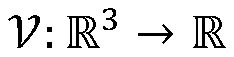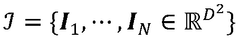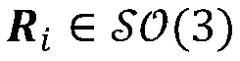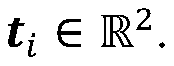Advanced Tomographic Reconstruction Methods For Cryo-EM
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Tomography Background and Objectives
Cryo-electron microscopy (Cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules in their native states at near-atomic resolution. The field has evolved significantly since its inception in the 1980s, transitioning from a niche technique to a mainstream approach for structural determination. The development trajectory shows a clear trend toward higher resolution, improved sample preparation methods, and more sophisticated computational reconstruction algorithms.
The evolution of Cryo-EM technology has been marked by several breakthrough moments, including the introduction of direct electron detectors in 2013, which dramatically improved signal-to-noise ratios and enabled routine sub-4Å resolution structures. More recently, the field has witnessed significant advancements in motion correction algorithms, automated data collection, and machine learning-based particle picking and classification.
Tomographic reconstruction in Cryo-EM represents a specialized approach that enables 3D visualization of cellular structures in their native context. Unlike single-particle analysis, which requires averaging of thousands of identical particles, tomography captures the structural heterogeneity and spatial relationships within complex biological systems. This capability is particularly valuable for studying cellular organelles, virus-host interactions, and macromolecular assemblies in situ.
The current technical objectives in advanced tomographic reconstruction for Cryo-EM focus on several key areas. First, improving resolution beyond the current limitations (typically 10-40Å) to approach the sub-nanometer range consistently. Second, developing more efficient algorithms to handle the inherently low signal-to-noise ratio in tomographic data, particularly for dose-limited specimens. Third, addressing the "missing wedge" problem resulting from limited tilt angles during data acquisition.
Additionally, there is a growing emphasis on integrating complementary techniques such as focused ion beam (FIB) milling with Cryo-ET to enable visualization of structures within thick cellular specimens. The field is also moving toward correlative approaches that combine the structural insights from Cryo-EM with functional information from light microscopy or other imaging modalities.
The ultimate goal of advanced tomographic reconstruction methods is to bridge the resolution gap between molecular and cellular structural biology, enabling researchers to visualize macromolecular complexes within their native cellular environments at resolutions sufficient for mechanistic interpretation. This would revolutionize our understanding of cellular processes and disease mechanisms at the molecular level.
The evolution of Cryo-EM technology has been marked by several breakthrough moments, including the introduction of direct electron detectors in 2013, which dramatically improved signal-to-noise ratios and enabled routine sub-4Å resolution structures. More recently, the field has witnessed significant advancements in motion correction algorithms, automated data collection, and machine learning-based particle picking and classification.
Tomographic reconstruction in Cryo-EM represents a specialized approach that enables 3D visualization of cellular structures in their native context. Unlike single-particle analysis, which requires averaging of thousands of identical particles, tomography captures the structural heterogeneity and spatial relationships within complex biological systems. This capability is particularly valuable for studying cellular organelles, virus-host interactions, and macromolecular assemblies in situ.
The current technical objectives in advanced tomographic reconstruction for Cryo-EM focus on several key areas. First, improving resolution beyond the current limitations (typically 10-40Å) to approach the sub-nanometer range consistently. Second, developing more efficient algorithms to handle the inherently low signal-to-noise ratio in tomographic data, particularly for dose-limited specimens. Third, addressing the "missing wedge" problem resulting from limited tilt angles during data acquisition.
Additionally, there is a growing emphasis on integrating complementary techniques such as focused ion beam (FIB) milling with Cryo-ET to enable visualization of structures within thick cellular specimens. The field is also moving toward correlative approaches that combine the structural insights from Cryo-EM with functional information from light microscopy or other imaging modalities.
The ultimate goal of advanced tomographic reconstruction methods is to bridge the resolution gap between molecular and cellular structural biology, enabling researchers to visualize macromolecular complexes within their native cellular environments at resolutions sufficient for mechanistic interpretation. This would revolutionize our understanding of cellular processes and disease mechanisms at the molecular level.
Market Analysis for Advanced Cryo-EM Solutions
The global market for advanced Cryo-EM solutions is experiencing robust growth, driven by increasing demand for high-resolution structural biology research across pharmaceutical, biotechnology, and academic sectors. Current market valuations place the Cryo-EM equipment segment at approximately 1.2 billion USD, with projected annual growth rates of 8-10% through 2028, significantly outpacing traditional electron microscopy markets.
Pharmaceutical companies represent the largest market segment, accounting for nearly 40% of Cryo-EM technology adoption. This dominance stems from the critical role these technologies play in drug discovery and development processes, particularly for structure-based drug design. Academic research institutions constitute the second-largest segment at 35%, while biotechnology companies represent about 20% of the market.
Geographically, North America leads with approximately 45% market share, benefiting from substantial research funding and the presence of major pharmaceutical companies. Europe follows at 30%, with particularly strong adoption in countries with established structural biology research centers such as the UK, Germany, and Switzerland. The Asia-Pacific region, currently at 20% market share, represents the fastest-growing market with annual growth rates exceeding 12%, primarily driven by increased research investments in China, Japan, and South Korea.
The advanced tomographic reconstruction methods segment specifically is projected to grow at 11-13% annually, outpacing the broader Cryo-EM market. This accelerated growth reflects the critical importance of computational methods in maximizing the utility of expensive Cryo-EM hardware investments. Software solutions for advanced reconstruction are estimated to represent a 300 million USD market currently, with particularly strong demand for AI-enhanced reconstruction algorithms.
Customer segmentation reveals distinct needs across different user groups. Large pharmaceutical companies prioritize throughput and integration with existing structural biology workflows, while academic institutions place greater emphasis on methodological flexibility and cost-effectiveness. Emerging biotechnology companies typically seek scalable solutions that can grow with their research programs.
Key market drivers include the increasing complexity of biological targets being studied, growing recognition of Cryo-EM's advantages for membrane protein and macromolecular complex visualization, and the continuous improvement in resolution capabilities. Market restraints primarily involve high equipment costs, technical expertise requirements, and computational infrastructure demands for processing the massive datasets generated.
Pharmaceutical companies represent the largest market segment, accounting for nearly 40% of Cryo-EM technology adoption. This dominance stems from the critical role these technologies play in drug discovery and development processes, particularly for structure-based drug design. Academic research institutions constitute the second-largest segment at 35%, while biotechnology companies represent about 20% of the market.
Geographically, North America leads with approximately 45% market share, benefiting from substantial research funding and the presence of major pharmaceutical companies. Europe follows at 30%, with particularly strong adoption in countries with established structural biology research centers such as the UK, Germany, and Switzerland. The Asia-Pacific region, currently at 20% market share, represents the fastest-growing market with annual growth rates exceeding 12%, primarily driven by increased research investments in China, Japan, and South Korea.
The advanced tomographic reconstruction methods segment specifically is projected to grow at 11-13% annually, outpacing the broader Cryo-EM market. This accelerated growth reflects the critical importance of computational methods in maximizing the utility of expensive Cryo-EM hardware investments. Software solutions for advanced reconstruction are estimated to represent a 300 million USD market currently, with particularly strong demand for AI-enhanced reconstruction algorithms.
Customer segmentation reveals distinct needs across different user groups. Large pharmaceutical companies prioritize throughput and integration with existing structural biology workflows, while academic institutions place greater emphasis on methodological flexibility and cost-effectiveness. Emerging biotechnology companies typically seek scalable solutions that can grow with their research programs.
Key market drivers include the increasing complexity of biological targets being studied, growing recognition of Cryo-EM's advantages for membrane protein and macromolecular complex visualization, and the continuous improvement in resolution capabilities. Market restraints primarily involve high equipment costs, technical expertise requirements, and computational infrastructure demands for processing the massive datasets generated.
Current Challenges in Tomographic Reconstruction
Despite significant advancements in cryo-electron microscopy (cryo-EM) tomographic reconstruction methods, several critical challenges persist that impede optimal structural determination of biological specimens. The primary obstacle remains the "missing wedge" problem, where limited tilt angles (typically ±60° due to physical constraints) result in incomplete sampling of Fourier space, causing elongation artifacts and resolution anisotropy in the final reconstruction.
Noise contamination presents another formidable challenge, as cryo-EM data inherently suffers from low signal-to-noise ratios (SNR) due to the necessity of using low electron doses to minimize radiation damage to sensitive biological specimens. This fundamental trade-off between structural preservation and image quality significantly complicates accurate reconstruction.
Beam-induced motion and specimen deformation during data acquisition introduce additional complexities. As electrons interact with the specimen, they induce movement and conformational changes that blur images and create inconsistencies between projections, compromising the fidelity of the reconstruction process. Current motion correction algorithms struggle with the complex, non-uniform nature of these movements in tomographic series.
Computational limitations also constrain reconstruction quality. The iterative nature of advanced reconstruction algorithms demands substantial computational resources, creating bottlenecks in processing the increasingly large datasets generated by modern detectors. This is particularly problematic for sub-tomogram averaging workflows that may involve thousands of sub-volumes.
Alignment accuracy remains a persistent challenge, with errors in tilt-series alignment propagating through the reconstruction process. The precise determination of the tilt axis and projection parameters becomes increasingly difficult at higher resolutions, limiting the achievable resolution of the final reconstruction.
The heterogeneity of biological specimens further complicates reconstruction efforts. Macromolecular complexes often exist in multiple conformational states, and current methods struggle to effectively classify and reconstruct these different states from tomographic data, particularly when conformational changes are subtle or continuous.
Finally, the integration of prior knowledge and complementary structural information into the reconstruction process remains underdeveloped. While approaches like constrained reconstruction show promise, effectively incorporating multi-scale or multi-modal data to guide and enhance tomographic reconstruction represents an ongoing challenge that requires sophisticated mathematical frameworks and validation methodologies.
Noise contamination presents another formidable challenge, as cryo-EM data inherently suffers from low signal-to-noise ratios (SNR) due to the necessity of using low electron doses to minimize radiation damage to sensitive biological specimens. This fundamental trade-off between structural preservation and image quality significantly complicates accurate reconstruction.
Beam-induced motion and specimen deformation during data acquisition introduce additional complexities. As electrons interact with the specimen, they induce movement and conformational changes that blur images and create inconsistencies between projections, compromising the fidelity of the reconstruction process. Current motion correction algorithms struggle with the complex, non-uniform nature of these movements in tomographic series.
Computational limitations also constrain reconstruction quality. The iterative nature of advanced reconstruction algorithms demands substantial computational resources, creating bottlenecks in processing the increasingly large datasets generated by modern detectors. This is particularly problematic for sub-tomogram averaging workflows that may involve thousands of sub-volumes.
Alignment accuracy remains a persistent challenge, with errors in tilt-series alignment propagating through the reconstruction process. The precise determination of the tilt axis and projection parameters becomes increasingly difficult at higher resolutions, limiting the achievable resolution of the final reconstruction.
The heterogeneity of biological specimens further complicates reconstruction efforts. Macromolecular complexes often exist in multiple conformational states, and current methods struggle to effectively classify and reconstruct these different states from tomographic data, particularly when conformational changes are subtle or continuous.
Finally, the integration of prior knowledge and complementary structural information into the reconstruction process remains underdeveloped. While approaches like constrained reconstruction show promise, effectively incorporating multi-scale or multi-modal data to guide and enhance tomographic reconstruction represents an ongoing challenge that requires sophisticated mathematical frameworks and validation methodologies.
State-of-the-Art Reconstruction Methods
01 Iterative reconstruction algorithms for improved resolution
Iterative reconstruction methods can significantly enhance tomographic image resolution by progressively refining the image through multiple iterations. These algorithms compare projected data with measured data and make adjustments to minimize differences. Techniques such as maximum likelihood expectation maximization (MLEM) and ordered subset expectation maximization (OSEM) are commonly used to achieve higher resolution while managing noise levels in the reconstructed images.- Iterative reconstruction algorithms for improved resolution: Iterative reconstruction methods can significantly enhance tomographic image resolution by progressively refining the image through multiple iterations. These algorithms compare projected data with measured data and make adjustments to minimize differences. Techniques such as maximum likelihood expectation maximization (MLEM) and ordered subset expectation maximization (OSEM) are commonly used to achieve higher resolution while managing noise levels in the reconstructed images.
- Resolution enhancement through super-resolution techniques: Super-resolution techniques can be applied to tomographic reconstruction to achieve resolution beyond the physical limitations of imaging systems. These methods combine multiple low-resolution images or projections to generate a higher resolution output. Advanced computational approaches including deep learning and neural networks can extract sub-pixel information from the projection data, resulting in reconstructions with significantly improved spatial resolution.
- Sparse sampling and compressed sensing for resolution preservation: Sparse sampling and compressed sensing techniques enable high-resolution tomographic reconstruction while using fewer projections or samples than traditional methods. These approaches leverage the inherent sparsity in many images and utilize mathematical optimization to recover missing information. By incorporating prior knowledge about the imaged object's structure, these methods can maintain or even enhance resolution while reducing acquisition time and radiation dose.
- Multi-scale and adaptive resolution reconstruction methods: Multi-scale and adaptive resolution approaches adjust the reconstruction parameters based on local image characteristics. These methods apply different levels of resolution to different regions of the image, focusing computational resources where higher detail is needed. By analyzing the data at multiple scales and adaptively refining the reconstruction process, these techniques can optimize the resolution-to-noise trade-off across the entire image volume.
- Hardware-based resolution enhancement techniques: Hardware modifications and specialized acquisition protocols can be implemented to improve the resolution of tomographic reconstruction. These include detector collimation adjustments, focal spot modulation, and specialized scanning geometries. Advanced detector technologies with higher spatial sensitivity and improved signal-to-noise characteristics directly contribute to enhanced resolution in the reconstructed images. Dual-energy and spectral imaging approaches can also provide additional information for resolution enhancement.
02 Resolution enhancement through super-resolution techniques
Super-resolution techniques can be applied to tomographic reconstruction to achieve resolution beyond the physical limitations of imaging systems. These methods combine multiple low-resolution images or projections to generate a higher resolution output. By utilizing information from slightly different views or time points, these algorithms can recover sub-pixel details and enhance the effective resolution of tomographic images.Expand Specific Solutions03 Filtered back-projection methods with resolution optimization
Filtered back-projection (FBP) remains a fundamental tomographic reconstruction method that can be optimized for resolution through careful filter selection and application. Advanced filtering techniques can enhance edge definition and fine details while suppressing noise. Modifications to traditional FBP, including weighted filtered back-projection and region-of-interest reconstruction, allow for targeted resolution improvements in specific areas of interest.Expand Specific Solutions04 Machine learning and AI-based reconstruction for resolution enhancement
Machine learning and artificial intelligence approaches are increasingly being applied to tomographic reconstruction to improve resolution. Deep learning networks can be trained to recognize patterns and features in tomographic data that traditional algorithms might miss. These methods can effectively reduce artifacts, enhance contrast, and improve spatial resolution by learning optimal reconstruction parameters from training datasets containing high-quality reference images.Expand Specific Solutions05 Multi-modal and sparse sampling techniques for resolution improvement
Multi-modal imaging and sparse sampling techniques can be combined with advanced reconstruction methods to enhance tomographic resolution. By incorporating prior information from complementary imaging modalities or utilizing compressed sensing principles, these approaches can recover high-resolution details from undersampled data. This enables higher resolution reconstruction while potentially reducing radiation dose or acquisition time in medical and industrial applications.Expand Specific Solutions
Leading Institutions and Companies in Cryo-EM Technology
The field of Advanced Tomographic Reconstruction Methods for Cryo-EM is currently in a growth phase, with the market expanding rapidly due to increasing applications in structural biology and drug discovery. The global market size for cryo-electron microscopy is projected to reach several billion dollars by 2025, driven by pharmaceutical research and academic investments. Technologically, the field is maturing with key players developing sophisticated algorithms and hardware solutions. Leading companies like Thermo Fisher Scientific (through its Bremen subsidiary) and FEI Co. dominate the hardware segment, while academic institutions including Oxford University, Peking University, and the University of California are pioneering advanced reconstruction algorithms. Research centers such as the New York Structural Biology Center and VIB provide specialized expertise, creating a competitive ecosystem where commercial solutions increasingly integrate with academic innovations to address resolution and processing challenges.
FEI Co.
Technical Solution: FEI Co. (now part of Thermo Fisher Scientific) has developed advanced tomographic reconstruction methods for cryo-electron microscopy (cryo-EM) that integrate hardware and software solutions. Their approach combines direct electron detectors with sophisticated image processing algorithms to enhance resolution and contrast in 3D reconstructions. FEI's technology employs iterative reconstruction techniques that account for beam-induced motion and sample heterogeneity, significantly improving the quality of structural data. Their Titan Krios microscope platform integrates with proprietary software that implements regularized maximum likelihood algorithms and Bayesian statistical frameworks to extract maximum information from noisy cryo-EM images[1]. FEI has pioneered methods for sub-tomogram averaging that allow researchers to resolve structures within complex cellular environments, and their automated data collection workflows enable high-throughput structural studies that were previously impossible[3].
Strengths: Integrated hardware-software solution provides end-to-end workflow optimization; high automation reduces user bias and increases reproducibility. Weaknesses: Proprietary nature of some algorithms limits customization for specialized research applications; computational demands require significant infrastructure investment.
Institute of Biophysics of Chinese Academy of Sciences
Technical Solution: The Institute of Biophysics of the Chinese Academy of Sciences has developed innovative tomographic reconstruction methods for cryo-EM that focus on addressing the challenges of low signal-to-noise ratio and beam-induced specimen damage. Their approach incorporates deep learning frameworks to enhance image contrast and feature detection prior to reconstruction. The institute has pioneered methods that combine convolutional neural networks with traditional reconstruction algorithms to improve resolution in subtomogram averaging[2]. Their THUNDER software platform implements novel motion correction algorithms that track particle movements with sub-pixel accuracy during exposure, significantly improving the quality of 3D reconstructions[4]. Additionally, they have developed specialized algorithms for handling structural heterogeneity through 3D classification techniques that can identify and sort multiple conformational states within a single dataset, enabling researchers to visualize dynamic molecular processes[5].
Strengths: Advanced deep learning integration provides superior noise reduction and feature enhancement; specialized algorithms for heterogeneity analysis reveal dynamic structural information. Weaknesses: High computational requirements limit accessibility for smaller research groups; some methods require extensive training data which may not be available for novel structural targets.
Key Innovations in Noise Reduction and Resolution Enhancement
Neural implicit function for end-to-end reconstruction of dynamic CRYO-em structures
PatentWO2023004558A1
Innovation
- End-to-end neural implicit function approach for reconstructing dynamic cryo-EM structures, addressing the challenges of low signal-to-noise ratio and unknown particle poses.
- Direct reconstruction of dynamic molecular structures from cryo-EM images without requiring intermediate steps like 2D classification or initial model building.
- Novel computational framework that can potentially reveal conformational heterogeneity in biomolecules without crystallization requirements.
Patent
Innovation
- Implementation of iterative reconstruction algorithms that incorporate prior knowledge about the specimen structure to enhance resolution in cryo-EM tomography.
- Development of motion correction techniques that account for beam-induced specimen movement during data acquisition, resulting in improved image quality and resolution.
- Novel sampling strategies in Fourier space that reduce the missing wedge artifact, leading to more isotropic resolution in the reconstructed volumes.
Data Management and Computational Infrastructure
The management of cryo-electron microscopy (cryo-EM) data presents significant challenges due to the massive volumes generated during experiments. A typical cryo-EM session can produce terabytes of raw data, necessitating robust storage solutions and efficient data transfer mechanisms. Modern facilities are increasingly implementing hierarchical storage architectures that combine high-speed solid-state drives for active processing with larger capacity hard disk arrays and tape archives for long-term storage.
Data compression techniques have become essential in this field, with specialized algorithms being developed to preserve the integrity of microscopy data while reducing storage requirements. Lossless compression methods are preferred for raw data, while carefully validated lossy compression can be applied to intermediate processing stages where appropriate.
The computational infrastructure required for advanced tomographic reconstruction in cryo-EM has evolved dramatically in recent years. GPU acceleration has revolutionized processing capabilities, with modern reconstruction pipelines leveraging multiple high-performance GPUs to handle the computationally intensive operations involved in 3D reconstruction. Cloud computing platforms have also emerged as viable solutions, offering scalable resources that can be dynamically allocated according to processing demands.
Workflow management systems specifically designed for cryo-EM data processing have become increasingly sophisticated. These systems coordinate the complex sequence of operations from data acquisition through motion correction, CTF estimation, particle picking, classification, and final reconstruction. Notable examples include Scipion, cryoSPARC, and RELION, which provide integrated environments for managing the entire processing pipeline.
Database solutions for organizing metadata and tracking processing history have become critical components of the infrastructure. These systems enable researchers to maintain provenance information, facilitate reproducibility, and support collaborative research efforts across institutions. The implementation of standardized data formats and metadata schemas, such as those proposed by the Electron Microscopy Public Image Archive (EMPIAR), has further enhanced interoperability between different software tools and facilities.
Real-time monitoring and quality control mechanisms are increasingly being integrated into data management systems, allowing researchers to assess data quality during acquisition and make informed decisions about experiment parameters. These capabilities are particularly valuable for optimizing the use of limited microscope time and ensuring that sufficient high-quality data is collected for successful reconstructions.
Data compression techniques have become essential in this field, with specialized algorithms being developed to preserve the integrity of microscopy data while reducing storage requirements. Lossless compression methods are preferred for raw data, while carefully validated lossy compression can be applied to intermediate processing stages where appropriate.
The computational infrastructure required for advanced tomographic reconstruction in cryo-EM has evolved dramatically in recent years. GPU acceleration has revolutionized processing capabilities, with modern reconstruction pipelines leveraging multiple high-performance GPUs to handle the computationally intensive operations involved in 3D reconstruction. Cloud computing platforms have also emerged as viable solutions, offering scalable resources that can be dynamically allocated according to processing demands.
Workflow management systems specifically designed for cryo-EM data processing have become increasingly sophisticated. These systems coordinate the complex sequence of operations from data acquisition through motion correction, CTF estimation, particle picking, classification, and final reconstruction. Notable examples include Scipion, cryoSPARC, and RELION, which provide integrated environments for managing the entire processing pipeline.
Database solutions for organizing metadata and tracking processing history have become critical components of the infrastructure. These systems enable researchers to maintain provenance information, facilitate reproducibility, and support collaborative research efforts across institutions. The implementation of standardized data formats and metadata schemas, such as those proposed by the Electron Microscopy Public Image Archive (EMPIAR), has further enhanced interoperability between different software tools and facilities.
Real-time monitoring and quality control mechanisms are increasingly being integrated into data management systems, allowing researchers to assess data quality during acquisition and make informed decisions about experiment parameters. These capabilities are particularly valuable for optimizing the use of limited microscope time and ensuring that sufficient high-quality data is collected for successful reconstructions.
Standardization and Validation Protocols
The standardization and validation of cryo-electron microscopy (cryo-EM) tomographic reconstruction methods remains a critical challenge in the field. Despite significant advancements in reconstruction algorithms, the lack of universally accepted protocols for evaluating these methods has hindered comparative analysis and reproducibility across research institutions. Current validation approaches often rely on subjective visual assessment or inconsistent metrics, making it difficult to establish the true efficacy of novel reconstruction techniques.
A comprehensive standardization framework must address multiple dimensions of the reconstruction process. Resolution assessment protocols require particular attention, as traditional Fourier Shell Correlation (FSC) metrics may not fully capture the complexities of tomographic data. The development of gold-standard datasets with known ground truth is essential for benchmarking different reconstruction algorithms under controlled conditions. These reference datasets should encompass various specimen types, imaging conditions, and noise levels to ensure robust validation across diverse experimental scenarios.
Quality control metrics need standardization to enable objective comparison between reconstruction methods. These metrics should evaluate not only resolution but also contrast, noise characteristics, artifact suppression, and feature preservation. Quantitative measures such as signal-to-noise ratio (SNR), contrast transfer function (CTF) correction accuracy, and structural similarity indices provide more comprehensive evaluation than visual inspection alone. Implementation of these metrics through automated validation pipelines would significantly enhance reproducibility.
Cross-laboratory validation protocols represent another crucial component of standardization efforts. Round-robin testing, where multiple laboratories apply their reconstruction methods to identical datasets, can reveal method-specific biases and establish confidence intervals for performance claims. Such collaborative validation approaches have proven valuable in related fields like single-particle cryo-EM but remain underutilized in tomographic reconstruction.
The integration of validation metrics into reconstruction software packages would streamline quality assessment and promote wider adoption of standardized protocols. Several initiatives are currently underway to develop open-source validation frameworks and reference datasets specifically designed for cryo-EM tomography. These efforts include the establishment of public repositories for benchmark datasets and the development of standardized reporting guidelines for publications describing new reconstruction methods.
Addressing these standardization challenges will accelerate progress in the field by enabling meaningful comparison between competing approaches and facilitating the identification of truly innovative methods. As reconstruction algorithms continue to evolve, particularly with the integration of machine learning approaches, robust validation protocols will become increasingly important to ensure that methodological advances translate to genuine improvements in biological insight.
A comprehensive standardization framework must address multiple dimensions of the reconstruction process. Resolution assessment protocols require particular attention, as traditional Fourier Shell Correlation (FSC) metrics may not fully capture the complexities of tomographic data. The development of gold-standard datasets with known ground truth is essential for benchmarking different reconstruction algorithms under controlled conditions. These reference datasets should encompass various specimen types, imaging conditions, and noise levels to ensure robust validation across diverse experimental scenarios.
Quality control metrics need standardization to enable objective comparison between reconstruction methods. These metrics should evaluate not only resolution but also contrast, noise characteristics, artifact suppression, and feature preservation. Quantitative measures such as signal-to-noise ratio (SNR), contrast transfer function (CTF) correction accuracy, and structural similarity indices provide more comprehensive evaluation than visual inspection alone. Implementation of these metrics through automated validation pipelines would significantly enhance reproducibility.
Cross-laboratory validation protocols represent another crucial component of standardization efforts. Round-robin testing, where multiple laboratories apply their reconstruction methods to identical datasets, can reveal method-specific biases and establish confidence intervals for performance claims. Such collaborative validation approaches have proven valuable in related fields like single-particle cryo-EM but remain underutilized in tomographic reconstruction.
The integration of validation metrics into reconstruction software packages would streamline quality assessment and promote wider adoption of standardized protocols. Several initiatives are currently underway to develop open-source validation frameworks and reference datasets specifically designed for cryo-EM tomography. These efforts include the establishment of public repositories for benchmark datasets and the development of standardized reporting guidelines for publications describing new reconstruction methods.
Addressing these standardization challenges will accelerate progress in the field by enabling meaningful comparison between competing approaches and facilitating the identification of truly innovative methods. As reconstruction algorithms continue to evolve, particularly with the integration of machine learning approaches, robust validation protocols will become increasingly important to ensure that methodological advances translate to genuine improvements in biological insight.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!