Cost-Benefit Analysis Of Establishing A Cryo-EM Facility
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Technology Background and Objectives
Cryo-electron microscopy (Cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules at near-atomic resolution without the need for crystallization. This technology emerged in the 1980s but underwent a "resolution revolution" around 2013 when direct electron detectors and improved computational methods dramatically enhanced image quality and resolution capabilities.
The evolution of Cryo-EM technology has progressed through several key phases: from initial negative staining techniques to vitrification methods, followed by the development of advanced detectors and sophisticated image processing algorithms. Recent advancements include the integration of artificial intelligence for data processing and the development of microED for crystalline samples.
Current state-of-the-art Cryo-EM facilities can achieve resolutions below 2 Å, enabling researchers to visualize molecular structures with unprecedented detail, including hydrogen atoms in some cases. This capability has transformed drug discovery, vaccine development, and fundamental biological research by providing insights into protein dynamics and conformational states.
The primary objective of establishing a Cryo-EM facility is to enable high-resolution structural analysis of complex biological molecules that are difficult or impossible to study using traditional methods like X-ray crystallography or NMR spectroscopy. Such facilities aim to support breakthrough research in areas including drug development, disease mechanisms, and fundamental cellular processes.
Technical trends indicate continued improvement in resolution capabilities, automation of sample preparation and data collection, and integration with complementary techniques such as cryo-electron tomography (cryo-ET) and correlative light and electron microscopy (CLEM). The field is moving toward more accessible and user-friendly systems, though high-end instruments remain complex and expensive.
Future technical goals include improving throughput and efficiency, reducing sample requirements, enhancing resolution for challenging specimens, and developing more automated workflows from sample preparation to structure determination. There is also significant interest in developing more affordable and compact systems to democratize access to this powerful technology.
The establishment of a Cryo-EM facility represents a substantial investment in infrastructure, expertise, and ongoing operational support. Any cost-benefit analysis must consider not only the immediate capital expenses but also the long-term scientific impact, potential for collaborative research, and competitive advantages in securing research funding and attracting top scientific talent.
The evolution of Cryo-EM technology has progressed through several key phases: from initial negative staining techniques to vitrification methods, followed by the development of advanced detectors and sophisticated image processing algorithms. Recent advancements include the integration of artificial intelligence for data processing and the development of microED for crystalline samples.
Current state-of-the-art Cryo-EM facilities can achieve resolutions below 2 Å, enabling researchers to visualize molecular structures with unprecedented detail, including hydrogen atoms in some cases. This capability has transformed drug discovery, vaccine development, and fundamental biological research by providing insights into protein dynamics and conformational states.
The primary objective of establishing a Cryo-EM facility is to enable high-resolution structural analysis of complex biological molecules that are difficult or impossible to study using traditional methods like X-ray crystallography or NMR spectroscopy. Such facilities aim to support breakthrough research in areas including drug development, disease mechanisms, and fundamental cellular processes.
Technical trends indicate continued improvement in resolution capabilities, automation of sample preparation and data collection, and integration with complementary techniques such as cryo-electron tomography (cryo-ET) and correlative light and electron microscopy (CLEM). The field is moving toward more accessible and user-friendly systems, though high-end instruments remain complex and expensive.
Future technical goals include improving throughput and efficiency, reducing sample requirements, enhancing resolution for challenging specimens, and developing more automated workflows from sample preparation to structure determination. There is also significant interest in developing more affordable and compact systems to democratize access to this powerful technology.
The establishment of a Cryo-EM facility represents a substantial investment in infrastructure, expertise, and ongoing operational support. Any cost-benefit analysis must consider not only the immediate capital expenses but also the long-term scientific impact, potential for collaborative research, and competitive advantages in securing research funding and attracting top scientific talent.
Market Demand for Structural Biology Services
The structural biology services market has experienced significant growth over the past decade, driven primarily by pharmaceutical and biotechnology companies seeking to understand protein structures for drug discovery and development. The global market for structural biology was valued at approximately $1.7 billion in 2022 and is projected to reach $3.2 billion by 2028, with a compound annual growth rate of 11.2% during this forecast period.
Cryo-electron microscopy (cryo-EM) services represent one of the fastest-growing segments within this market. Following the "resolution revolution" that began around 2013 with the introduction of direct electron detectors, demand for cryo-EM services has surged dramatically. Academic institutions, pharmaceutical companies, and biotechnology firms are increasingly seeking access to this technology for determining complex macromolecular structures that were previously inaccessible through traditional methods like X-ray crystallography.
Industry surveys indicate that approximately 65% of pharmaceutical companies now incorporate cryo-EM data in their drug discovery pipelines, compared to just 20% five years ago. This shift reflects the growing recognition of cryo-EM's unique capabilities in visualizing membrane proteins, large protein complexes, and dynamic molecular assemblies that are critical drug targets.
The demand is particularly strong in therapeutic areas focusing on neurological disorders, infectious diseases, and cancer research. For instance, cryo-EM has become instrumental in studying ion channels, G-protein coupled receptors (GPCRs), and viral proteins, which collectively represent targets for over 40% of FDA-approved drugs.
Regional analysis shows that North America currently dominates the structural biology services market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and South Korea.
Commercial cryo-EM service providers report waiting periods of 3-6 months for project slots, indicating significant unmet demand. Academic core facilities similarly report utilization rates exceeding 90%, with many institutions implementing prioritization systems to manage access to their limited resources.
The cost structure of the market reveals that clients are willing to pay premium prices for high-quality structural data, with typical commercial cryo-EM structure determination projects ranging from $50,000 to $200,000 depending on complexity. This price elasticity suggests that the market values the unique insights provided by cryo-EM technology and recognizes its potential to accelerate drug discovery timelines and reduce development costs.
Cryo-electron microscopy (cryo-EM) services represent one of the fastest-growing segments within this market. Following the "resolution revolution" that began around 2013 with the introduction of direct electron detectors, demand for cryo-EM services has surged dramatically. Academic institutions, pharmaceutical companies, and biotechnology firms are increasingly seeking access to this technology for determining complex macromolecular structures that were previously inaccessible through traditional methods like X-ray crystallography.
Industry surveys indicate that approximately 65% of pharmaceutical companies now incorporate cryo-EM data in their drug discovery pipelines, compared to just 20% five years ago. This shift reflects the growing recognition of cryo-EM's unique capabilities in visualizing membrane proteins, large protein complexes, and dynamic molecular assemblies that are critical drug targets.
The demand is particularly strong in therapeutic areas focusing on neurological disorders, infectious diseases, and cancer research. For instance, cryo-EM has become instrumental in studying ion channels, G-protein coupled receptors (GPCRs), and viral proteins, which collectively represent targets for over 40% of FDA-approved drugs.
Regional analysis shows that North America currently dominates the structural biology services market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and South Korea.
Commercial cryo-EM service providers report waiting periods of 3-6 months for project slots, indicating significant unmet demand. Academic core facilities similarly report utilization rates exceeding 90%, with many institutions implementing prioritization systems to manage access to their limited resources.
The cost structure of the market reveals that clients are willing to pay premium prices for high-quality structural data, with typical commercial cryo-EM structure determination projects ranging from $50,000 to $200,000 depending on complexity. This price elasticity suggests that the market values the unique insights provided by cryo-EM technology and recognizes its potential to accelerate drug discovery timelines and reduce development costs.
Current Cryo-EM Landscape and Technical Challenges
Cryo-electron microscopy (Cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules at near-atomic resolution. The global landscape of Cryo-EM facilities has expanded significantly over the past decade, with major research institutions in North America, Europe, and Asia establishing dedicated centers. Currently, there are approximately 200 high-end Cryo-EM facilities worldwide, with the majority concentrated in the United States, United Kingdom, Germany, China, and Japan.
Despite its transformative potential, Cryo-EM technology faces several significant technical challenges. Sample preparation remains a critical bottleneck, requiring specialized expertise to achieve optimal vitrification without ice crystal formation. The process is highly manual, time-consuming, and subject to variability, with success rates often below 30% for complex specimens. This challenge directly impacts facility throughput and operational efficiency.
Data acquisition represents another major hurdle, as modern Cryo-EM instruments generate massive datasets—often exceeding 10 terabytes per week. This necessitates substantial computational infrastructure for data storage, processing, and analysis. The computational requirements for image processing have grown exponentially with increasing resolution demands, requiring specialized hardware configurations and software solutions.
Instrument stability and reliability present ongoing challenges for facility operations. High-end Cryo-EM systems require precisely controlled environments with minimal electromagnetic interference, temperature fluctuations, and vibrations. Maintaining these conditions demands sophisticated facility design and continuous monitoring systems, significantly increasing establishment and operational costs.
The economic landscape for Cryo-EM facilities is characterized by high capital expenditure and substantial ongoing operational costs. A single high-end Cryo-EM instrument typically costs between $5-7 million, with additional expenses for supporting equipment, facility modifications, and computational infrastructure potentially doubling this investment. Annual maintenance contracts alone can exceed $300,000 per instrument, not including staffing costs for specialized personnel.
Access models vary globally, with some facilities operating as national or regional resources with shared access, while others function as institutional or commercial service providers. The sustainability of these different models depends heavily on funding mechanisms, utilization rates, and the ability to demonstrate scientific impact and return on investment.
Technological evolution continues to shape the Cryo-EM landscape, with recent advances in automation, direct electron detectors, and image processing algorithms improving accessibility and capabilities. However, these advances have not yet significantly reduced the overall cost burden, maintaining the high barrier to entry for establishing new facilities.
Despite its transformative potential, Cryo-EM technology faces several significant technical challenges. Sample preparation remains a critical bottleneck, requiring specialized expertise to achieve optimal vitrification without ice crystal formation. The process is highly manual, time-consuming, and subject to variability, with success rates often below 30% for complex specimens. This challenge directly impacts facility throughput and operational efficiency.
Data acquisition represents another major hurdle, as modern Cryo-EM instruments generate massive datasets—often exceeding 10 terabytes per week. This necessitates substantial computational infrastructure for data storage, processing, and analysis. The computational requirements for image processing have grown exponentially with increasing resolution demands, requiring specialized hardware configurations and software solutions.
Instrument stability and reliability present ongoing challenges for facility operations. High-end Cryo-EM systems require precisely controlled environments with minimal electromagnetic interference, temperature fluctuations, and vibrations. Maintaining these conditions demands sophisticated facility design and continuous monitoring systems, significantly increasing establishment and operational costs.
The economic landscape for Cryo-EM facilities is characterized by high capital expenditure and substantial ongoing operational costs. A single high-end Cryo-EM instrument typically costs between $5-7 million, with additional expenses for supporting equipment, facility modifications, and computational infrastructure potentially doubling this investment. Annual maintenance contracts alone can exceed $300,000 per instrument, not including staffing costs for specialized personnel.
Access models vary globally, with some facilities operating as national or regional resources with shared access, while others function as institutional or commercial service providers. The sustainability of these different models depends heavily on funding mechanisms, utilization rates, and the ability to demonstrate scientific impact and return on investment.
Technological evolution continues to shape the Cryo-EM landscape, with recent advances in automation, direct electron detectors, and image processing algorithms improving accessibility and capabilities. However, these advances have not yet significantly reduced the overall cost burden, maintaining the high barrier to entry for establishing new facilities.
Existing Facility Implementation Models
01 Equipment and infrastructure costs for Cryo-EM facilities
Establishing a Cryo-EM facility requires significant capital investment in specialized equipment and infrastructure. This includes the electron microscope itself, sample preparation equipment, computing resources for data processing, and facility modifications to ensure proper environmental conditions. The high initial costs must be weighed against the long-term scientific benefits and potential for collaborative research opportunities.- Equipment and infrastructure costs for Cryo-EM facilities: Establishing a Cryo-EM facility requires significant capital investment in specialized equipment including electron microscopes, sample preparation tools, and computing infrastructure. The high acquisition costs of these instruments, along with facility construction requirements such as vibration isolation, electromagnetic shielding, and temperature control systems, represent major financial considerations. Ongoing maintenance contracts and regular upgrades also contribute to the total cost of ownership over the facility's lifecycle.
- Operational efficiency and resource management: Maximizing the return on investment for Cryo-EM facilities requires efficient scheduling systems, resource allocation, and utilization tracking. Implementing management software for instrument time booking, sample queuing, and data storage optimization helps increase throughput and reduce downtime. Strategic staffing models with specialized technicians and researchers can further enhance operational efficiency while balancing access between different user groups and research priorities.
- Collaborative models and shared facility approaches: To distribute the high costs of Cryo-EM technology, various collaborative models have emerged including multi-institution partnerships, regional facilities, and industry-academic collaborations. These shared resource approaches allow smaller institutions to access advanced structural biology capabilities without bearing the full financial burden. Service-based models where facilities charge usage fees based on instrument time, technical support, and data processing requirements help sustain operations while expanding access to a broader scientific community.
- Data management and computational infrastructure: Cryo-EM generates massive datasets requiring substantial computational resources for processing, analysis, and storage. Investments in high-performance computing clusters, specialized software, and long-term data archiving solutions represent significant but essential costs. Cloud-based solutions and distributed computing networks offer scalable alternatives to on-premises infrastructure, potentially reducing capital expenditures while maintaining processing capabilities. Effective data management strategies are crucial for maximizing the scientific output from expensive microscope time.
- Scientific return on investment metrics: Evaluating the cost-benefit ratio of Cryo-EM facilities requires defining appropriate metrics for scientific impact. These may include publication output, structural determinations deposited in public databases, patents filed, grants secured, and educational outcomes. Long-term benefits such as accelerated drug discovery, advanced materials development, and training of skilled workforce in cutting-edge techniques should be considered alongside direct financial returns. Quantitative and qualitative assessment frameworks help justify continued investment and identify opportunities for enhancing facility value.
02 Resource management and operational efficiency
Effective management of Cryo-EM facilities involves optimizing resource allocation, scheduling, and utilization to maximize return on investment. This includes implementing systems for user training, equipment maintenance schedules, and sample throughput optimization. Operational efficiency metrics help justify the high costs of maintaining these facilities and ensure sustainable operation over time.Expand Specific Solutions03 Cost-benefit analysis methodologies for scientific facilities
Specialized methodologies for evaluating the cost-benefit ratio of Cryo-EM facilities have been developed. These approaches consider factors such as research output quality, publication impact, grant funding attracted, and educational benefits. Quantitative and qualitative assessment tools help institutions make informed decisions about investing in and maintaining expensive scientific infrastructure.Expand Specific Solutions04 Collaborative models and shared facility approaches
To distribute the high costs of Cryo-EM facilities, various collaborative models have emerged. These include multi-institution partnerships, regional facilities, and fee-for-service arrangements. Shared access models allow smaller institutions to benefit from advanced technology while contributing to operational costs, creating economies of scale that improve the overall cost-benefit ratio.Expand Specific Solutions05 Technological advancements improving cost-effectiveness
Recent technological innovations in Cryo-EM are improving the cost-benefit ratio of these facilities. Developments include more efficient detectors, automated sample handling, improved software for image processing, and remote operation capabilities. These advancements increase sample throughput, reduce operational costs, and extend the useful life of equipment, enhancing the return on investment for institutions operating Cryo-EM facilities.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The cryo-electron microscopy (cryo-EM) facility market is currently in a growth phase, with increasing adoption across academic and commercial sectors. The global market size for cryo-EM equipment and services is expanding rapidly, estimated to reach several billion dollars by 2025. Technologically, the field has matured significantly with companies like FEI Co. (now part of Thermo Fisher) and Bruker Switzerland AG leading commercial development of high-resolution instruments. Academic institutions including University of Washington, University of California, and Oxford University are advancing research applications, while specialized players such as Ion Dx and Phononic are developing complementary technologies. Chinese institutions like the Institute of Biophysics CAS and companies like Shenzhen Mindray are increasingly competitive, suggesting a globalizing market with opportunities for cost optimization in facility establishment.
Institute of Biophysics of Chinese Academy of Sciences
Technical Solution: The Institute of Biophysics (IBP) of the Chinese Academy of Sciences has pioneered a comprehensive cost-benefit framework for cryo-EM facilities that balances scientific output with financial sustainability. Their approach incorporates a multi-tier facility model that combines high-end instruments with mid-range systems to optimize resource allocation. IBP's analysis demonstrates that a well-designed facility can achieve break-even within 5-7 years through a combination of grant funding, industrial collaborations, and service fees. Their model includes detailed operational metrics showing that a facility operating at 70-80% capacity can support approximately 30-40 research projects annually, potentially generating 15-20 high-impact publications. IBP has developed specialized training programs that reduce the learning curve for new users by approximately 40%, maximizing facility productivity. Their cost-benefit analysis incorporates both quantitative metrics (publications, grants secured) and qualitative outcomes (research breakthroughs, talent attraction) to provide a holistic evaluation framework for institutional decision-makers.
Strengths: Proven sustainable operational model; balanced approach to high-end and mid-range instrumentation; strong training component to maximize user base. Weaknesses: Model optimized for large research institutions; may require adaptation for smaller organizations; significant initial investment in staff development and training infrastructure.
FEI Co.
Technical Solution: FEI Co. (now part of Thermo Fisher Scientific) has developed comprehensive cryo-EM facility solutions that integrate their Titan Krios microscopes with automated sample handling systems. Their approach focuses on maximizing operational efficiency through integrated workflows that combine sample preparation, data acquisition, and image processing. FEI's technology enables high-resolution structural analysis of biological macromolecules at near-atomic resolution (2-3 Å), allowing researchers to visualize protein structures that were previously inaccessible. Their cost-benefit analysis framework evaluates facility establishment based on initial capital expenditure against projected scientific output and potential for breakthrough discoveries. FEI provides detailed ROI models that account for equipment lifespan (typically 7-10 years), maintenance costs (approximately 7-10% of instrument cost annually), and staffing requirements (typically 2-3 specialized technicians per facility).
Strengths: Industry-leading resolution capabilities; comprehensive service infrastructure; established track record with major research institutions. Weaknesses: High initial capital investment (typically $5-7 million for complete facility); significant ongoing operational costs; requires specialized expertise for operation and maintenance.
Key Technological Innovations in Cryo-EM
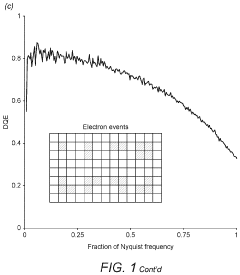
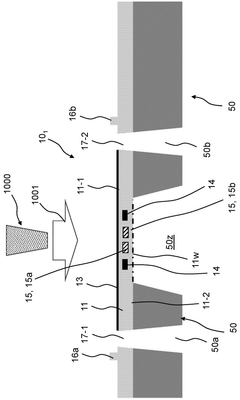
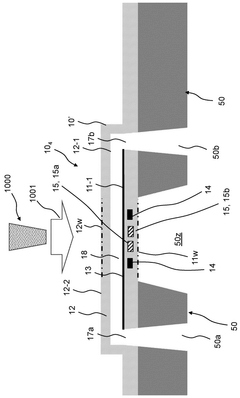
System and method for electron cryomicroscopy
PatentPendingUS20230135352A1
Innovation
- An electron cryomicroscopy system with a field-emission gun generating an 80 keV to 120 keV electron beam, an objective lens with selected chromatic aberration for high resolution, a cryostage for specimen cooling, and a direct electron detector with pixels capable of detecting incident electrons, optimizing the system for reduced radiation damage and cost while enhancing information content.
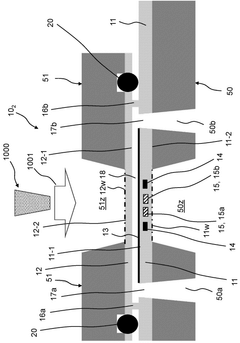
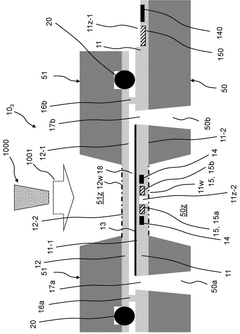
A microfluidic reactor device for use in a cryo-electron microscope, a mounting stage for such microfluidic reactor device and a method of analysing a sample under cryogen temperature conditions in a cryo-electron microscope.
PatentWO2025127920A1
Innovation
- A MEMS-based microfluidic reactor device with a transparent reactor layer for electron beam access, integrated heating means, and optional electrodes for monitoring sample characteristics, allowing for efficient, automated, and reproducible vitrification of samples within a compact and cost-effective system.
Financial Investment and ROI Analysis
Establishing a cryo-electron microscopy (cryo-EM) facility requires substantial financial investment, with comprehensive cost considerations spanning initial capital expenditure through ongoing operational expenses. The initial investment typically ranges from $5-10 million, encompassing the core microscope system ($3-7 million), specimen preparation equipment ($500,000-1 million), computational infrastructure ($500,000-1.5 million), and facility renovation costs ($1-2 million) for vibration isolation, electromagnetic shielding, and temperature control systems.
Operational expenses present an equally significant financial commitment, averaging $500,000-1 million annually. This includes specialized staff salaries ($250,000-500,000), maintenance contracts ($150,000-300,000 per year), liquid nitrogen and helium consumption ($50,000-100,000), and energy costs for continuous operation ($50,000-150,000). Additionally, computational storage and processing requirements generate ongoing expenses of $50,000-200,000 annually.
Return on investment analysis reveals multiple value streams beyond direct financial returns. Academic institutions benefit from enhanced research capabilities, increased grant funding opportunities (estimated 20-40% improvement in competitive grant applications), and elevated institutional prestige attracting top-tier researchers. For pharmaceutical and biotechnology companies, ROI manifests through accelerated drug development timelines, potentially reducing development cycles by 1-3 years and saving $100-500 million per successful therapeutic.
The payback period varies significantly by institution type. Commercial enterprises may achieve financial breakeven within 3-5 years through direct revenue generation or cost savings in drug development. Academic institutions typically require 5-8 years to realize equivalent value through grant funding, collaborative opportunities, and intellectual property development.
Cost-sharing models present viable strategies for optimizing financial sustainability. Multi-institutional partnerships can reduce per-institution investment by 40-60%, while industry-academic collaborations offer mutual benefits through shared access and expertise. Fee-for-service operational models generate $500,000-2 million annually depending on utilization rates, helping offset operational costs while maximizing facility usage.
Risk mitigation strategies should include technology obsolescence planning with 15-20% of initial investment reserved for mid-life upgrades, and diversified funding sources to ensure sustainable operations beyond initial establishment phases.
Operational expenses present an equally significant financial commitment, averaging $500,000-1 million annually. This includes specialized staff salaries ($250,000-500,000), maintenance contracts ($150,000-300,000 per year), liquid nitrogen and helium consumption ($50,000-100,000), and energy costs for continuous operation ($50,000-150,000). Additionally, computational storage and processing requirements generate ongoing expenses of $50,000-200,000 annually.
Return on investment analysis reveals multiple value streams beyond direct financial returns. Academic institutions benefit from enhanced research capabilities, increased grant funding opportunities (estimated 20-40% improvement in competitive grant applications), and elevated institutional prestige attracting top-tier researchers. For pharmaceutical and biotechnology companies, ROI manifests through accelerated drug development timelines, potentially reducing development cycles by 1-3 years and saving $100-500 million per successful therapeutic.
The payback period varies significantly by institution type. Commercial enterprises may achieve financial breakeven within 3-5 years through direct revenue generation or cost savings in drug development. Academic institutions typically require 5-8 years to realize equivalent value through grant funding, collaborative opportunities, and intellectual property development.
Cost-sharing models present viable strategies for optimizing financial sustainability. Multi-institutional partnerships can reduce per-institution investment by 40-60%, while industry-academic collaborations offer mutual benefits through shared access and expertise. Fee-for-service operational models generate $500,000-2 million annually depending on utilization rates, helping offset operational costs while maximizing facility usage.
Risk mitigation strategies should include technology obsolescence planning with 15-20% of initial investment reserved for mid-life upgrades, and diversified funding sources to ensure sustainable operations beyond initial establishment phases.
Operational Requirements and Staffing Considerations
Operating a cryo-electron microscopy (cryo-EM) facility requires careful consideration of both technical infrastructure and human resources. The facility must maintain optimal environmental conditions, including stable temperature (20-22°C), controlled humidity (below 40%), minimal vibration, and electromagnetic field protection. These parameters are critical for achieving high-resolution imaging and preventing data quality degradation.
Power requirements for a cryo-EM facility are substantial, with microscopes typically consuming 5-10 kW during operation. Uninterrupted power supply systems are essential to prevent data loss and equipment damage during power fluctuations. Additionally, the facility must incorporate specialized cooling systems for both the microscopes and the computing infrastructure that processes the generated data.
Staffing represents one of the most significant operational considerations. A fully functional cryo-EM facility requires at minimum: a facility manager with extensive experience in cryo-EM techniques, 1-2 dedicated microscope operators with specialized training, a sample preparation specialist, and an IT/computational expert to manage data processing pipelines. Depending on facility size and usage demands, additional support staff may be necessary.
Training requirements are substantial, with new staff typically requiring 6-12 months to become fully proficient in independent microscope operation. Ongoing professional development is essential as cryo-EM technology continues to evolve rapidly. Budget allocations should include provisions for staff to attend conferences, workshops, and manufacturer training sessions.
Maintenance schedules must be rigorously observed, with preventative maintenance performed quarterly and major service annually. Service contracts typically cost 5-10% of the initial equipment purchase price annually. Consumables, including liquid nitrogen (50-100 liters daily), sample grids, and preparation materials, represent ongoing operational expenses that must be factored into long-term budgeting.
Data management infrastructure represents another critical operational requirement. A typical cryo-EM session can generate 1-5 TB of data, necessitating robust storage solutions and high-performance computing resources for image processing. Cloud-based solutions versus on-premises infrastructure should be evaluated based on institutional capabilities, data security requirements, and budget constraints.
Power requirements for a cryo-EM facility are substantial, with microscopes typically consuming 5-10 kW during operation. Uninterrupted power supply systems are essential to prevent data loss and equipment damage during power fluctuations. Additionally, the facility must incorporate specialized cooling systems for both the microscopes and the computing infrastructure that processes the generated data.
Staffing represents one of the most significant operational considerations. A fully functional cryo-EM facility requires at minimum: a facility manager with extensive experience in cryo-EM techniques, 1-2 dedicated microscope operators with specialized training, a sample preparation specialist, and an IT/computational expert to manage data processing pipelines. Depending on facility size and usage demands, additional support staff may be necessary.
Training requirements are substantial, with new staff typically requiring 6-12 months to become fully proficient in independent microscope operation. Ongoing professional development is essential as cryo-EM technology continues to evolve rapidly. Budget allocations should include provisions for staff to attend conferences, workshops, and manufacturer training sessions.
Maintenance schedules must be rigorously observed, with preventative maintenance performed quarterly and major service annually. Service contracts typically cost 5-10% of the initial equipment purchase price annually. Consumables, including liquid nitrogen (50-100 liters daily), sample grids, and preparation materials, represent ongoing operational expenses that must be factored into long-term budgeting.
Data management infrastructure represents another critical operational requirement. A typical cryo-EM session can generate 1-5 TB of data, necessitating robust storage solutions and high-performance computing resources for image processing. Cloud-based solutions versus on-premises infrastructure should be evaluated based on institutional capabilities, data security requirements, and budget constraints.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!