentrations, and cell density. The platform incorporates advanced spectroscopic techniques such as Raman and near-infrared spectroscopy to provide non-destructive, real-time analysis of viral vector attributes during production. Janssen's technology features a sophisticated data integration system that correlates process parameters with product quality attributes using multivariate statistical models, enabling predictive quality control. Their approach includes automated feedback control mechanisms that adjust process conditions in response to deviations from optimal parameters, maintaining consistent vector quality throughout production runs. The company has also implemented machine learning algorithms that continuously improve process understanding by analyzing historical production data alongside real-time measurements.
Strengths: Backed by extensive R&D resources of Johnson & Johnson; strong integration with downstream processing systems; robust data analytics capabilities for process understanding. Weaknesses: System complexity may require significant technical expertise to implement and maintain; potentially less flexible for adaptation to different vector types compared to specialized providers.
Optimal Industrial Technologies Ltd.
Technical Solution: Optimal Industrial Technologies has developed synTQ, a comprehensive PAT knowledge management platform specifically designed for real-time monitoring of biopharmaceutical processes including viral vector production. The system integrates multiple analytical instruments and sensors to provide continuous multivariate data analysis of critical quality attributes (CQAs). Their technology implements a closed-loop control system that automatically adjusts process parameters based on real-time measurements of viral vector quality attributes such as vector integrity, purity, and potency. The platform incorporates machine learning algorithms that can detect process deviations before they affect product quality, enabling predictive control rather than reactive adjustments. synTQ facilitates regulatory compliance by maintaining complete data integrity and audit trails while supporting 21 CFR Part 11 requirements for electronic records in pharmaceutical manufacturing.
Strengths: Purpose-built for PAT implementation with extensive experience in pharmaceutical applications; offers seamless integration with multiple analytical instruments; provides comprehensive data management and regulatory compliance features. Weaknesses: May require significant customization for specific viral vector processes; implementation costs can be substantial; requires specialized expertise to fully utilize advanced features.
Critical Technologies for Real-time Viral Vector Monitoring
Method, device, medium and equipment for monitoring solid precipitation in transparent container
PatentPendingCN117058102A
Innovation
- The Mask R-CNN detection model is used to obtain the ROI grayscale image in the transparent container through the video frame, extract the image of the suspected crystallization area, perform high-resolution enhancement and binarization processing, and combine the intersection ratio calculation to determine whether there is solid precipitation.
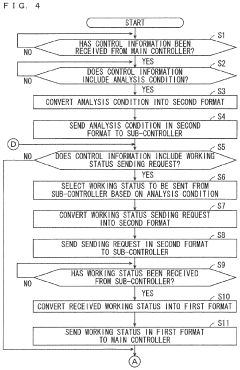
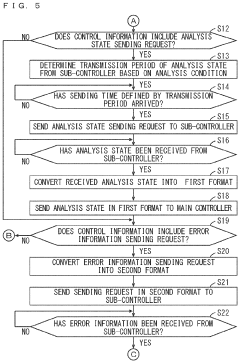
Monitoring system and monitoring method
PatentPendingUS20240201658A1
Innovation
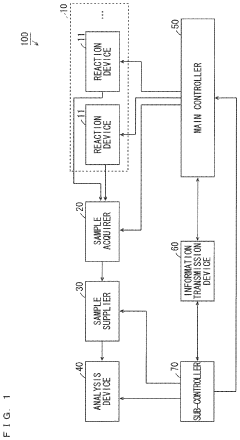
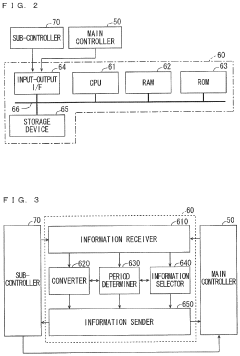
- A monitoring system comprising a first controller for the reactor, an analyzer, and an information transmission device that converts and transmits information between the controllers, enabling cooperative operation despite differences in programming languages and hardware.
Regulatory Framework for Viral Vector Manufacturing
The regulatory landscape for viral vector manufacturing is complex and continuously evolving as advanced technologies like Process Analytical Technology (PAT) become more integrated into production processes. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established frameworks that manufacturers must navigate to ensure compliance while implementing real-time monitoring systems for viral vector quality attributes.
The FDA's guidance on PAT, initially published in 2004, provides a foundation for implementing real-time monitoring in pharmaceutical manufacturing, including viral vectors. This framework emphasizes the importance of quality by design (QbD) principles and encourages manufacturers to develop process understanding through continuous monitoring rather than relying solely on end-product testing.
In the European context, the EMA has developed specific guidelines for advanced therapy medicinal products (ATMPs), which include viral vector-based gene therapies. These guidelines outline requirements for process validation, in-process controls, and specifications that must be considered when implementing PAT systems for viral vector production.
Regulatory expectations for viral vector manufacturing focus on several critical quality attributes that must be monitored, including vector titer, empty/full capsid ratio, aggregation, identity, purity, and potency. PAT implementation must demonstrate the ability to reliably monitor these attributes in real-time while maintaining data integrity and system validation.
Recent regulatory developments have shown increased acceptance of PAT approaches, with agencies providing more detailed guidance on validation requirements for analytical methods used in real-time monitoring. The FDA's CBER and CDER divisions have published recommendations specifically addressing the implementation of PAT in biologics manufacturing, including viral vectors.
Manufacturers implementing PAT systems must develop comprehensive validation protocols that demonstrate the equivalence between real-time measurements and traditional release testing methods. This includes establishing appropriate acceptance criteria, defining control strategies, and implementing robust data management systems that comply with 21 CFR Part 11 for electronic records.
Global harmonization efforts, including those through the International Council for Harmonisation (ICH), are working to standardize regulatory expectations for advanced manufacturing technologies like PAT across different regions. These initiatives aim to reduce regulatory barriers while maintaining stringent quality and safety standards for viral vector products.
The regulatory pathway for implementing PAT in viral vector manufacturing typically involves early engagement with regulatory authorities through scientific advice meetings, where manufacturers can discuss their proposed monitoring strategies and receive feedback before full implementation.
The FDA's guidance on PAT, initially published in 2004, provides a foundation for implementing real-time monitoring in pharmaceutical manufacturing, including viral vectors. This framework emphasizes the importance of quality by design (QbD) principles and encourages manufacturers to develop process understanding through continuous monitoring rather than relying solely on end-product testing.
In the European context, the EMA has developed specific guidelines for advanced therapy medicinal products (ATMPs), which include viral vector-based gene therapies. These guidelines outline requirements for process validation, in-process controls, and specifications that must be considered when implementing PAT systems for viral vector production.
Regulatory expectations for viral vector manufacturing focus on several critical quality attributes that must be monitored, including vector titer, empty/full capsid ratio, aggregation, identity, purity, and potency. PAT implementation must demonstrate the ability to reliably monitor these attributes in real-time while maintaining data integrity and system validation.
Recent regulatory developments have shown increased acceptance of PAT approaches, with agencies providing more detailed guidance on validation requirements for analytical methods used in real-time monitoring. The FDA's CBER and CDER divisions have published recommendations specifically addressing the implementation of PAT in biologics manufacturing, including viral vectors.
Manufacturers implementing PAT systems must develop comprehensive validation protocols that demonstrate the equivalence between real-time measurements and traditional release testing methods. This includes establishing appropriate acceptance criteria, defining control strategies, and implementing robust data management systems that comply with 21 CFR Part 11 for electronic records.
Global harmonization efforts, including those through the International Council for Harmonisation (ICH), are working to standardize regulatory expectations for advanced manufacturing technologies like PAT across different regions. These initiatives aim to reduce regulatory barriers while maintaining stringent quality and safety standards for viral vector products.
The regulatory pathway for implementing PAT in viral vector manufacturing typically involves early engagement with regulatory authorities through scientific advice meetings, where manufacturers can discuss their proposed monitoring strategies and receive feedback before full implementation.
Cost-Benefit Analysis of PAT Implementation
Implementing Process Analytical Technology (PAT) for viral vector production requires significant initial investment but offers substantial long-term returns. The upfront costs include specialized equipment acquisition (spectroscopic analyzers, biosensors, and multivariate data analysis systems) ranging from $500,000 to $2 million depending on production scale. Additional expenses encompass facility modifications ($100,000-300,000), software integration ($50,000-150,000), and staff training ($30,000-80,000 annually).
Personnel requirements represent an ongoing cost factor, with specialized PAT scientists commanding salaries of $80,000-120,000 annually, plus additional data analysts and validation specialists. Regulatory compliance activities add $50,000-200,000 for initial validation and $20,000-50,000 for annual maintenance.
Against these investments, PAT implementation delivers quantifiable benefits through reduced batch failures. With traditional methods, viral vector production experiences 15-20% batch rejection rates, each representing $250,000-1,000,000 in lost production value. PAT monitoring can reduce these failures by 50-70%, translating to annual savings of $750,000-3,500,000 for medium-scale operations.
Process optimization enabled by real-time monitoring typically increases production yields by 10-25%, while reducing production cycle times by 15-30%. This efficiency improvement allows manufacturers to process more batches annually with existing infrastructure, effectively increasing capacity utilization by 20-40%.
Quality assurance costs decrease significantly as PAT reduces reliance on extensive post-production testing. Traditional quality control requires approximately $50,000-150,000 per batch, while PAT implementation can reduce these costs by 30-50% through earlier detection of quality deviations.
The return on investment timeline varies by implementation scale, with most facilities achieving breakeven within 12-24 months. Small-scale operations (≤10 batches annually) may require 2-3 years, while large-scale facilities (≥50 batches annually) often recoup investments within 6-12 months.
Beyond quantifiable returns, PAT implementation delivers strategic advantages through enhanced regulatory compliance, improved process understanding, and accelerated technology transfer capabilities. These benefits, while difficult to monetize directly, contribute significantly to competitive positioning and organizational resilience in the rapidly evolving cell and gene therapy manufacturing landscape.
Personnel requirements represent an ongoing cost factor, with specialized PAT scientists commanding salaries of $80,000-120,000 annually, plus additional data analysts and validation specialists. Regulatory compliance activities add $50,000-200,000 for initial validation and $20,000-50,000 for annual maintenance.
Against these investments, PAT implementation delivers quantifiable benefits through reduced batch failures. With traditional methods, viral vector production experiences 15-20% batch rejection rates, each representing $250,000-1,000,000 in lost production value. PAT monitoring can reduce these failures by 50-70%, translating to annual savings of $750,000-3,500,000 for medium-scale operations.
Process optimization enabled by real-time monitoring typically increases production yields by 10-25%, while reducing production cycle times by 15-30%. This efficiency improvement allows manufacturers to process more batches annually with existing infrastructure, effectively increasing capacity utilization by 20-40%.
Quality assurance costs decrease significantly as PAT reduces reliance on extensive post-production testing. Traditional quality control requires approximately $50,000-150,000 per batch, while PAT implementation can reduce these costs by 30-50% through earlier detection of quality deviations.
The return on investment timeline varies by implementation scale, with most facilities achieving breakeven within 12-24 months. Small-scale operations (≤10 batches annually) may require 2-3 years, while large-scale facilities (≥50 batches annually) often recoup investments within 6-12 months.
Beyond quantifiable returns, PAT implementation delivers strategic advantages through enhanced regulatory compliance, improved process understanding, and accelerated technology transfer capabilities. These benefits, while difficult to monetize directly, contribute significantly to competitive positioning and organizational resilience in the rapidly evolving cell and gene therapy manufacturing landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!