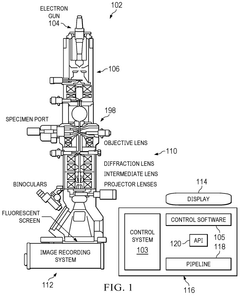
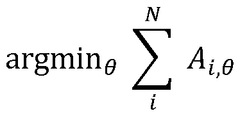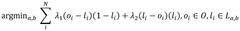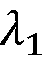Cryo-EM Throughput Improvements: Automation And Robotics
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Automation Background and Objectives
Cryo-electron microscopy (Cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules at near-atomic resolution. Since its inception in the 1980s, the technique has evolved from a niche method to a mainstream approach for structural determination, culminating in the 2017 Nobel Prize in Chemistry awarded to Jacques Dubochet, Joachim Frank, and Richard Henderson for their pioneering contributions.
The historical trajectory of Cryo-EM has been marked by significant technological advancements, from the development of vitrification methods to the introduction of direct electron detectors. However, despite these improvements, traditional Cryo-EM workflows remain labor-intensive and time-consuming, with significant bottlenecks in sample preparation, grid handling, and data acquisition processes.
Current Cryo-EM operations typically require extensive manual intervention, leading to inconsistent results, operator fatigue, and limited throughput. The preparation of a single grid can take several hours, while screening and data collection may extend over days or weeks. This operational inefficiency represents a critical limitation in the broader application of Cryo-EM across research and industry.
The global trend in scientific instrumentation is moving decisively toward automation, with robotics playing an increasingly central role in laboratory workflows. This shift is particularly evident in adjacent fields such as X-ray crystallography, where automated sample handling and data collection have become standard practice, dramatically increasing throughput and reproducibility.
The primary objective of Cryo-EM automation and robotics integration is to address these efficiency constraints by developing systems that can perform repetitive tasks with minimal human intervention. Specific goals include reducing sample-to-structure time from weeks to days, increasing grid preparation consistency, minimizing sample waste, and enabling 24/7 operation of expensive Cryo-EM facilities.
Additionally, automation aims to democratize access to Cryo-EM technology by lowering the expertise barrier required for successful implementation. This would expand the user base beyond specialized structural biology laboratories to include pharmaceutical companies, biotechnology firms, and academic institutions with limited prior experience in the field.
The technical objectives extend to developing integrated workflows that seamlessly connect sample preparation, vitrification, grid loading, screening, and data acquisition. These systems must maintain or improve the quality of structural data while significantly increasing the quantity of processable samples, ultimately transforming Cryo-EM from a boutique technique to a high-throughput structural biology platform.
The historical trajectory of Cryo-EM has been marked by significant technological advancements, from the development of vitrification methods to the introduction of direct electron detectors. However, despite these improvements, traditional Cryo-EM workflows remain labor-intensive and time-consuming, with significant bottlenecks in sample preparation, grid handling, and data acquisition processes.
Current Cryo-EM operations typically require extensive manual intervention, leading to inconsistent results, operator fatigue, and limited throughput. The preparation of a single grid can take several hours, while screening and data collection may extend over days or weeks. This operational inefficiency represents a critical limitation in the broader application of Cryo-EM across research and industry.
The global trend in scientific instrumentation is moving decisively toward automation, with robotics playing an increasingly central role in laboratory workflows. This shift is particularly evident in adjacent fields such as X-ray crystallography, where automated sample handling and data collection have become standard practice, dramatically increasing throughput and reproducibility.
The primary objective of Cryo-EM automation and robotics integration is to address these efficiency constraints by developing systems that can perform repetitive tasks with minimal human intervention. Specific goals include reducing sample-to-structure time from weeks to days, increasing grid preparation consistency, minimizing sample waste, and enabling 24/7 operation of expensive Cryo-EM facilities.
Additionally, automation aims to democratize access to Cryo-EM technology by lowering the expertise barrier required for successful implementation. This would expand the user base beyond specialized structural biology laboratories to include pharmaceutical companies, biotechnology firms, and academic institutions with limited prior experience in the field.
The technical objectives extend to developing integrated workflows that seamlessly connect sample preparation, vitrification, grid loading, screening, and data acquisition. These systems must maintain or improve the quality of structural data while significantly increasing the quantity of processable samples, ultimately transforming Cryo-EM from a boutique technique to a high-throughput structural biology platform.
Market Demand Analysis for High-Throughput Cryo-EM
The global market for Cryo-Electron Microscopy (Cryo-EM) has experienced substantial growth in recent years, driven primarily by increasing demand for structural biology research in pharmaceutical development and academic institutions. The market size for Cryo-EM equipment and services was valued at approximately $650 million in 2021 and is projected to reach $1.2 billion by 2026, representing a compound annual growth rate of 13%.
This growth is largely fueled by the pharmaceutical and biotechnology sectors, where high-throughput Cryo-EM has become essential for drug discovery and development processes. These industries require rapid structural determination of complex biomolecules to accelerate therapeutic development timelines. According to industry reports, over 70% of major pharmaceutical companies have either established in-house Cryo-EM facilities or formed partnerships with academic institutions to access this technology.
Academic and research institutions constitute another significant market segment, accounting for approximately 60% of the current Cryo-EM installations worldwide. The demand in this sector is driven by fundamental research in structural biology and the need to remain competitive in securing research grants.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). China, in particular, has demonstrated aggressive growth, increasing its Cryo-EM installations by 200% over the past five years as part of its strategic investment in life sciences research infrastructure.
The demand for high-throughput solutions is particularly acute, as traditional Cryo-EM workflows suffer from significant bottlenecks. Current manual sample preparation and data collection processes can take days to weeks per structure, severely limiting throughput. Market surveys indicate that 85% of Cryo-EM users identify sample preparation automation and robotic handling as their highest priority needs.
Healthcare applications represent an emerging market segment with substantial growth potential. The use of Cryo-EM in precision medicine and diagnostic applications is expected to expand at 18% annually over the next decade, outpacing the overall market growth rate.
Cost remains a significant barrier to wider adoption, with complete Cryo-EM systems typically priced between $5-7 million, plus substantial operational costs. This has created a growing market for shared facilities and contract research organizations offering Cryo-EM services, which is expanding at 15% annually.
Industry analysts predict that automation and robotics innovations that can increase throughput by at least 5-10 fold while reducing operational costs would potentially double the addressable market within three years by making the technology accessible to mid-sized research institutions and biotechnology companies.
This growth is largely fueled by the pharmaceutical and biotechnology sectors, where high-throughput Cryo-EM has become essential for drug discovery and development processes. These industries require rapid structural determination of complex biomolecules to accelerate therapeutic development timelines. According to industry reports, over 70% of major pharmaceutical companies have either established in-house Cryo-EM facilities or formed partnerships with academic institutions to access this technology.
Academic and research institutions constitute another significant market segment, accounting for approximately 60% of the current Cryo-EM installations worldwide. The demand in this sector is driven by fundamental research in structural biology and the need to remain competitive in securing research grants.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). China, in particular, has demonstrated aggressive growth, increasing its Cryo-EM installations by 200% over the past five years as part of its strategic investment in life sciences research infrastructure.
The demand for high-throughput solutions is particularly acute, as traditional Cryo-EM workflows suffer from significant bottlenecks. Current manual sample preparation and data collection processes can take days to weeks per structure, severely limiting throughput. Market surveys indicate that 85% of Cryo-EM users identify sample preparation automation and robotic handling as their highest priority needs.
Healthcare applications represent an emerging market segment with substantial growth potential. The use of Cryo-EM in precision medicine and diagnostic applications is expected to expand at 18% annually over the next decade, outpacing the overall market growth rate.
Cost remains a significant barrier to wider adoption, with complete Cryo-EM systems typically priced between $5-7 million, plus substantial operational costs. This has created a growing market for shared facilities and contract research organizations offering Cryo-EM services, which is expanding at 15% annually.
Industry analysts predict that automation and robotics innovations that can increase throughput by at least 5-10 fold while reducing operational costs would potentially double the addressable market within three years by making the technology accessible to mid-sized research institutions and biotechnology companies.
Current Challenges in Cryo-EM Automation
Despite significant advancements in cryo-electron microscopy (cryo-EM) technology, several critical challenges persist in automation systems that limit throughput and efficiency. The current generation of automated cryo-EM workflows faces substantial bottlenecks in sample preparation, which remains labor-intensive and requires extensive human intervention. Vitrification processes, while partially automated, still demand expert supervision to ensure optimal ice thickness and sample distribution, creating a significant workflow constraint.
Grid handling and transfer systems represent another major challenge, with existing robotics struggling to maintain the required cryogenic conditions consistently during sample transfers between preparation stations and microscopes. Temperature fluctuations during these transfers can introduce ice contamination and sample degradation, compromising structural integrity and data quality.
Data acquisition automation, though considerably advanced, continues to face difficulties in real-time quality assessment. Current systems often collect substantial amounts of data from suboptimal areas, resulting in computational resource waste and extended processing times. The lack of sophisticated feedback mechanisms between data quality assessment and acquisition parameters adjustment represents a significant limitation in maximizing microscope utilization efficiency.
Image processing pipelines present computational bottlenecks, with existing automated workflows unable to effectively prioritize promising particles or terminate unproductive processing paths early. This results in substantial computational overhead and delayed results, particularly for challenging or heterogeneous samples.
Maintenance requirements for automated systems pose additional challenges, with current robotics requiring frequent calibration and alignment procedures that interrupt continuous operation. The complexity of these systems often necessitates specialized technical expertise, limiting their adoption in smaller research facilities.
Integration between different automation components remains problematic, with many facilities utilizing solutions from multiple vendors that lack standardized interfaces. This creates compatibility issues and requires custom integration efforts, resulting in suboptimal system performance and reliability concerns.
User interface complexity presents a barrier to wider adoption, as many current automation systems require significant training and experience to operate effectively. The learning curve associated with these interfaces limits the accessibility of cryo-EM technology to new users and smaller research groups without dedicated microscopy specialists.
Human-machine collaboration models need refinement, as current systems either require too much human intervention or attempt full automation without adequate error detection and recovery mechanisms. Finding the optimal balance between automated operation and expert oversight remains an ongoing challenge in the field.
Grid handling and transfer systems represent another major challenge, with existing robotics struggling to maintain the required cryogenic conditions consistently during sample transfers between preparation stations and microscopes. Temperature fluctuations during these transfers can introduce ice contamination and sample degradation, compromising structural integrity and data quality.
Data acquisition automation, though considerably advanced, continues to face difficulties in real-time quality assessment. Current systems often collect substantial amounts of data from suboptimal areas, resulting in computational resource waste and extended processing times. The lack of sophisticated feedback mechanisms between data quality assessment and acquisition parameters adjustment represents a significant limitation in maximizing microscope utilization efficiency.
Image processing pipelines present computational bottlenecks, with existing automated workflows unable to effectively prioritize promising particles or terminate unproductive processing paths early. This results in substantial computational overhead and delayed results, particularly for challenging or heterogeneous samples.
Maintenance requirements for automated systems pose additional challenges, with current robotics requiring frequent calibration and alignment procedures that interrupt continuous operation. The complexity of these systems often necessitates specialized technical expertise, limiting their adoption in smaller research facilities.
Integration between different automation components remains problematic, with many facilities utilizing solutions from multiple vendors that lack standardized interfaces. This creates compatibility issues and requires custom integration efforts, resulting in suboptimal system performance and reliability concerns.
User interface complexity presents a barrier to wider adoption, as many current automation systems require significant training and experience to operate effectively. The learning curve associated with these interfaces limits the accessibility of cryo-EM technology to new users and smaller research groups without dedicated microscopy specialists.
Human-machine collaboration models need refinement, as current systems either require too much human intervention or attempt full automation without adequate error detection and recovery mechanisms. Finding the optimal balance between automated operation and expert oversight remains an ongoing challenge in the field.
Current Robotic Solutions for Cryo-EM Throughput
01 Automated sample preparation and handling systems
Automated systems for sample preparation and handling significantly improve cryo-EM throughput by reducing manual intervention and standardizing processes. These systems include robotic sample loading, automated grid preparation, and integrated workflows that minimize human error and sample contamination. Advanced automation enables continuous operation with minimal downtime, allowing for higher sample processing rates and more efficient use of microscope time.- Automated sample preparation and handling systems: Automated systems for cryo-EM sample preparation significantly increase throughput by reducing manual handling steps. These systems include robotic sample loading, grid preparation automation, and integrated workflows that minimize human intervention. Such automation reduces preparation time, improves reproducibility, and allows for continuous operation, thereby increasing the number of samples that can be processed in a given timeframe.
- Advanced data acquisition and processing techniques: Enhanced data acquisition methods and processing algorithms improve cryo-EM throughput by optimizing image collection and analysis. These techniques include parallel processing of multiple samples, real-time image analysis, and machine learning approaches for automated particle picking and classification. By reducing the time required for data collection and processing, these advancements allow for faster structure determination and higher sample throughput.
- Hardware improvements for faster imaging: Hardware innovations in cryo-EM technology enhance throughput by improving microscope performance and efficiency. These include advanced electron detectors with higher sensitivity and frame rates, improved stage designs for faster sample exchange, and enhanced cooling systems that maintain sample integrity during extended imaging sessions. Such hardware improvements reduce imaging time per sample and enable continuous operation, significantly increasing overall throughput.
- Integrated workflow solutions: Comprehensive workflow solutions that integrate sample preparation, data acquisition, and analysis into a seamless process substantially improve cryo-EM throughput. These integrated systems coordinate multiple steps of the cryo-EM pipeline, reducing bottlenecks and idle time between processes. By optimizing the entire workflow from sample to structure, these solutions maximize efficiency and enable higher throughput operations in structural biology research.
- High-throughput screening applications: Specialized cryo-EM applications designed for high-throughput screening enable rapid analysis of multiple samples or conditions. These approaches include miniaturized sample formats, parallel imaging strategies, and automated comparative analysis of structural data. Such screening applications are particularly valuable for drug discovery, protein engineering, and structural genomics projects where large numbers of samples need to be analyzed efficiently.
02 High-speed data acquisition and processing techniques
Enhanced data acquisition and processing methods dramatically increase cryo-EM throughput by optimizing image capture rates and computational analysis. These techniques include parallel processing algorithms, GPU-accelerated image reconstruction, and real-time data analysis that reduce the time required for structure determination. Advanced detector technologies with improved sensitivity and frame rates allow for faster image collection while maintaining high resolution.Expand Specific Solutions03 Multi-grid and batch processing methodologies
Multi-grid and batch processing approaches enable simultaneous analysis of multiple samples, substantially increasing cryo-EM throughput. These methodologies incorporate specialized sample holders that accommodate multiple grids, coordinated imaging protocols for sequential analysis, and batch processing software that manages large datasets efficiently. By reducing the time spent on individual sample loading and setup, these approaches maximize microscope utilization and overall productivity.Expand Specific Solutions04 Intelligent screening and targeting systems
Intelligent screening and targeting systems enhance cryo-EM throughput by automatically identifying optimal imaging areas and focusing on the most promising samples. These systems employ machine learning algorithms to recognize high-quality regions on grids, automate focusing and alignment procedures, and prioritize data collection from the most informative areas. By reducing time spent on manual screening and targeting, these systems significantly increase the efficiency of microscope usage.Expand Specific Solutions05 Integrated workflow management platforms
Integrated workflow management platforms optimize cryo-EM throughput by coordinating all aspects of the experimental process from sample preparation to structure determination. These platforms incorporate scheduling algorithms, resource allocation tools, and progress tracking systems that minimize downtime between experiments. By providing comprehensive oversight of the entire workflow, these platforms identify bottlenecks, suggest process improvements, and ensure efficient utilization of equipment and personnel.Expand Specific Solutions
Leading Companies in Cryo-EM Automation Industry
The Cryo-EM throughput improvement market is currently in a growth phase, with increasing adoption across pharmaceutical and research sectors. The market size is expanding rapidly, driven by demand for higher efficiency in structural biology research. Technologically, the field is advancing from early automation to sophisticated robotics integration. Companies like New York Structural Biology Center and F. Hoffmann-La Roche are leading in research applications, while robotics specialists such as YASKAWA Electric, ABB Group, and Toyota Motor Corp. provide automation solutions. Emerging players like DENSsolutions are developing specialized tools for sample preparation and handling. The convergence of life sciences expertise with industrial automation capabilities is creating a competitive landscape where cross-industry partnerships are becoming increasingly valuable.
YASKAWA Electric Corp.
Technical Solution: YASKAWA Electric has applied their extensive robotics expertise to develop specialized automation solutions for cryo-EM facilities. Their system centers around high-precision, multi-axis robotic arms specifically designed to handle the delicate and temperature-sensitive requirements of cryo-EM sample preparation and transfer. YASKAWA's solution features custom end effectors capable of manipulating electron microscopy grids with nanometer precision while maintaining the critical cryogenic temperatures required for sample integrity. The company has integrated advanced motion control algorithms that minimize vibration during critical operations, ensuring sample quality is preserved throughout the workflow. Their automation platform includes sophisticated environmental monitoring systems that continuously track temperature, humidity, and contaminant levels, automatically adjusting handling parameters to maintain optimal conditions.
Strengths: Leverages decades of industrial robotics expertise to deliver exceptional reliability and precision; modular design allows for customization to specific facility requirements and gradual implementation. Weaknesses: Primarily focused on the mechanical aspects of automation rather than offering a complete end-to-end solution; integration with existing microscope control software may require additional development.
New York Structural Biology Center, Inc.
Technical Solution: The New York Structural Biology Center (NYSBC) has developed an integrated automation system for their cryo-EM workflow that combines robotic sample handling with AI-driven data acquisition. Their solution features a robotic arm system that automates grid transfer between storage, preparation stations, and microscopes, minimizing human intervention and sample exposure to atmospheric conditions. The system incorporates machine learning algorithms for automated targeting and focusing, which can identify optimal imaging areas on grids and adjust focus parameters in real-time. NYSBC's platform includes a centralized laboratory information management system (LIMS) that coordinates sample tracking, microscope scheduling, and data management, creating a seamless pipeline from sample preparation to data collection.
Strengths: Significantly reduces hands-on time for researchers, minimizes human error in delicate sample handling procedures, and increases microscope utilization by enabling 24/7 operation. Weaknesses: Requires substantial initial investment in both hardware and software integration, and the system may need frequent calibration to maintain precision in sample handling.
Key Patents and Innovations in Cryo-EM Automation
Cryogenic electron microscopy fully automated acquisition for single particle and tomography
PatentPendingUS20240038488A1
Innovation
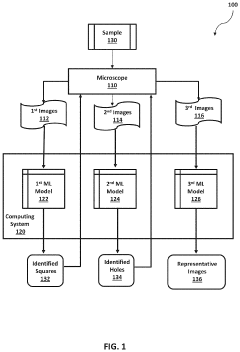
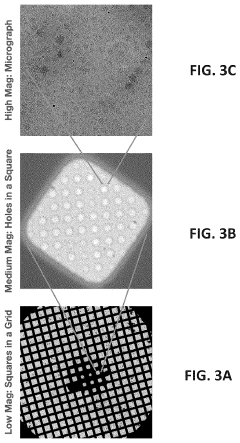
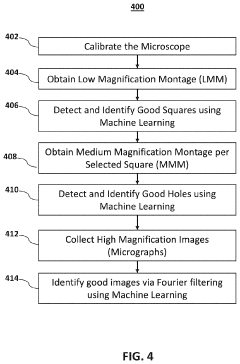
- The implementation of machine learning models for automated data acquisition and analysis, including training models to detect high-quality grid squares and holes, and performing Fourier transformations to identify suitable micrographs for 3D modeling, thereby reducing manual intervention and enhancing data processing efficiency.
Automating cryo-electron microscopy data collection
PatentWO2024229329A1
Innovation
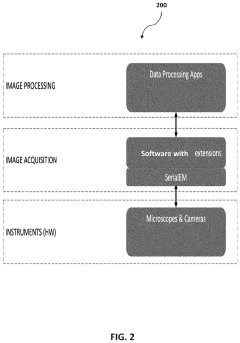
- A software pipeline utilizing machine learning models for automated navigation of cryo-EM grids, including square and hole localization, scoring, and on-the-fly learning, to determine high-quality targeting locations without human input, using pretrained models and active learning techniques like Gaussian Process regression.
Cost-Benefit Analysis of Automated Cryo-EM Systems
The implementation of automated systems in cryo-electron microscopy represents a significant financial investment that requires thorough cost-benefit analysis. Initial capital expenditure for robotic sample handling systems, automated data collection software, and integrated workflow platforms typically ranges from $500,000 to $2 million, depending on the sophistication and scale of automation desired.
Operating costs must also be considered, including maintenance contracts (approximately $50,000-100,000 annually), software licensing fees, and specialized training for staff. However, these expenses should be weighed against quantifiable benefits in throughput enhancement. Automated systems can increase sample processing capacity by 300-500%, with some facilities reporting the ability to screen up to 200 grids per day compared to 40-50 with manual methods.
Labor cost reduction represents another significant advantage, with automated systems requiring approximately 60% less hands-on time from highly skilled technicians. This translates to annual savings of $100,000-150,000 in personnel costs for a medium-sized facility, allowing reallocation of human resources to more complex analytical tasks.
The return on investment timeline typically shows that most facilities achieve financial break-even within 2-3 years of implementing automated cryo-EM systems. Facilities processing more than 1,000 samples annually tend to realize ROI more quickly, often within 18 months.
Quality improvements must also factor into the analysis, as automation reduces human error and improves reproducibility. Studies indicate that automated systems achieve 25-30% higher consistency in ice thickness and particle distribution, leading to higher resolution structures and fewer failed experiments. This reduction in sample wastage and repeated experiments represents significant cost savings, estimated at $50,000-75,000 annually for an active research facility.
Scalability considerations reveal that modular automated systems allow for phased implementation, enabling facilities to distribute costs over multiple budget cycles while incrementally improving capabilities. This approach typically reduces initial capital requirements by 40-50% compared to comprehensive one-time installations.
When evaluating vendor options, total cost of ownership calculations should include not only purchase price but also compatibility with existing infrastructure, upgrade pathways, and vendor support quality. Facilities report 15-25% variation in five-year total cost of ownership among leading vendors, highlighting the importance of comprehensive evaluation beyond initial purchase price.
Operating costs must also be considered, including maintenance contracts (approximately $50,000-100,000 annually), software licensing fees, and specialized training for staff. However, these expenses should be weighed against quantifiable benefits in throughput enhancement. Automated systems can increase sample processing capacity by 300-500%, with some facilities reporting the ability to screen up to 200 grids per day compared to 40-50 with manual methods.
Labor cost reduction represents another significant advantage, with automated systems requiring approximately 60% less hands-on time from highly skilled technicians. This translates to annual savings of $100,000-150,000 in personnel costs for a medium-sized facility, allowing reallocation of human resources to more complex analytical tasks.
The return on investment timeline typically shows that most facilities achieve financial break-even within 2-3 years of implementing automated cryo-EM systems. Facilities processing more than 1,000 samples annually tend to realize ROI more quickly, often within 18 months.
Quality improvements must also factor into the analysis, as automation reduces human error and improves reproducibility. Studies indicate that automated systems achieve 25-30% higher consistency in ice thickness and particle distribution, leading to higher resolution structures and fewer failed experiments. This reduction in sample wastage and repeated experiments represents significant cost savings, estimated at $50,000-75,000 annually for an active research facility.
Scalability considerations reveal that modular automated systems allow for phased implementation, enabling facilities to distribute costs over multiple budget cycles while incrementally improving capabilities. This approach typically reduces initial capital requirements by 40-50% compared to comprehensive one-time installations.
When evaluating vendor options, total cost of ownership calculations should include not only purchase price but also compatibility with existing infrastructure, upgrade pathways, and vendor support quality. Facilities report 15-25% variation in five-year total cost of ownership among leading vendors, highlighting the importance of comprehensive evaluation beyond initial purchase price.
Integration with AI and Machine Learning Workflows
The integration of artificial intelligence and machine learning workflows with cryo-electron microscopy (cryo-EM) represents a transformative approach to addressing throughput limitations. Recent advancements in deep learning algorithms have enabled automated particle picking, classification, and image processing, significantly reducing the time required for data analysis from weeks to days or even hours.
Machine learning models, particularly convolutional neural networks (CNNs), have demonstrated remarkable efficiency in identifying and classifying particles from noisy cryo-EM micrographs. Systems like cryoSPARC, RELION, and DeepPicker leverage these capabilities to perform real-time data processing as images are being collected, eliminating bottlenecks in the analytical pipeline.
Predictive maintenance algorithms are being implemented to monitor robotic systems and automate troubleshooting processes. These AI-driven systems can detect potential failures before they occur, schedule maintenance during non-critical periods, and optimize instrument performance parameters based on historical data patterns, thereby maximizing operational uptime.
Decision support systems powered by reinforcement learning are emerging as valuable tools for optimizing sample preparation and imaging conditions. These systems continuously learn from previous experiments, suggesting optimal grid preparation parameters, blotting times, and imaging settings based on sample characteristics and desired resolution outcomes.
Natural language processing (NLP) interfaces are being developed to facilitate seamless human-robot interaction, allowing researchers to issue complex commands through intuitive verbal instructions rather than programming sequences manually. This democratizes access to advanced cryo-EM technology for researchers without specialized training in robotics or automation.
Cloud-based AI platforms are enabling distributed processing of cryo-EM data across multiple computing resources, creating virtual processing pipelines that can scale dynamically based on workload demands. These systems integrate with laboratory information management systems (LIMS) to track samples, experiments, and results throughout the entire workflow.
Edge computing implementations are bringing AI capabilities directly to cryo-EM instruments, enabling real-time decision-making without the latency associated with cloud processing. This approach is particularly valuable for adaptive data collection strategies where imaging parameters need to be adjusted based on initial results.
The convergence of robotics, automation, and AI/ML workflows in cryo-EM is creating intelligent systems capable of autonomous operation with minimal human intervention, potentially increasing throughput by an order of magnitude while simultaneously improving data quality and experimental reproducibility.
Machine learning models, particularly convolutional neural networks (CNNs), have demonstrated remarkable efficiency in identifying and classifying particles from noisy cryo-EM micrographs. Systems like cryoSPARC, RELION, and DeepPicker leverage these capabilities to perform real-time data processing as images are being collected, eliminating bottlenecks in the analytical pipeline.
Predictive maintenance algorithms are being implemented to monitor robotic systems and automate troubleshooting processes. These AI-driven systems can detect potential failures before they occur, schedule maintenance during non-critical periods, and optimize instrument performance parameters based on historical data patterns, thereby maximizing operational uptime.
Decision support systems powered by reinforcement learning are emerging as valuable tools for optimizing sample preparation and imaging conditions. These systems continuously learn from previous experiments, suggesting optimal grid preparation parameters, blotting times, and imaging settings based on sample characteristics and desired resolution outcomes.
Natural language processing (NLP) interfaces are being developed to facilitate seamless human-robot interaction, allowing researchers to issue complex commands through intuitive verbal instructions rather than programming sequences manually. This democratizes access to advanced cryo-EM technology for researchers without specialized training in robotics or automation.
Cloud-based AI platforms are enabling distributed processing of cryo-EM data across multiple computing resources, creating virtual processing pipelines that can scale dynamically based on workload demands. These systems integrate with laboratory information management systems (LIMS) to track samples, experiments, and results throughout the entire workflow.
Edge computing implementations are bringing AI capabilities directly to cryo-EM instruments, enabling real-time decision-making without the latency associated with cloud processing. This approach is particularly valuable for adaptive data collection strategies where imaging parameters need to be adjusted based on initial results.
The convergence of robotics, automation, and AI/ML workflows in cryo-EM is creating intelligent systems capable of autonomous operation with minimal human intervention, potentially increasing throughput by an order of magnitude while simultaneously improving data quality and experimental reproducibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!