Cryo-EM For Nanoporous Polymer Characterization
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Technology Evolution and Objectives
Cryo-electron microscopy (Cryo-EM) has emerged as a revolutionary technique in the field of structural biology, enabling researchers to visualize biological macromolecules at near-atomic resolution. The evolution of this technology began in the 1970s with the development of electron crystallography, but it wasn't until the 1980s that Jacques Dubochet and colleagues pioneered the vitrification process, allowing biological samples to be preserved in their native state without the formation of ice crystals.
The technological breakthrough that truly transformed Cryo-EM came in the 2010s with the development of direct electron detectors, which significantly improved signal-to-noise ratios and enabled higher resolution imaging. This advancement, coupled with sophisticated image processing algorithms, led to the "resolution revolution" that earned Jacques Dubochet, Joachim Frank, and Richard Henderson the Nobel Prize in Chemistry in 2017.
While Cryo-EM has been predominantly applied to biological macromolecules, its application to nanoporous polymer characterization represents an emerging frontier with significant potential. Nanoporous polymers, with their complex three-dimensional structures and diverse pore architectures, present unique characterization challenges that conventional techniques struggle to address comprehensively.
The primary objective of applying Cryo-EM to nanoporous polymer characterization is to achieve high-resolution, three-dimensional visualization of pore structures, connectivity, and spatial distribution within polymer matrices. This would enable researchers to establish structure-property relationships that are crucial for optimizing these materials for applications in catalysis, separation, energy storage, and drug delivery.
Current technological trends indicate a growing interest in adapting Cryo-EM techniques specifically for synthetic materials. Innovations in sample preparation protocols, contrast enhancement methods, and specialized image processing algorithms tailored for non-biological specimens are actively being developed. The integration of machine learning approaches for image analysis and reconstruction is also accelerating progress in this field.
Looking forward, the evolution of Cryo-EM for nanoporous polymer characterization aims to achieve sub-nanometer resolution imaging of polymer networks, real-time observation of dynamic processes within pores, and quantitative analysis of pore characteristics across multiple length scales. These advancements would bridge the gap between molecular-level design and macroscopic performance of nanoporous polymer materials.
The convergence of Cryo-EM with complementary techniques such as tomography, spectroscopy, and computational modeling represents another important trend, enabling multi-modal characterization approaches that provide more comprehensive insights into the structure and function of nanoporous polymers.
The technological breakthrough that truly transformed Cryo-EM came in the 2010s with the development of direct electron detectors, which significantly improved signal-to-noise ratios and enabled higher resolution imaging. This advancement, coupled with sophisticated image processing algorithms, led to the "resolution revolution" that earned Jacques Dubochet, Joachim Frank, and Richard Henderson the Nobel Prize in Chemistry in 2017.
While Cryo-EM has been predominantly applied to biological macromolecules, its application to nanoporous polymer characterization represents an emerging frontier with significant potential. Nanoporous polymers, with their complex three-dimensional structures and diverse pore architectures, present unique characterization challenges that conventional techniques struggle to address comprehensively.
The primary objective of applying Cryo-EM to nanoporous polymer characterization is to achieve high-resolution, three-dimensional visualization of pore structures, connectivity, and spatial distribution within polymer matrices. This would enable researchers to establish structure-property relationships that are crucial for optimizing these materials for applications in catalysis, separation, energy storage, and drug delivery.
Current technological trends indicate a growing interest in adapting Cryo-EM techniques specifically for synthetic materials. Innovations in sample preparation protocols, contrast enhancement methods, and specialized image processing algorithms tailored for non-biological specimens are actively being developed. The integration of machine learning approaches for image analysis and reconstruction is also accelerating progress in this field.
Looking forward, the evolution of Cryo-EM for nanoporous polymer characterization aims to achieve sub-nanometer resolution imaging of polymer networks, real-time observation of dynamic processes within pores, and quantitative analysis of pore characteristics across multiple length scales. These advancements would bridge the gap between molecular-level design and macroscopic performance of nanoporous polymer materials.
The convergence of Cryo-EM with complementary techniques such as tomography, spectroscopy, and computational modeling represents another important trend, enabling multi-modal characterization approaches that provide more comprehensive insights into the structure and function of nanoporous polymers.
Market Applications for Nanoporous Polymer Analysis
The market for nanoporous polymer analysis is experiencing significant growth driven by increasing demand across multiple industries. Cryo-EM technology for characterizing nanoporous polymers addresses critical needs in pharmaceutical development, where precise understanding of drug delivery systems is essential for controlled release formulations. The ability to visualize nanopore structures at near-atomic resolution enables pharmaceutical companies to optimize drug encapsulation efficiency and release kinetics, potentially reducing development cycles by 30-40% compared to traditional characterization methods.
In the energy sector, nanoporous polymer membranes are revolutionizing battery technology and hydrogen storage solutions. The market for advanced battery separators alone is projected to reach $7.3 billion by 2027, with nanoporous polymers representing a key growth segment. Cryo-EM characterization provides crucial insights into ion transport pathways and degradation mechanisms, directly impacting battery performance and longevity.
Environmental applications represent another substantial market opportunity, particularly in water purification and air filtration. The global water treatment membrane market, heavily reliant on nanoporous polymers, continues to expand as water scarcity concerns intensify worldwide. Cryo-EM analysis enables manufacturers to develop more efficient filtration membranes with precisely controlled pore size distributions, significantly improving contaminant removal rates while reducing energy consumption.
The semiconductor industry has emerged as a high-value application area, where nanoporous low-k dielectric materials are essential for next-generation microchips. As chip manufacturers pursue increasingly smaller node sizes, the characterization of nanoporous structures becomes critical for maintaining signal integrity and reducing crosstalk. This segment represents a premium market where the high cost of Cryo-EM analysis is justified by the substantial value it delivers in process optimization.
Textile and consumer goods industries are also adopting nanoporous polymers for advanced functional materials, including breathable waterproof fabrics and controlled-release packaging. These applications benefit from Cryo-EM's ability to correlate structure with performance characteristics, driving innovation in consumer products.
The biomedical field presents perhaps the most promising growth opportunity, with applications ranging from tissue engineering scaffolds to biosensors. The global biomaterials market, valued at $106.5 billion in 2022, is increasingly incorporating nanoporous polymers to enhance biocompatibility and functionality. Cryo-EM characterization provides essential insights into cell-material interactions at the nanoscale, accelerating the development of next-generation medical devices and implants.
In the energy sector, nanoporous polymer membranes are revolutionizing battery technology and hydrogen storage solutions. The market for advanced battery separators alone is projected to reach $7.3 billion by 2027, with nanoporous polymers representing a key growth segment. Cryo-EM characterization provides crucial insights into ion transport pathways and degradation mechanisms, directly impacting battery performance and longevity.
Environmental applications represent another substantial market opportunity, particularly in water purification and air filtration. The global water treatment membrane market, heavily reliant on nanoporous polymers, continues to expand as water scarcity concerns intensify worldwide. Cryo-EM analysis enables manufacturers to develop more efficient filtration membranes with precisely controlled pore size distributions, significantly improving contaminant removal rates while reducing energy consumption.
The semiconductor industry has emerged as a high-value application area, where nanoporous low-k dielectric materials are essential for next-generation microchips. As chip manufacturers pursue increasingly smaller node sizes, the characterization of nanoporous structures becomes critical for maintaining signal integrity and reducing crosstalk. This segment represents a premium market where the high cost of Cryo-EM analysis is justified by the substantial value it delivers in process optimization.
Textile and consumer goods industries are also adopting nanoporous polymers for advanced functional materials, including breathable waterproof fabrics and controlled-release packaging. These applications benefit from Cryo-EM's ability to correlate structure with performance characteristics, driving innovation in consumer products.
The biomedical field presents perhaps the most promising growth opportunity, with applications ranging from tissue engineering scaffolds to biosensors. The global biomaterials market, valued at $106.5 billion in 2022, is increasingly incorporating nanoporous polymers to enhance biocompatibility and functionality. Cryo-EM characterization provides essential insights into cell-material interactions at the nanoscale, accelerating the development of next-generation medical devices and implants.
Current Limitations in Nanoporous Material Imaging
Despite significant advancements in imaging technologies, current methods for characterizing nanoporous polymers face substantial limitations that impede comprehensive structural analysis. Conventional techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) provide high-resolution surface imaging but struggle to capture the three-dimensional internal architecture of nanoporous materials without destructive sample preparation. This preparation often alters the native structure of delicate polymer networks, leading to artifacts and misinterpretation of data.
Resolution constraints represent another significant challenge in nanoporous polymer characterization. While atomic force microscopy (AFM) offers nanometer-scale resolution for surface topography, it cannot effectively probe the internal pore networks that define functionality in these materials. Similarly, gas adsorption techniques provide valuable information about pore size distribution and surface area but fail to reveal spatial arrangements and interconnectivity of pores.
Sample preparation for nanoporous polymers presents unique difficulties across imaging platforms. Hydrated polymers, particularly those used in biomedical applications, suffer from dehydration artifacts during conventional imaging processes. Staining techniques employed to enhance contrast often introduce chemical modifications that alter the native polymer structure, while sectioning methods can collapse delicate pore networks.
Contrast generation remains problematic for polymer materials due to their predominantly carbon-based composition, resulting in poor natural contrast in electron microscopy. This limitation is particularly acute when attempting to distinguish between the polymer matrix and void spaces in nanoporous structures, as both consist primarily of light elements with similar electron densities.
Dynamic processes within nanoporous polymers, such as swelling, diffusion, and responsive behavior to environmental stimuli, are difficult to capture with current static imaging approaches. Real-time visualization of these phenomena requires specialized environmental chambers that are not widely available or compatible with high-resolution imaging techniques.
Quantitative analysis of three-dimensional pore networks presents computational challenges, as reconstructing complete pore architectures from limited imaging data requires sophisticated algorithms and significant computational resources. The heterogeneity inherent in many nanoporous polymer systems further complicates this analysis, as statistical representations must account for variations across different spatial scales.
These limitations collectively hinder the development and optimization of nanoporous polymers for applications ranging from filtration membranes to drug delivery systems, highlighting the urgent need for advanced characterization methodologies that can overcome these constraints.
Resolution constraints represent another significant challenge in nanoporous polymer characterization. While atomic force microscopy (AFM) offers nanometer-scale resolution for surface topography, it cannot effectively probe the internal pore networks that define functionality in these materials. Similarly, gas adsorption techniques provide valuable information about pore size distribution and surface area but fail to reveal spatial arrangements and interconnectivity of pores.
Sample preparation for nanoporous polymers presents unique difficulties across imaging platforms. Hydrated polymers, particularly those used in biomedical applications, suffer from dehydration artifacts during conventional imaging processes. Staining techniques employed to enhance contrast often introduce chemical modifications that alter the native polymer structure, while sectioning methods can collapse delicate pore networks.
Contrast generation remains problematic for polymer materials due to their predominantly carbon-based composition, resulting in poor natural contrast in electron microscopy. This limitation is particularly acute when attempting to distinguish between the polymer matrix and void spaces in nanoporous structures, as both consist primarily of light elements with similar electron densities.
Dynamic processes within nanoporous polymers, such as swelling, diffusion, and responsive behavior to environmental stimuli, are difficult to capture with current static imaging approaches. Real-time visualization of these phenomena requires specialized environmental chambers that are not widely available or compatible with high-resolution imaging techniques.
Quantitative analysis of three-dimensional pore networks presents computational challenges, as reconstructing complete pore architectures from limited imaging data requires sophisticated algorithms and significant computational resources. The heterogeneity inherent in many nanoporous polymer systems further complicates this analysis, as statistical representations must account for variations across different spatial scales.
These limitations collectively hinder the development and optimization of nanoporous polymers for applications ranging from filtration membranes to drug delivery systems, highlighting the urgent need for advanced characterization methodologies that can overcome these constraints.
Established Cryo-EM Protocols for Polymer Characterization
01 Sample preparation techniques for Cryo-EM
Various sample preparation methods are crucial for successful cryo-electron microscopy characterization. These techniques include vitrification processes that rapidly freeze specimens to preserve their native structure, grid preparation protocols that optimize specimen distribution, and methods to prevent ice contamination. Advanced preparation approaches enable the visualization of biological macromolecules in their near-native states without the need for crystallization, allowing for structural determination of complex biomolecules and assemblies.- Sample preparation techniques for Cryo-EM: Various sample preparation methods are crucial for successful cryo-electron microscopy characterization. These techniques include vitrification processes that rapidly freeze specimens to preserve their native structure, grid preparation protocols that optimize specimen distribution, and methods to prevent ice contamination. Advanced preparation approaches enable the visualization of biological macromolecules in their near-native states without the need for crystallization, allowing for high-resolution structural analysis of complex biomolecules and assemblies.
- Image processing and 3D reconstruction methods: Sophisticated computational methods are essential for processing cryo-EM data and reconstructing three-dimensional structures. These include particle picking algorithms to identify individual molecules in micrographs, motion correction techniques to account for beam-induced movement, and classification approaches to sort heterogeneous particle populations. Advanced reconstruction algorithms integrate multiple 2D projections to generate 3D density maps, while refinement procedures enhance resolution by iteratively improving alignment parameters and addressing microscope aberrations.
- Hardware innovations for Cryo-EM characterization: Technological advancements in cryo-EM hardware have significantly improved imaging capabilities. These innovations include direct electron detectors with enhanced sensitivity and frame rates, phase plates that increase contrast for biological specimens, automated sample loading systems for high-throughput analysis, and improved electron sources for more coherent beams. Energy filters and aberration correctors further enhance image quality by reducing noise and distortion, enabling atomic-resolution characterization of biological and materials science specimens.
- Applications in structural biology and drug discovery: Cryo-EM has revolutionized structural biology and pharmaceutical research by enabling the visualization of complex biological structures at near-atomic resolution. This technique allows researchers to characterize protein-ligand interactions, membrane proteins, large macromolecular assemblies, and conformational states that were previously inaccessible. The structural insights gained from cryo-EM facilitate rational drug design by revealing binding sites and mechanisms of action, accelerating the development of therapeutics for various diseases including viral infections, cancer, and neurodegenerative disorders.
- Integration with complementary analytical techniques: Combining cryo-EM with other analytical methods creates powerful hybrid approaches for comprehensive structural characterization. Integration with mass spectrometry provides compositional information alongside structural data, while correlative light and electron microscopy enables targeted imaging of specific cellular components. Computational modeling and molecular dynamics simulations complement cryo-EM by predicting dynamic behaviors and filling in missing information. These integrated approaches yield more complete understanding of molecular structures and functions across multiple spatial and temporal scales.
02 Image processing and 3D reconstruction methods
Sophisticated computational methods are essential for processing cryo-EM data and reconstructing three-dimensional structures. These include particle picking algorithms, motion correction techniques, contrast transfer function estimation, and classification methods to sort heterogeneous particles. Advanced reconstruction algorithms enable high-resolution structure determination from multiple 2D projections, while refinement techniques improve the resolution of the final 3D models, allowing researchers to visualize molecular structures at near-atomic resolution.Expand Specific Solutions03 Hardware innovations for Cryo-EM
Technological advancements in cryo-EM hardware have significantly improved imaging capabilities. These innovations include direct electron detectors with enhanced sensitivity and speed, phase plates that improve contrast without defocusing, automated data collection systems, and stable specimen stages that minimize drift during imaging. Cold field emission guns and energy filters further enhance resolution by providing more coherent electron beams and reducing inelastic scattering, enabling visualization of biological structures at unprecedented resolutions.Expand Specific Solutions04 Applications in structural biology and drug discovery
Cryo-EM has revolutionized structural biology and pharmaceutical research by enabling the visualization of complex biological assemblies in their native states. This technique allows for the characterization of membrane proteins, large macromolecular complexes, and dynamic molecular interactions that are challenging to study using other methods. In drug discovery, cryo-EM facilitates structure-based drug design by revealing binding sites and conformational changes upon ligand binding, accelerating the development of therapeutics targeting previously undruggable proteins.Expand Specific Solutions05 Integration with complementary techniques
Combining cryo-EM with other analytical methods enhances structural characterization capabilities. Hybrid approaches integrating cryo-EM with X-ray crystallography, NMR spectroscopy, mass spectrometry, or computational modeling provide comprehensive structural insights. Correlative light and electron microscopy (CLEM) allows for targeted imaging of specific cellular components, while time-resolved cryo-EM captures structural intermediates during biological processes. These integrated approaches overcome limitations of individual techniques and provide multi-scale structural information from atomic to cellular levels.Expand Specific Solutions
Leading Research Groups and Commercial Entities
The cryo-electron microscopy (cryo-EM) market for nanoporous polymer characterization is in its growth phase, with increasing adoption across materials science and polymer research. The market size is expanding as researchers recognize cryo-EM's unique capabilities for visualizing nanoporous structures with minimal sample damage. Technologically, the field is advancing rapidly but remains specialized, with key players demonstrating varying levels of expertise. Leading research institutions like The Francis Crick Institute, Max Planck Society, and multiple Chinese universities (Tsinghua, Southeast) are driving academic innovation, while commercial entities such as FEI Co., Quantifoil Micro Tools, and DENSsolutions provide essential instrumentation and sample preparation technologies. Pharmaceutical companies like Sanofi are exploring applications in drug delivery systems, indicating growing industrial interest in this specialized characterization technique.
FEI Co.
Technical Solution: FEI Co. has developed advanced cryo-electron microscopy (cryo-EM) solutions specifically optimized for nanoporous polymer characterization. Their technology combines high-resolution imaging capabilities with specialized sample preparation techniques to preserve the delicate structure of nanoporous polymers during analysis. FEI's Titan Krios cryo-EM platform features direct electron detectors with single-electron sensitivity and automated data collection workflows that enable visualization of nanoporous polymer structures at near-atomic resolution (2-3 Å). The company has implemented phase plate technology to enhance contrast for beam-sensitive polymer samples without increasing electron dose, and their energy-filtered TEM capabilities allow for elemental mapping within nanoporous polymer frameworks. FEI's workflow includes vitrification protocols specifically designed to prevent structural collapse of nanoporous polymers during sample preparation, maintaining the native pore architecture for accurate characterization[1][3].
Strengths: Industry-leading resolution capabilities for visualizing nanoporous structures; comprehensive workflow from sample preparation to analysis; advanced phase contrast technology for beam-sensitive materials. Weaknesses: High equipment costs limit accessibility; requires significant expertise for operation and data interpretation; sample preparation remains challenging for certain polymer types.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to cryo-EM for nanoporous polymer characterization centers on their pioneering work in low-dose imaging techniques and advanced computational methods. Their research teams have developed specialized protocols that minimize beam damage while maximizing structural information obtained from nanoporous polymer samples. Harvard's methodology incorporates machine learning algorithms for image processing that can extract meaningful structural data from noisy cryo-EM micrographs of polymer networks. They have established correlative microscopy workflows that combine cryo-EM with complementary techniques such as atomic force microscopy and small-angle X-ray scattering to provide comprehensive characterization of pore size distribution, connectivity, and surface chemistry. Harvard researchers have also developed in situ environmental chambers that allow for direct observation of dynamic processes within nanoporous polymers, such as swelling, degradation, or guest molecule interactions under controlled conditions[2][5]. Their approach emphasizes quantitative analysis of pore morphology and connectivity to establish structure-property relationships.
Strengths: Cutting-edge computational methods for image analysis; multidisciplinary approach combining complementary techniques; focus on structure-property relationships for functional understanding. Weaknesses: Research-oriented approach may lack standardization needed for industrial applications; techniques often require specialized expertise beyond standard cryo-EM operations; methods may be optimized for specific polymer systems rather than broadly applicable.
Breakthrough Techniques in Nanoscale Structural Analysis
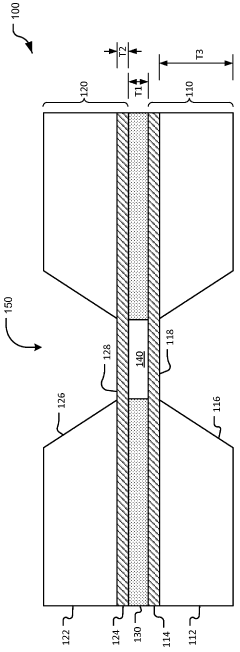
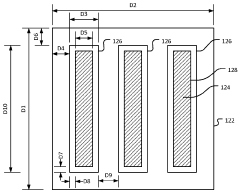
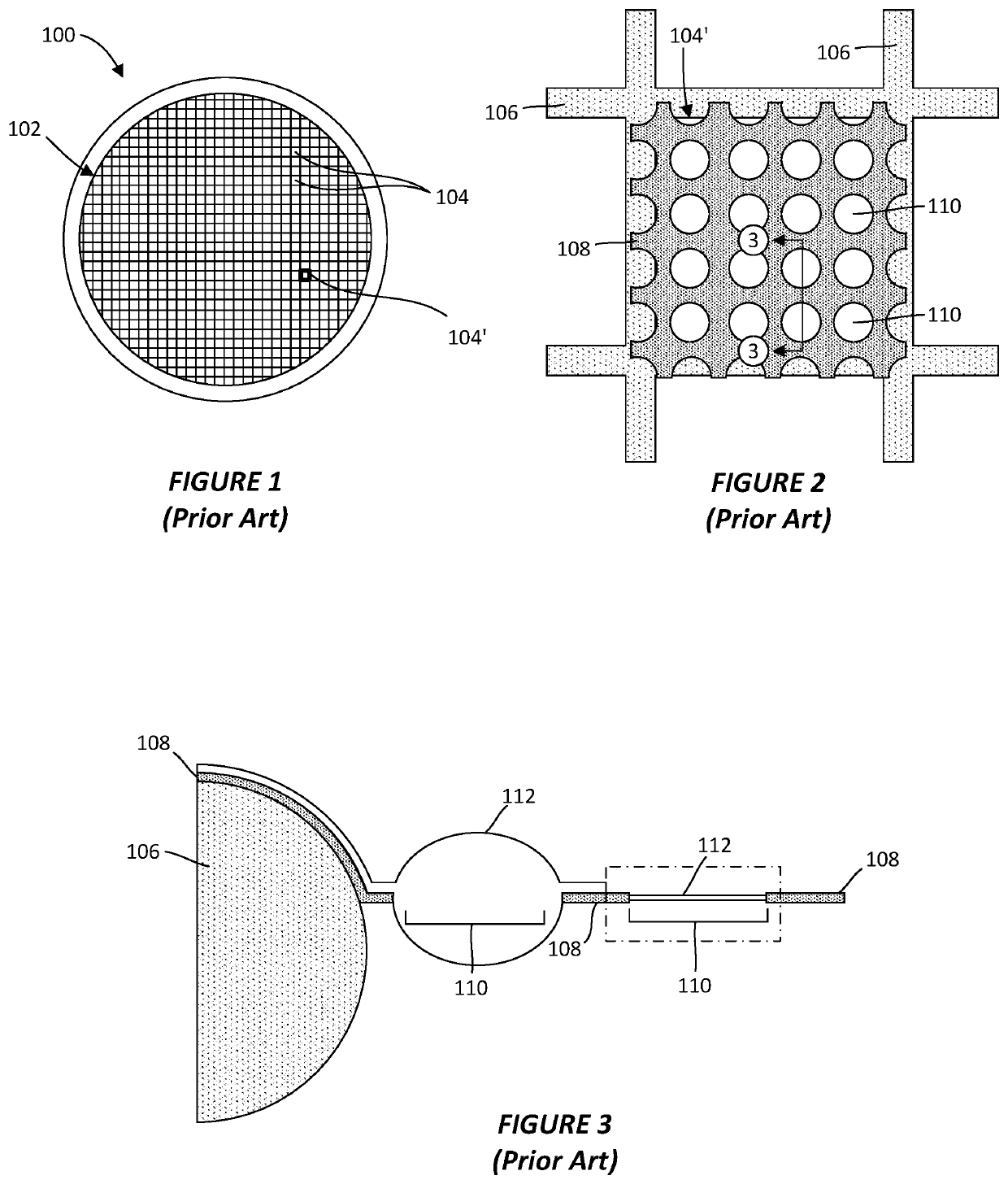
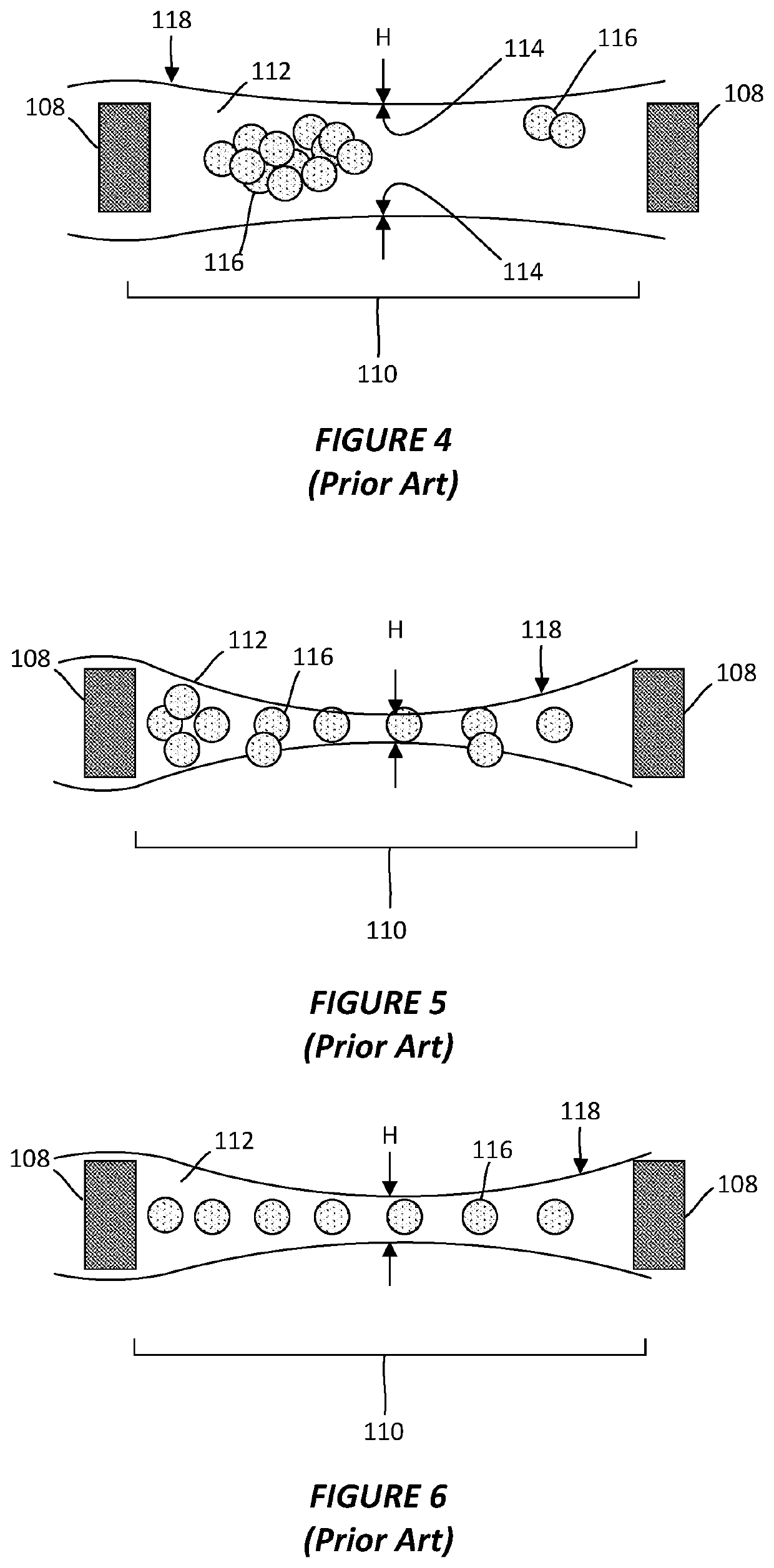
Thin-ice grid assembly for CRYO-electron microscopy
PatentWO2015134575A1
Innovation
- A thin-ice grid assembly is developed, comprising two electron-transparent support members with a rigid spacer layer, allowing precise control of ice thickness between them, enabling consistent vitrification and improved imaging conditions.
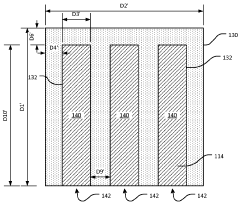
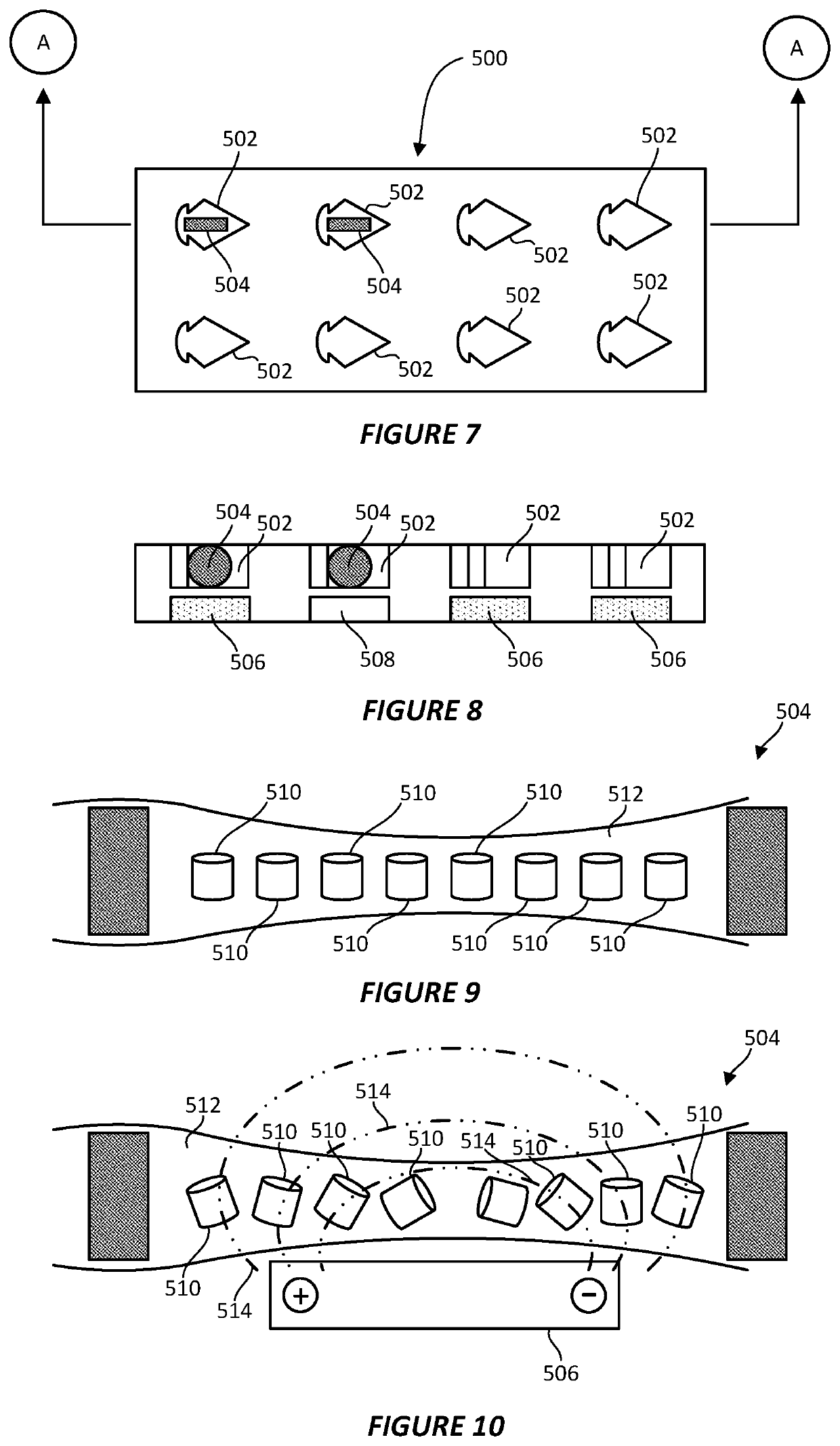
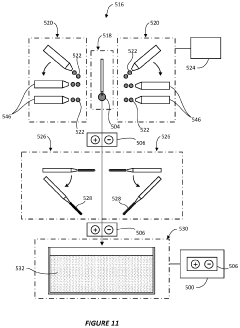
System and method for preparing CRYO-em grids
PatentActiveUS20200363301A1
Innovation
- A method and apparatus that utilize an electromagnetic field (EMF) to re-orient proteins and a system with a sample shaping element and cryogenic vitrifying element to deposit, thin, and vitrify samples on cryo-EM grids, allowing for precise control over sample thickness and orientation, and incorporating a storage device to further randomize protein orientations post-vitrification.
Sample Preparation Challenges and Innovations
Sample preparation represents one of the most critical challenges in applying cryo-electron microscopy (cryo-EM) to nanoporous polymer characterization. The intrinsic complexity of polymer structures, combined with their sensitivity to electron beam damage, necessitates innovative approaches to specimen preparation that preserve the native structure while enabling high-resolution imaging.
Traditional sample preparation methods often lead to structural collapse or deformation of nanoporous polymers during the vitrification process. This occurs primarily because the surface tension forces during conventional plunge-freezing can compress or distort the delicate pore architecture. Recent innovations have focused on developing gentler vitrification protocols that maintain pore integrity, including controlled humidity environments and specialized blotting parameters tailored specifically for polymer materials.
Contrast enhancement presents another significant challenge, as polymers typically exhibit low electron density differences compared to biological specimens. Researchers have addressed this through selective staining techniques using heavy metal compounds that preferentially bind to specific polymer functional groups. Novel phase plate technologies have also emerged as non-invasive alternatives for improving contrast without chemical modification of the sample.
The hydrophobic nature of many nanoporous polymers creates additional complications during grid preparation, often resulting in uneven specimen distribution or aggregation. Surface modification strategies for EM grids have shown promising results, including plasma treatment protocols and the application of amphiphilic coatings that improve polymer adhesion while maintaining structural integrity. These innovations have significantly enhanced sample distribution uniformity across the grid surface.
Beam-induced damage remains perhaps the most formidable challenge, as polymers are particularly susceptible to radiation damage. Advanced dose-fractionation schemes have been developed specifically for polymer materials, allowing for the collection of structural information before significant damage occurs. Complementary to this, new computational approaches can extrapolate undamaged structures from dose series data, effectively "rewinding" the radiation damage.
Cryogenic focused ion beam (cryo-FIB) milling has revolutionized sample preparation for thicker polymer specimens by enabling precise thinning without introducing mechanical artifacts. This technique has proven especially valuable for hierarchically structured nanoporous polymers where internal architecture analysis is crucial. The integration of correlative light and electron microscopy workflows further enhances targeting capabilities, allowing researchers to precisely locate regions of interest within heterogeneous polymer samples.
Traditional sample preparation methods often lead to structural collapse or deformation of nanoporous polymers during the vitrification process. This occurs primarily because the surface tension forces during conventional plunge-freezing can compress or distort the delicate pore architecture. Recent innovations have focused on developing gentler vitrification protocols that maintain pore integrity, including controlled humidity environments and specialized blotting parameters tailored specifically for polymer materials.
Contrast enhancement presents another significant challenge, as polymers typically exhibit low electron density differences compared to biological specimens. Researchers have addressed this through selective staining techniques using heavy metal compounds that preferentially bind to specific polymer functional groups. Novel phase plate technologies have also emerged as non-invasive alternatives for improving contrast without chemical modification of the sample.
The hydrophobic nature of many nanoporous polymers creates additional complications during grid preparation, often resulting in uneven specimen distribution or aggregation. Surface modification strategies for EM grids have shown promising results, including plasma treatment protocols and the application of amphiphilic coatings that improve polymer adhesion while maintaining structural integrity. These innovations have significantly enhanced sample distribution uniformity across the grid surface.
Beam-induced damage remains perhaps the most formidable challenge, as polymers are particularly susceptible to radiation damage. Advanced dose-fractionation schemes have been developed specifically for polymer materials, allowing for the collection of structural information before significant damage occurs. Complementary to this, new computational approaches can extrapolate undamaged structures from dose series data, effectively "rewinding" the radiation damage.
Cryogenic focused ion beam (cryo-FIB) milling has revolutionized sample preparation for thicker polymer specimens by enabling precise thinning without introducing mechanical artifacts. This technique has proven especially valuable for hierarchically structured nanoporous polymers where internal architecture analysis is crucial. The integration of correlative light and electron microscopy workflows further enhances targeting capabilities, allowing researchers to precisely locate regions of interest within heterogeneous polymer samples.
Data Processing and Computational Analysis Methods
The processing of cryo-electron microscopy (cryo-EM) data for nanoporous polymer characterization requires sophisticated computational approaches to extract meaningful structural information. Image acquisition in cryo-EM generates terabytes of raw data that must undergo multiple processing stages before yielding interpretable results. The initial step involves motion correction to account for beam-induced specimen movement during exposure, followed by contrast transfer function (CTF) estimation to correct for microscope lens aberrations.
Particle picking represents a critical phase in the workflow, where algorithms identify and select individual polymer structures from micrographs. Traditional approaches relied on template matching, but modern deep learning methods have significantly improved accuracy and efficiency. Convolutional neural networks (CNNs) trained on manually annotated datasets can now automatically detect nanoporous features with minimal human intervention, reducing processing time from weeks to days.
2D classification techniques organize similar particle images into classes, enhancing signal-to-noise ratio through averaging. For nanoporous polymers, this step is particularly valuable as it reveals characteristic pore patterns and network architectures. Advanced algorithms like RELION and cryoSPARC implement maximum likelihood approaches that have become industry standards, though specialized modifications are often necessary to accommodate the unique contrast properties of polymer materials.
3D reconstruction algorithms transform 2D projections into volumetric representations, with approaches ranging from direct Fourier methods to iterative refinement techniques. For nanoporous polymers, the heterogeneity in pore size distribution presents unique challenges, requiring specialized algorithms that can handle structural variability. Multi-reference refinement and 3D classification have proven effective in distinguishing different conformational states within polymer networks.
Post-processing steps include sharpening to enhance high-resolution features and local resolution estimation to identify regions of varying structural definition. Specialized software tools for pore analysis have emerged, capable of quantifying parameters such as pore diameter distribution, connectivity, and tortuosity. These metrics provide crucial insights into structure-property relationships that determine material performance in applications like filtration, catalysis, and drug delivery.
Integration with molecular dynamics simulations represents an emerging frontier, where cryo-EM structures serve as starting points for computational modeling of polymer behavior under various conditions. This hybrid approach bridges static structural information with dynamic functional insights, enabling more comprehensive characterization of nanoporous polymer systems.
Particle picking represents a critical phase in the workflow, where algorithms identify and select individual polymer structures from micrographs. Traditional approaches relied on template matching, but modern deep learning methods have significantly improved accuracy and efficiency. Convolutional neural networks (CNNs) trained on manually annotated datasets can now automatically detect nanoporous features with minimal human intervention, reducing processing time from weeks to days.
2D classification techniques organize similar particle images into classes, enhancing signal-to-noise ratio through averaging. For nanoporous polymers, this step is particularly valuable as it reveals characteristic pore patterns and network architectures. Advanced algorithms like RELION and cryoSPARC implement maximum likelihood approaches that have become industry standards, though specialized modifications are often necessary to accommodate the unique contrast properties of polymer materials.
3D reconstruction algorithms transform 2D projections into volumetric representations, with approaches ranging from direct Fourier methods to iterative refinement techniques. For nanoporous polymers, the heterogeneity in pore size distribution presents unique challenges, requiring specialized algorithms that can handle structural variability. Multi-reference refinement and 3D classification have proven effective in distinguishing different conformational states within polymer networks.
Post-processing steps include sharpening to enhance high-resolution features and local resolution estimation to identify regions of varying structural definition. Specialized software tools for pore analysis have emerged, capable of quantifying parameters such as pore diameter distribution, connectivity, and tortuosity. These metrics provide crucial insights into structure-property relationships that determine material performance in applications like filtration, catalysis, and drug delivery.
Integration with molecular dynamics simulations represents an emerging frontier, where cryo-EM structures serve as starting points for computational modeling of polymer behavior under various conditions. This hybrid approach bridges static structural information with dynamic functional insights, enabling more comprehensive characterization of nanoporous polymer systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!