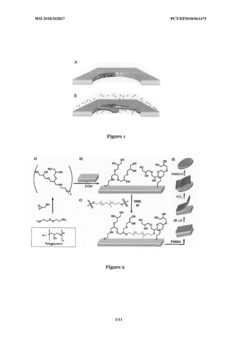
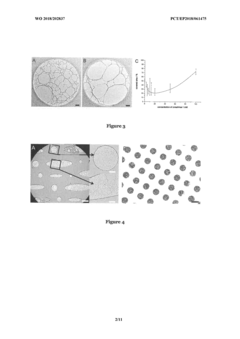
Cryo-EM For Characterizing Soft Matter Biomimetic Materials
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Technology Evolution and Research Objectives
Cryo-electron microscopy (Cryo-EM) has emerged as a revolutionary technique in structural biology, enabling researchers to visualize biological macromolecules at near-atomic resolution. The evolution of this technology began in the 1980s with the development of vitrification methods by Jacques Dubochet and colleagues, allowing biological samples to be preserved in their native state without the formation of ice crystals. This breakthrough laid the foundation for modern Cryo-EM applications.
The technological trajectory of Cryo-EM has been marked by significant advancements in both hardware and software components. The introduction of direct electron detectors around 2012 represented a pivotal moment, dramatically improving signal-to-noise ratios and enabling higher resolution imaging. Concurrently, sophisticated image processing algorithms and computational methods have evolved to extract maximum structural information from the collected data.
In recent years, Cryo-EM has expanded beyond traditional protein structure determination to characterize soft matter and biomimetic materials. These materials, which include hydrogels, polymer assemblies, and synthetic cellular structures, present unique challenges for structural characterization due to their dynamic nature, heterogeneity, and sensitivity to conventional imaging conditions.
The application of Cryo-EM to soft matter biomimetic materials represents a frontier in materials science and bioengineering. These materials often mimic biological structures and functions, serving as platforms for drug delivery, tissue engineering, and biosensing applications. Understanding their structural organization at the nanoscale is crucial for optimizing their performance and developing new functionalities.
Current research objectives in this field focus on several key areas. First, improving sample preparation techniques to better preserve the native state of soft materials during the vitrification process. Second, developing specialized image processing algorithms capable of handling the inherent heterogeneity and flexibility of these systems. Third, integrating complementary techniques such as cryo-electron tomography and correlative light and electron microscopy to provide multi-scale structural information.
The ultimate goal of this research is to establish Cryo-EM as a standard tool for characterizing soft matter biomimetic materials across different length scales, from molecular assemblies to macroscopic properties. This would enable rational design of advanced materials with tailored functionalities for biomedical and technological applications. Additionally, understanding the structure-function relationships in these materials could provide insights into fundamental biological processes and inspire new biomimetic designs.
As we look toward the future, the continued evolution of Cryo-EM technology, particularly in terms of automation, throughput, and resolution, will further expand its capabilities for soft matter characterization, potentially revolutionizing our approach to designing and optimizing biomimetic materials.
The technological trajectory of Cryo-EM has been marked by significant advancements in both hardware and software components. The introduction of direct electron detectors around 2012 represented a pivotal moment, dramatically improving signal-to-noise ratios and enabling higher resolution imaging. Concurrently, sophisticated image processing algorithms and computational methods have evolved to extract maximum structural information from the collected data.
In recent years, Cryo-EM has expanded beyond traditional protein structure determination to characterize soft matter and biomimetic materials. These materials, which include hydrogels, polymer assemblies, and synthetic cellular structures, present unique challenges for structural characterization due to their dynamic nature, heterogeneity, and sensitivity to conventional imaging conditions.
The application of Cryo-EM to soft matter biomimetic materials represents a frontier in materials science and bioengineering. These materials often mimic biological structures and functions, serving as platforms for drug delivery, tissue engineering, and biosensing applications. Understanding their structural organization at the nanoscale is crucial for optimizing their performance and developing new functionalities.
Current research objectives in this field focus on several key areas. First, improving sample preparation techniques to better preserve the native state of soft materials during the vitrification process. Second, developing specialized image processing algorithms capable of handling the inherent heterogeneity and flexibility of these systems. Third, integrating complementary techniques such as cryo-electron tomography and correlative light and electron microscopy to provide multi-scale structural information.
The ultimate goal of this research is to establish Cryo-EM as a standard tool for characterizing soft matter biomimetic materials across different length scales, from molecular assemblies to macroscopic properties. This would enable rational design of advanced materials with tailored functionalities for biomedical and technological applications. Additionally, understanding the structure-function relationships in these materials could provide insights into fundamental biological processes and inspire new biomimetic designs.
As we look toward the future, the continued evolution of Cryo-EM technology, particularly in terms of automation, throughput, and resolution, will further expand its capabilities for soft matter characterization, potentially revolutionizing our approach to designing and optimizing biomimetic materials.
Market Applications for Soft Matter Biomimetic Materials
The market for soft matter biomimetic materials is experiencing significant growth driven by their unique properties that mimic biological systems. These materials, characterized by their responsiveness to environmental stimuli and structural complexity, are finding applications across multiple industries due to their biocompatibility, adaptability, and sustainable nature.
In the healthcare sector, soft biomimetic materials are revolutionizing drug delivery systems, tissue engineering, and regenerative medicine. The global market for biomaterials in healthcare is projected to grow substantially as these materials enable more targeted drug delivery, reduce side effects, and improve patient outcomes. Cryo-EM characterization has been instrumental in developing these applications by providing detailed structural information that guides material design.
The cosmetics and personal care industry represents another significant market opportunity. Biomimetic materials that replicate natural skin structures or deliver active ingredients through biomimetic mechanisms are gaining popularity. Companies are increasingly investing in soft matter technologies that can provide enhanced moisturizing properties, controlled release of active ingredients, and improved skin compatibility.
Environmental remediation and water treatment constitute emerging application areas. Soft biomimetic materials designed to mimic natural filtering systems or selectively capture pollutants are being developed to address growing environmental concerns. The ability to characterize these materials at the nanoscale using Cryo-EM has accelerated innovation in this sector.
The food industry is adopting soft biomimetic materials for food preservation, texture enhancement, and nutrient delivery systems. These materials can extend shelf life, improve sensory properties, and enhance nutritional profiles of food products. The market for such applications is growing as consumers demand more natural and functional food products.
Soft robotics represents a cutting-edge application area with significant market potential. Biomimetic materials that can change shape, respond to stimuli, or self-heal are enabling the development of robots that can interact safely with humans and navigate complex environments. Cryo-EM characterization helps engineers understand the structural dynamics of these materials under various conditions.
The electronics industry is exploring soft biomimetic materials for flexible electronics, sensors, and energy storage devices. These materials offer advantages in wearable technology and implantable devices where traditional rigid electronics are unsuitable. The market for flexible electronics is expected to expand rapidly as these technologies mature.
Agricultural applications include controlled release fertilizers, soil conditioners, and crop protection products based on biomimetic principles. These materials can improve agricultural efficiency while reducing environmental impact, addressing growing concerns about sustainable farming practices.
In the healthcare sector, soft biomimetic materials are revolutionizing drug delivery systems, tissue engineering, and regenerative medicine. The global market for biomaterials in healthcare is projected to grow substantially as these materials enable more targeted drug delivery, reduce side effects, and improve patient outcomes. Cryo-EM characterization has been instrumental in developing these applications by providing detailed structural information that guides material design.
The cosmetics and personal care industry represents another significant market opportunity. Biomimetic materials that replicate natural skin structures or deliver active ingredients through biomimetic mechanisms are gaining popularity. Companies are increasingly investing in soft matter technologies that can provide enhanced moisturizing properties, controlled release of active ingredients, and improved skin compatibility.
Environmental remediation and water treatment constitute emerging application areas. Soft biomimetic materials designed to mimic natural filtering systems or selectively capture pollutants are being developed to address growing environmental concerns. The ability to characterize these materials at the nanoscale using Cryo-EM has accelerated innovation in this sector.
The food industry is adopting soft biomimetic materials for food preservation, texture enhancement, and nutrient delivery systems. These materials can extend shelf life, improve sensory properties, and enhance nutritional profiles of food products. The market for such applications is growing as consumers demand more natural and functional food products.
Soft robotics represents a cutting-edge application area with significant market potential. Biomimetic materials that can change shape, respond to stimuli, or self-heal are enabling the development of robots that can interact safely with humans and navigate complex environments. Cryo-EM characterization helps engineers understand the structural dynamics of these materials under various conditions.
The electronics industry is exploring soft biomimetic materials for flexible electronics, sensors, and energy storage devices. These materials offer advantages in wearable technology and implantable devices where traditional rigid electronics are unsuitable. The market for flexible electronics is expected to expand rapidly as these technologies mature.
Agricultural applications include controlled release fertilizers, soil conditioners, and crop protection products based on biomimetic principles. These materials can improve agricultural efficiency while reducing environmental impact, addressing growing concerns about sustainable farming practices.
Current Limitations in Soft Matter Characterization
Despite significant advancements in characterization techniques, soft matter biomimetic materials present unique challenges for conventional analytical methods. Traditional electron microscopy techniques often cause structural damage to these delicate materials due to high vacuum conditions and electron beam radiation. The inherent complexity of soft matter systems—featuring hierarchical structures spanning multiple length scales from nanometers to micrometers—further complicates comprehensive characterization.
Water content in hydrogels and other biomimetic materials poses a particular challenge, as dehydration during sample preparation can dramatically alter their native structure. This creates a fundamental dilemma: preserving the material in its natural hydrated state while achieving high-resolution imaging. Current sample preparation protocols often fail to maintain the original morphology and spatial arrangement of components.
Contrast generation represents another significant limitation. Soft matter biomimetic materials typically consist of light elements (C, H, O, N) with minimal density differences between components, resulting in poor natural contrast. While staining techniques can enhance contrast, they may introduce artifacts or alter the material's intrinsic properties, compromising the validity of subsequent analyses.
Dynamic behavior characterization remains particularly challenging. Many biomimetic materials exhibit time-dependent responses to environmental stimuli—a key feature for their functionality. However, most high-resolution imaging techniques provide only static snapshots rather than continuous monitoring of structural evolution, limiting our understanding of their dynamic properties.
Quantitative analysis of mechanical properties at the nanoscale presents additional difficulties. While techniques like atomic force microscopy offer localized mechanical measurements, they struggle to provide comprehensive mechanical mapping across different hierarchical levels simultaneously. This gap hinders the establishment of structure-property relationships crucial for rational design of biomimetic materials.
The integration of multiple characterization techniques often leads to data correlation challenges. Information obtained from different methods may represent different sample states or preparation conditions, making it difficult to construct a unified understanding of the material's structure and properties. This fragmentation of data impedes the development of predictive models for biomimetic material behavior.
Finally, the field faces reproducibility issues due to the inherent heterogeneity of soft matter biomimetic materials and the sensitivity of their properties to subtle variations in processing conditions. This variability complicates standardization efforts and slows the translation of laboratory discoveries into practical applications.
Water content in hydrogels and other biomimetic materials poses a particular challenge, as dehydration during sample preparation can dramatically alter their native structure. This creates a fundamental dilemma: preserving the material in its natural hydrated state while achieving high-resolution imaging. Current sample preparation protocols often fail to maintain the original morphology and spatial arrangement of components.
Contrast generation represents another significant limitation. Soft matter biomimetic materials typically consist of light elements (C, H, O, N) with minimal density differences between components, resulting in poor natural contrast. While staining techniques can enhance contrast, they may introduce artifacts or alter the material's intrinsic properties, compromising the validity of subsequent analyses.
Dynamic behavior characterization remains particularly challenging. Many biomimetic materials exhibit time-dependent responses to environmental stimuli—a key feature for their functionality. However, most high-resolution imaging techniques provide only static snapshots rather than continuous monitoring of structural evolution, limiting our understanding of their dynamic properties.
Quantitative analysis of mechanical properties at the nanoscale presents additional difficulties. While techniques like atomic force microscopy offer localized mechanical measurements, they struggle to provide comprehensive mechanical mapping across different hierarchical levels simultaneously. This gap hinders the establishment of structure-property relationships crucial for rational design of biomimetic materials.
The integration of multiple characterization techniques often leads to data correlation challenges. Information obtained from different methods may represent different sample states or preparation conditions, making it difficult to construct a unified understanding of the material's structure and properties. This fragmentation of data impedes the development of predictive models for biomimetic material behavior.
Finally, the field faces reproducibility issues due to the inherent heterogeneity of soft matter biomimetic materials and the sensitivity of their properties to subtle variations in processing conditions. This variability complicates standardization efforts and slows the translation of laboratory discoveries into practical applications.
State-of-the-Art Cryo-EM Techniques for Soft Materials
01 Sample preparation techniques for Cryo-EM
Various sample preparation methods have been developed to optimize specimens for cryogenic electron microscopy analysis. These techniques include vitrification processes that rapidly freeze samples to preserve their native structure, grid preparation protocols that enhance sample distribution, and specialized treatments that improve contrast and resolution. Advanced preparation methods allow for better visualization of biological macromolecules and complexes in their near-native states without the artifacts introduced by traditional sample preparation methods.- Sample preparation techniques for cryo-EM: Various sample preparation methods are crucial for successful cryo-EM characterization. These techniques include vitrification processes that rapidly freeze specimens to preserve their native structure, grid preparation protocols that optimize specimen distribution, and specialized handling procedures to maintain sample integrity during the freezing process. Advanced preparation methods enable the visualization of biological macromolecules in their near-native states without the artifacts introduced by traditional sample preparation methods.
- Hardware innovations in cryo-EM instrumentation: Recent advancements in cryo-EM hardware have significantly improved imaging capabilities. These innovations include enhanced electron detectors with higher sensitivity and faster frame rates, improved electron sources that provide more coherent electron beams, and sophisticated stage designs that reduce specimen drift and vibration. Additionally, automated data collection systems enable high-throughput imaging, while specialized lens configurations minimize aberrations and improve resolution.
- Image processing and 3D reconstruction algorithms: Sophisticated computational methods are essential for processing cryo-EM data and reconstructing three-dimensional structures. These algorithms include motion correction to account for beam-induced movement, contrast transfer function estimation and correction, particle picking and classification, and iterative refinement procedures. Machine learning approaches have been integrated to improve particle selection and classification, while advanced statistical methods enhance the resolution of the final reconstructions.
- Applications in structural biology and drug discovery: Cryo-EM has become an invaluable tool for structural biology and pharmaceutical research. It enables the visualization of large macromolecular complexes that are difficult to crystallize, such as membrane proteins, viruses, and dynamic molecular assemblies. In drug discovery, cryo-EM facilitates structure-based drug design by revealing binding sites and conformational changes upon ligand binding. The technique has been particularly impactful in understanding disease mechanisms and developing targeted therapeutics.
- Integration with complementary analytical techniques: Combining cryo-EM with other analytical methods creates powerful hybrid approaches for comprehensive structural characterization. These integrative strategies include correlative light and electron microscopy (CLEM), which merges fluorescence microscopy with cryo-EM; cryo-electron tomography for visualizing cellular ultrastructure; and computational integration with data from X-ray crystallography, NMR spectroscopy, and mass spectrometry. These hybrid approaches provide multi-scale structural information from atomic to cellular levels.
02 Hardware innovations in Cryo-EM instrumentation
Recent advancements in Cryo-EM hardware have significantly improved imaging capabilities. These innovations include enhanced electron detectors with higher sensitivity and faster frame rates, improved stage designs that reduce specimen drift and vibration, and more stable electron sources that provide coherent illumination. Automated data collection systems have also been developed to increase throughput and efficiency while maintaining high-quality image acquisition, enabling researchers to collect larger datasets for improved 3D reconstructions.Expand Specific Solutions03 Image processing and computational methods for Cryo-EM
Sophisticated computational algorithms and software tools have been developed to process and analyze Cryo-EM data. These methods include motion correction to account for beam-induced movement, contrast transfer function estimation and correction, particle picking and classification, and 3D reconstruction techniques. Machine learning and artificial intelligence approaches are increasingly being applied to automate and enhance various steps in the Cryo-EM workflow, improving resolution and enabling the analysis of heterogeneous samples and conformational states.Expand Specific Solutions04 Applications of Cryo-EM in structural biology and drug discovery
Cryo-EM has become an essential tool for determining the structures of biological macromolecules and their complexes at near-atomic resolution. This technique is particularly valuable for studying membrane proteins, large molecular assemblies, and flexible biomolecules that are challenging to analyze using traditional structural biology methods. In drug discovery, Cryo-EM enables the visualization of drug-target interactions, facilitates structure-based drug design, and provides insights into the mechanisms of action of therapeutic compounds, accelerating the development of novel pharmaceuticals.Expand Specific Solutions05 Integration of Cryo-EM with complementary techniques
The integration of Cryo-EM with other analytical methods has expanded its capabilities and applications. Hybrid approaches combining Cryo-EM with techniques such as X-ray crystallography, nuclear magnetic resonance spectroscopy, mass spectrometry, and computational modeling provide comprehensive structural information across different resolution scales. Time-resolved Cryo-EM methods have been developed to capture dynamic processes and conformational changes in biomolecules. Additionally, correlative light and electron microscopy approaches enable the localization and structural characterization of specific cellular components within their native context.Expand Specific Solutions
Leading Research Institutions and Industry Collaborations
Cryo-electron microscopy (Cryo-EM) for soft matter biomimetic materials is currently in a growth phase, with the market expanding rapidly due to increasing applications in structural biology and materials science. The global market is estimated to reach several billion dollars by 2025, driven by pharmaceutical research and nanomaterial development. Technologically, the field is advancing from early adoption to mainstream implementation, with key players demonstrating varying levels of maturity. Leading research institutions like Tsinghua University, Oxford University, and Max Planck Society are pioneering academic advancements, while commercial entities such as FEI Co. (microscopy equipment), Quantifoil Micro Tools (specialized grids), and Genentech (pharmaceutical applications) are developing complementary technologies. The ecosystem shows a balanced distribution between academic innovation and industrial implementation, with significant cross-sector collaboration accelerating technological maturity.
FEI Co.
Technical Solution: FEI Co. (now part of Thermo Fisher Scientific) has developed advanced cryo-EM systems specifically optimized for soft matter biomimetic materials characterization. Their Titan Krios platform incorporates automated specimen loading and data collection capabilities with energy-filtered imaging to enhance contrast in radiation-sensitive soft materials. FEI's technology employs direct electron detectors with high detective quantum efficiency (DQE) that significantly improve signal-to-noise ratios when imaging delicate biomimetic structures. Their integrated workflow solutions include Vitrobot sample preparation systems that ensure consistent vitrification of hydrated specimens, critical for maintaining native structures of soft biomimetic materials. FEI has pioneered phase plate technologies that enhance contrast without requiring defocus, allowing better visualization of internal structures in low-contrast biomimetic assemblies. Their systems also feature advanced motion correction algorithms that compensate for beam-induced movement, particularly problematic in soft matter imaging.
Strengths: Industry-leading resolution capabilities (below 2Å); comprehensive integrated workflow from sample preparation to image processing; extensive global support network. Weaknesses: High acquisition and maintenance costs; significant expertise required for operation; large physical footprint requiring specialized facilities.
Quantifoil Micro Tools GmbH
Technical Solution: Quantifoil has developed specialized cryo-EM support films optimized for soft matter biomimetic materials research. Their holey carbon films feature precisely engineered hole patterns with controlled size distributions (ranging from 0.6 to 3.5 μm) and spacings, allowing researchers to position soft biomimetic samples optimally for imaging. The company has pioneered gold-coated support films that minimize charging effects and provide enhanced stability during high-resolution imaging of radiation-sensitive biomimetic assemblies. Quantifoil's R2/2 and R1.2/1.3 grids have become industry standards for cryo-EM of soft materials due to their optimal ice thickness control capabilities. Their UltrAuFoil® grids incorporate gold support films that exhibit minimal beam-induced movement, critical for high-resolution imaging of dynamic soft matter systems. Quantifoil has also developed specialized surface treatments that control hydrophilicity/hydrophobicity, enabling better interactions between grid surfaces and various biomimetic constructs such as lipid assemblies, protein-polymer conjugates, and synthetic cell mimics.
Strengths: Unparalleled precision in support film manufacturing; specialized products for specific biomimetic material applications; compatibility with automated data collection systems. Weaknesses: Limited to support film technology rather than complete imaging solutions; requires complementary technologies for full workflow implementation; higher cost compared to traditional EM grids.
Breakthrough Studies in Biomimetic Material Visualization
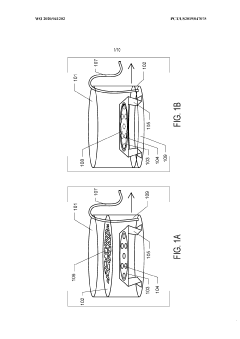
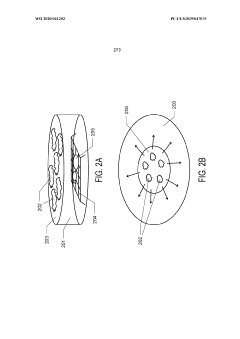
Graphene oxide affinity sample grids for cryo-em
PatentWO2020041202A1
Innovation
- The use of graphene oxide affinity grids with functionalized surfaces, including polyethylene glycol spacers, to immobilize samples away from the air-water interface and substrate interactions, enabling diverse target species capture and storage, and facilitating high-quality sample preparation for cryo-EM imaging.
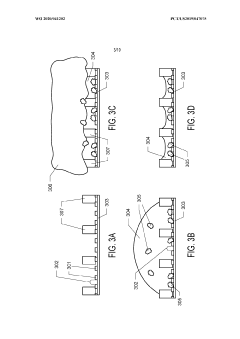
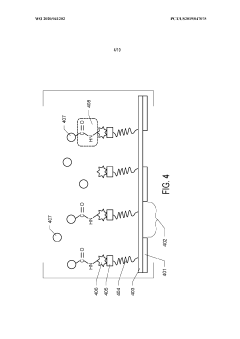
Method for preparing a cross-linked hydrogel nanomembrane, the cross-linked hydrogel nanomembrane, TEM grid comprising the same and use thereof
PatentWO2018202837A1
Innovation
- A method for preparing cross-linked hydrogel nanomembranes, specifically self-perforating hydrogel nanomembranes (SPHyNs), which stabilize the thin water film, minimize protein adsorption, and facilitate uniform distribution by transferring the nanomembranes to a TEM grid, allowing for improved specimen preparation and high-resolution data collection.
Sample Preparation Challenges and Innovations
Sample preparation for cryo-electron microscopy (cryo-EM) of soft matter biomimetic materials presents unique challenges due to the inherent structural complexity and environmental sensitivity of these materials. The primary challenge lies in preserving the native state of soft biomimetic structures during the vitrification process. Unlike crystalline materials, soft matter systems such as lipid assemblies, polymer networks, and hydrogels can undergo significant structural alterations when exposed to the rapid temperature changes required for vitrification.
The vitrification process itself introduces several technical hurdles. The formation of crystalline ice must be prevented as it can damage delicate soft matter structures and create imaging artifacts. This necessitates precise control of cooling rates, typically requiring specialized equipment such as automated plunge freezers that can achieve cooling rates exceeding 10,000°C/second. Even with such equipment, sample thickness remains a critical parameter, with optimal imaging requiring specimens under 200 nm in thickness.
Recent innovations in sample preparation techniques have significantly advanced the field. The development of graphene oxide supports has proven particularly valuable for soft biomimetic materials, as they provide minimal background signal while offering superior mechanical stability compared to traditional carbon films. These supports help maintain structural integrity during the freezing process and reduce beam-induced movement during imaging.
Correlative light and electron microscopy (CLEM) approaches have emerged as powerful tools for navigating the complex morphologies of soft biomimetic systems. By combining fluorescence microscopy with cryo-EM, researchers can precisely locate regions of interest within heterogeneous samples before detailed structural analysis, substantially improving workflow efficiency and targeting specific functional domains within complex assemblies.
Focused ion beam (FIB) milling has revolutionized sample preparation for thicker soft matter specimens. This technique allows for the precise thinning of vitrified samples to electron-transparent thicknesses, enabling the visualization of internal structures within bulk biomimetic materials that would otherwise be inaccessible to conventional cryo-EM approaches. The integration of cryo-FIB with tomographic imaging has opened new avenues for three-dimensional characterization of soft matter interfaces and hierarchical structures.
Environmental control during sample transfer represents another frontier in preparation methodology. Advanced transfer systems that maintain vitrified samples under controlled humidity and temperature conditions minimize contamination and structural alterations. These systems are particularly crucial for biomimetic materials that mimic the dynamic behavior of living systems, where even minor environmental fluctuations can trigger phase transitions or conformational changes.
The vitrification process itself introduces several technical hurdles. The formation of crystalline ice must be prevented as it can damage delicate soft matter structures and create imaging artifacts. This necessitates precise control of cooling rates, typically requiring specialized equipment such as automated plunge freezers that can achieve cooling rates exceeding 10,000°C/second. Even with such equipment, sample thickness remains a critical parameter, with optimal imaging requiring specimens under 200 nm in thickness.
Recent innovations in sample preparation techniques have significantly advanced the field. The development of graphene oxide supports has proven particularly valuable for soft biomimetic materials, as they provide minimal background signal while offering superior mechanical stability compared to traditional carbon films. These supports help maintain structural integrity during the freezing process and reduce beam-induced movement during imaging.
Correlative light and electron microscopy (CLEM) approaches have emerged as powerful tools for navigating the complex morphologies of soft biomimetic systems. By combining fluorescence microscopy with cryo-EM, researchers can precisely locate regions of interest within heterogeneous samples before detailed structural analysis, substantially improving workflow efficiency and targeting specific functional domains within complex assemblies.
Focused ion beam (FIB) milling has revolutionized sample preparation for thicker soft matter specimens. This technique allows for the precise thinning of vitrified samples to electron-transparent thicknesses, enabling the visualization of internal structures within bulk biomimetic materials that would otherwise be inaccessible to conventional cryo-EM approaches. The integration of cryo-FIB with tomographic imaging has opened new avenues for three-dimensional characterization of soft matter interfaces and hierarchical structures.
Environmental control during sample transfer represents another frontier in preparation methodology. Advanced transfer systems that maintain vitrified samples under controlled humidity and temperature conditions minimize contamination and structural alterations. These systems are particularly crucial for biomimetic materials that mimic the dynamic behavior of living systems, where even minor environmental fluctuations can trigger phase transitions or conformational changes.
Interdisciplinary Applications in Biomedicine and Materials Science
Cryo-electron microscopy (Cryo-EM) has emerged as a revolutionary tool bridging the gap between biomedicine and materials science, offering unprecedented insights into soft matter biomimetic materials. The interdisciplinary applications of this technology span multiple domains, creating synergies that accelerate innovation in both fields.
In biomedicine, Cryo-EM enables detailed visualization of biomimetic drug delivery systems, revealing crucial structural information about liposomes, polymersomes, and other nanocarriers. This visualization capability has transformed pharmaceutical development by allowing researchers to optimize drug encapsulation efficiency and release kinetics based on direct structural observations rather than indirect measurements.
The technology has proven particularly valuable in tissue engineering, where biomimetic scaffolds require precise characterization at the nanoscale. Cryo-EM provides detailed information about porosity, fiber orientation, and surface topography of these materials, directly correlating structure with biological performance in ways previously impossible with conventional imaging techniques.
Regenerative medicine has benefited significantly from Cryo-EM's ability to characterize biomimetic extracellular matrices. By revealing the intricate architecture of these materials, researchers can better understand cell-matrix interactions and design improved substrates for tissue regeneration. This has led to advances in wound healing technologies and artificial organ development.
In materials science, Cryo-EM has enabled the development of novel biomimetic polymers with precisely engineered properties. By characterizing these materials at near-atomic resolution, scientists can establish structure-property relationships that guide the design of next-generation sustainable materials inspired by natural systems.
The integration of Cryo-EM with artificial intelligence and machine learning algorithms has further expanded its applications. These computational approaches can identify patterns in biomimetic material structures that correlate with specific functional properties, accelerating materials discovery and optimization processes.
Notably, Cryo-EM has facilitated cross-disciplinary collaboration between materials scientists and biomedical researchers. This collaboration has led to innovations such as biomimetic sensors for medical diagnostics, smart materials that respond to biological stimuli, and advanced implantable devices with improved biocompatibility profiles.
The technology's ability to preserve and visualize soft matter in its native hydrated state has been particularly transformative for understanding biomimetic membranes and interfaces, which are critical components in biosensors, bioelectronics, and other hybrid technologies that bridge the biological-synthetic divide.
In biomedicine, Cryo-EM enables detailed visualization of biomimetic drug delivery systems, revealing crucial structural information about liposomes, polymersomes, and other nanocarriers. This visualization capability has transformed pharmaceutical development by allowing researchers to optimize drug encapsulation efficiency and release kinetics based on direct structural observations rather than indirect measurements.
The technology has proven particularly valuable in tissue engineering, where biomimetic scaffolds require precise characterization at the nanoscale. Cryo-EM provides detailed information about porosity, fiber orientation, and surface topography of these materials, directly correlating structure with biological performance in ways previously impossible with conventional imaging techniques.
Regenerative medicine has benefited significantly from Cryo-EM's ability to characterize biomimetic extracellular matrices. By revealing the intricate architecture of these materials, researchers can better understand cell-matrix interactions and design improved substrates for tissue regeneration. This has led to advances in wound healing technologies and artificial organ development.
In materials science, Cryo-EM has enabled the development of novel biomimetic polymers with precisely engineered properties. By characterizing these materials at near-atomic resolution, scientists can establish structure-property relationships that guide the design of next-generation sustainable materials inspired by natural systems.
The integration of Cryo-EM with artificial intelligence and machine learning algorithms has further expanded its applications. These computational approaches can identify patterns in biomimetic material structures that correlate with specific functional properties, accelerating materials discovery and optimization processes.
Notably, Cryo-EM has facilitated cross-disciplinary collaboration between materials scientists and biomedical researchers. This collaboration has led to innovations such as biomimetic sensors for medical diagnostics, smart materials that respond to biological stimuli, and advanced implantable devices with improved biocompatibility profiles.
The technology's ability to preserve and visualize soft matter in its native hydrated state has been particularly transformative for understanding biomimetic membranes and interfaces, which are critical components in biosensors, bioelectronics, and other hybrid technologies that bridge the biological-synthetic divide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!