Multimodal Cryo-EM: Combining X-Ray And Electron Imaging
AUG 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM and X-Ray Integration Background and Objectives
Cryo-electron microscopy (Cryo-EM) and X-ray crystallography have emerged as complementary techniques in structural biology, each with distinct advantages and limitations. Cryo-EM has revolutionized the field by enabling visualization of macromolecular structures in near-native states without crystallization requirements, while X-ray crystallography offers superior resolution for well-ordered samples. The integration of these modalities represents a significant technological advancement with potential to overcome the individual limitations of each approach.
The evolution of structural biology techniques has followed a trajectory from early X-ray diffraction studies in the 1950s to the "resolution revolution" in Cryo-EM during the 2010s. Recent technological breakthroughs, particularly in direct electron detectors and image processing algorithms, have elevated Cryo-EM to achieve near-atomic resolution comparable to X-ray methods. This convergence creates an unprecedented opportunity for multimodal integration.
Historically, researchers have treated these techniques as separate methodologies, often using them sequentially rather than synergistically. The concept of multimodal Cryo-EM represents a paradigm shift toward simultaneous or complementary data acquisition and analysis, potentially yielding more comprehensive structural insights than either method alone.
The primary objective of multimodal Cryo-EM development is to create integrated systems capable of collecting both electron microscopy and X-ray diffraction data from identical or similar specimens, enabling multi-scale structural characterization from atomic to cellular levels. This approach aims to leverage the high-resolution capabilities of X-ray crystallography with the flexibility and contextual information provided by Cryo-EM.
Secondary goals include developing computational frameworks for data fusion, addressing sample preparation challenges for dual-modality imaging, and establishing standardized protocols for correlative analysis. The ultimate vision is to enable researchers to seamlessly transition between modalities within a single experimental workflow, maximizing information extraction from precious biological samples.
Current technological trends suggest continued improvements in detector sensitivity, computational processing power, and automation capabilities will further drive this integration. The field is moving toward comprehensive structural biology platforms that incorporate multiple imaging modalities, potentially including additional techniques such as tomography and spectroscopy.
The successful development of multimodal Cryo-EM systems would significantly impact drug discovery, vaccine development, and fundamental understanding of cellular machinery by providing unprecedented insights into macromolecular structure and dynamics across multiple spatial and temporal scales.
The evolution of structural biology techniques has followed a trajectory from early X-ray diffraction studies in the 1950s to the "resolution revolution" in Cryo-EM during the 2010s. Recent technological breakthroughs, particularly in direct electron detectors and image processing algorithms, have elevated Cryo-EM to achieve near-atomic resolution comparable to X-ray methods. This convergence creates an unprecedented opportunity for multimodal integration.
Historically, researchers have treated these techniques as separate methodologies, often using them sequentially rather than synergistically. The concept of multimodal Cryo-EM represents a paradigm shift toward simultaneous or complementary data acquisition and analysis, potentially yielding more comprehensive structural insights than either method alone.
The primary objective of multimodal Cryo-EM development is to create integrated systems capable of collecting both electron microscopy and X-ray diffraction data from identical or similar specimens, enabling multi-scale structural characterization from atomic to cellular levels. This approach aims to leverage the high-resolution capabilities of X-ray crystallography with the flexibility and contextual information provided by Cryo-EM.
Secondary goals include developing computational frameworks for data fusion, addressing sample preparation challenges for dual-modality imaging, and establishing standardized protocols for correlative analysis. The ultimate vision is to enable researchers to seamlessly transition between modalities within a single experimental workflow, maximizing information extraction from precious biological samples.
Current technological trends suggest continued improvements in detector sensitivity, computational processing power, and automation capabilities will further drive this integration. The field is moving toward comprehensive structural biology platforms that incorporate multiple imaging modalities, potentially including additional techniques such as tomography and spectroscopy.
The successful development of multimodal Cryo-EM systems would significantly impact drug discovery, vaccine development, and fundamental understanding of cellular machinery by providing unprecedented insights into macromolecular structure and dynamics across multiple spatial and temporal scales.
Market Applications and Demand Analysis for Multimodal Imaging
The market for multimodal imaging technologies combining cryo-electron microscopy (cryo-EM) and X-ray techniques is experiencing significant growth driven by increasing demand across multiple sectors. Pharmaceutical and biotechnology companies represent the largest market segment, with an expanding need for high-resolution structural biology tools to accelerate drug discovery and development processes. These companies are increasingly investing in multimodal imaging capabilities to enhance their understanding of protein-drug interactions and complex biological systems.
Academic and research institutions constitute another major market segment, where multimodal cryo-EM technologies are essential for advancing fundamental scientific understanding in structural biology, cell biology, and materials science. The ability to correlate information from both X-ray and electron imaging provides researchers with unprecedented insights into biological structures and functions.
Healthcare providers and diagnostic centers are emerging as a growing market for multimodal imaging applications, particularly for advanced disease research and personalized medicine approaches. The detailed structural information provided by combined X-ray and electron imaging techniques enables more precise characterization of disease mechanisms at the molecular level.
Contract research organizations (CROs) specializing in structural biology services represent a rapidly expanding market segment. These organizations are increasingly adopting multimodal cryo-EM technologies to offer comprehensive analytical services to pharmaceutical companies and research institutions that may not have in-house capabilities or expertise.
Geographically, North America currently dominates the market for multimodal cryo-EM technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing research investments in countries like China, Japan, and South Korea, along with the expansion of biotechnology and pharmaceutical sectors in these regions.
The demand for multimodal imaging is primarily driven by the need for higher resolution structural information that cannot be achieved through single-modality approaches. The complementary nature of X-ray and electron imaging techniques allows researchers to overcome limitations inherent to each individual method, providing more comprehensive structural insights.
Market analysis indicates that the integration of artificial intelligence and machine learning with multimodal imaging technologies is creating new opportunities for automated data analysis and interpretation, further driving market growth. Additionally, the increasing focus on structural vaccinology and therapeutic antibody development has created specialized niches within the market where multimodal cryo-EM technologies offer significant advantages over traditional structural biology approaches.
Academic and research institutions constitute another major market segment, where multimodal cryo-EM technologies are essential for advancing fundamental scientific understanding in structural biology, cell biology, and materials science. The ability to correlate information from both X-ray and electron imaging provides researchers with unprecedented insights into biological structures and functions.
Healthcare providers and diagnostic centers are emerging as a growing market for multimodal imaging applications, particularly for advanced disease research and personalized medicine approaches. The detailed structural information provided by combined X-ray and electron imaging techniques enables more precise characterization of disease mechanisms at the molecular level.
Contract research organizations (CROs) specializing in structural biology services represent a rapidly expanding market segment. These organizations are increasingly adopting multimodal cryo-EM technologies to offer comprehensive analytical services to pharmaceutical companies and research institutions that may not have in-house capabilities or expertise.
Geographically, North America currently dominates the market for multimodal cryo-EM technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing research investments in countries like China, Japan, and South Korea, along with the expansion of biotechnology and pharmaceutical sectors in these regions.
The demand for multimodal imaging is primarily driven by the need for higher resolution structural information that cannot be achieved through single-modality approaches. The complementary nature of X-ray and electron imaging techniques allows researchers to overcome limitations inherent to each individual method, providing more comprehensive structural insights.
Market analysis indicates that the integration of artificial intelligence and machine learning with multimodal imaging technologies is creating new opportunities for automated data analysis and interpretation, further driving market growth. Additionally, the increasing focus on structural vaccinology and therapeutic antibody development has created specialized niches within the market where multimodal cryo-EM technologies offer significant advantages over traditional structural biology approaches.
Current Technological Landscape and Challenges in Multimodal Cryo-EM
Multimodal Cryo-EM technology represents a significant advancement in structural biology, combining the strengths of both X-ray crystallography and electron microscopy. Currently, the global landscape shows rapid development with major research institutions in North America, Europe, and Asia investing heavily in this field. The integration of these complementary imaging modalities has demonstrated remarkable potential for resolving complex biological structures at unprecedented resolution.
The current technological implementation typically involves sequential or parallel acquisition of data from both X-ray and electron-based systems. Leading research facilities have developed specialized sample holders and transfer mechanisms that maintain cryogenic conditions while moving between different imaging platforms. Software integration for correlative analysis has also seen significant progress, with several commercial and open-source solutions emerging in recent years.
Despite these advances, significant challenges persist in the multimodal Cryo-EM field. Sample preparation remains a critical bottleneck, as specimens must withstand both imaging modalities without structural degradation. The vitrification process must be optimized to prevent ice crystal formation while ensuring compatibility with both X-ray and electron beam exposure. Additionally, the differential radiation damage mechanisms between X-rays and electrons complicate integrated analysis.
Data integration presents another major hurdle. The fundamentally different contrast mechanisms and resolution characteristics between X-ray and electron imaging create significant computational challenges for meaningful correlation. Current registration algorithms struggle with the multi-scale nature of the combined datasets, often requiring extensive manual intervention and expertise.
Hardware limitations also constrain wider adoption. Most existing systems require physical transfer between separate X-ray and electron microscopy instruments, introducing potential for sample damage and alignment errors. The few integrated systems available commercially come with prohibitive costs, limiting access primarily to elite research institutions.
Beam-induced sample damage represents perhaps the most fundamental physical constraint. Both imaging modalities inevitably cause radiation damage, albeit through different mechanisms. Managing the cumulative dose across both modalities requires sophisticated dose fractionation strategies that are still being optimized. The development of more radiation-resistant sample preparation techniques remains an active area of research.
Standardization issues further complicate the landscape, with different research groups employing varied protocols for data acquisition, processing, and interpretation. This hampers reproducibility and slows the establishment of best practices across the field. International collaborations are beginning to address this through consensus guidelines, though widespread adoption remains limited.
The current technological implementation typically involves sequential or parallel acquisition of data from both X-ray and electron-based systems. Leading research facilities have developed specialized sample holders and transfer mechanisms that maintain cryogenic conditions while moving between different imaging platforms. Software integration for correlative analysis has also seen significant progress, with several commercial and open-source solutions emerging in recent years.
Despite these advances, significant challenges persist in the multimodal Cryo-EM field. Sample preparation remains a critical bottleneck, as specimens must withstand both imaging modalities without structural degradation. The vitrification process must be optimized to prevent ice crystal formation while ensuring compatibility with both X-ray and electron beam exposure. Additionally, the differential radiation damage mechanisms between X-rays and electrons complicate integrated analysis.
Data integration presents another major hurdle. The fundamentally different contrast mechanisms and resolution characteristics between X-ray and electron imaging create significant computational challenges for meaningful correlation. Current registration algorithms struggle with the multi-scale nature of the combined datasets, often requiring extensive manual intervention and expertise.
Hardware limitations also constrain wider adoption. Most existing systems require physical transfer between separate X-ray and electron microscopy instruments, introducing potential for sample damage and alignment errors. The few integrated systems available commercially come with prohibitive costs, limiting access primarily to elite research institutions.
Beam-induced sample damage represents perhaps the most fundamental physical constraint. Both imaging modalities inevitably cause radiation damage, albeit through different mechanisms. Managing the cumulative dose across both modalities requires sophisticated dose fractionation strategies that are still being optimized. The development of more radiation-resistant sample preparation techniques remains an active area of research.
Standardization issues further complicate the landscape, with different research groups employing varied protocols for data acquisition, processing, and interpretation. This hampers reproducibility and slows the establishment of best practices across the field. International collaborations are beginning to address this through consensus guidelines, though widespread adoption remains limited.
Current Multimodal Integration Solutions and Workflows
01 Advanced image processing algorithms for Cryo-EM resolution enhancement
Various computational algorithms have been developed to enhance the resolution of cryo-electron microscopy images. These include machine learning approaches, deep neural networks, and specialized image processing techniques that can extract more detailed structural information from raw cryo-EM data. These algorithms help in noise reduction, contrast enhancement, and feature extraction, ultimately leading to higher resolution reconstructions of biological macromolecules.- Advanced image processing techniques for Cryo-EM resolution enhancement: Various computational methods are employed to enhance the resolution of cryo-electron microscopy images. These techniques include machine learning algorithms, deep neural networks, and specialized image processing frameworks that can extract more detailed structural information from raw cryo-EM data. These approaches help overcome limitations in traditional imaging by applying sophisticated mathematical transformations and filtering methods to improve signal-to-noise ratio and overall image clarity.
- Multimodal data integration for improved structural analysis: Integration of multiple imaging modalities and data sources enhances cryo-EM resolution by combining complementary information. This approach merges cryo-EM data with other structural biology techniques such as X-ray crystallography, NMR spectroscopy, or mass spectrometry. The multimodal integration allows for more comprehensive structural determination by leveraging the strengths of each technique while compensating for individual limitations, resulting in more accurate and higher resolution molecular models.
- Hardware innovations for high-resolution cryo-EM imaging: Technological advancements in cryo-EM hardware components significantly improve image resolution. These innovations include enhanced electron detectors with higher sensitivity and faster frame rates, improved electron optics systems, more stable sample stages, and advanced aberration correctors. Such hardware developments reduce beam-induced motion and specimen damage while increasing signal detection efficiency, ultimately enabling atomic or near-atomic resolution imaging of biological macromolecules.
- Sample preparation techniques for optimal cryo-EM resolution: Specialized sample preparation methods are crucial for achieving high-resolution cryo-EM imaging. These techniques focus on optimizing specimen vitrification, grid preparation, and ice thickness control to minimize background noise and sample degradation. Advanced approaches include the use of specialized grid materials, automated vitrification systems, and methods to ensure uniform ice distribution, all contributing to better preservation of the native structure of biological specimens and enhanced image quality.
- Real-time processing and visualization systems for cryo-EM data: Real-time processing and visualization systems enable immediate analysis and quality assessment of cryo-EM data during acquisition. These systems incorporate parallel computing architectures, GPU acceleration, and specialized software frameworks to process the massive datasets generated during cryo-EM sessions. By providing immediate feedback on image quality and resolution, these approaches allow researchers to optimize data collection parameters on-the-fly, resulting in more efficient use of microscope time and higher quality structural information.
02 Multimodal data integration techniques for improved resolution
Integration of multiple imaging modalities with cryo-EM data can significantly enhance structural resolution. By combining data from different sources such as X-ray crystallography, NMR spectroscopy, or light microscopy with cryo-EM, researchers can overcome limitations of individual techniques. These multimodal approaches provide complementary structural information, allowing for more complete and higher resolution reconstructions of biological specimens.Expand Specific Solutions03 Hardware innovations for high-resolution cryo-EM imaging
Technological advancements in cryo-EM hardware have significantly contributed to resolution improvements. These innovations include direct electron detectors with improved sensitivity, more stable specimen stages, better electron sources, and advanced optical systems. Hardware developments enable the collection of higher quality raw data with improved signal-to-noise ratios, which is fundamental for achieving atomic-level resolution in structural studies.Expand Specific Solutions04 Sample preparation methods for enhanced cryo-EM resolution
Novel sample preparation techniques have been developed to improve cryo-EM image resolution. These methods focus on optimizing specimen vitrification, reducing beam-induced motion, minimizing ice thickness variations, and enhancing contrast. Advanced grid technologies, better vitrification protocols, and specialized sample supports contribute to preserving the native structure of biological specimens during imaging, resulting in higher resolution structural data.Expand Specific Solutions05 AI-driven approaches for cryo-EM image analysis and reconstruction
Artificial intelligence and deep learning methods are revolutionizing cryo-EM image processing and 3D reconstruction. These approaches include automated particle picking, classification, alignment, and density map improvement. AI systems can learn to recognize patterns in noisy cryo-EM data, extract meaningful features, and generate high-resolution reconstructions with reduced computational time. These methods are particularly valuable for processing large datasets and resolving heterogeneous structural states.Expand Specific Solutions
Leading Research Institutions and Equipment Manufacturers
Multimodal Cryo-EM technology, combining X-ray and electron imaging, is currently in an early growth phase with significant research momentum. The market is expanding rapidly, projected to reach substantial value as structural biology applications increase. Technologically, academic institutions lead fundamental research, with Wisconsin Alumni Research Foundation, University of California, and Peking University making significant contributions. Commercial development is advancing through established players like Koninklijke Philips, Siemens Healthineers, and FEI Co. (microscopy specialists), who are translating research into practical applications. Instrument manufacturers such as Direct Electron, Canon, and Bruker AXS are developing specialized hardware, while software companies like iTomography focus on computational aspects. This collaborative ecosystem between academia and industry is accelerating the technology toward broader adoption in pharmaceutical research and materials science.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced multimodal imaging solutions that combine X-ray and electron microscopy technologies for cryo-EM applications. Their platform integrates proprietary X-ray detectors with electron imaging systems to provide complementary structural information across different resolution scales. Philips' technology employs correlative workflows where samples are first examined using phase-contrast X-ray imaging to identify regions of interest before transitioning to high-resolution cryo-EM analysis. Their system incorporates specialized sample stages that maintain cryogenic conditions while allowing precise positioning between different imaging modalities. Philips has implemented advanced image processing algorithms that enable registration and fusion of data from X-ray and electron sources, creating comprehensive 3D reconstructions. Their technology utilizes energy-dispersive X-ray spectroscopy alongside electron imaging to provide elemental composition data in addition to structural information, offering researchers a more complete understanding of complex biological specimens[9][10].
Strengths: Extensive experience in medical imaging technologies; strong global support infrastructure; advanced detector technologies optimized for low-dose applications. Weaknesses: Less specialized in pure research applications compared to dedicated cryo-EM companies; integration between modalities still faces technical challenges; requires substantial facility infrastructure.
FEI Co.
Technical Solution: FEI Co. (now part of Thermo Fisher Scientific) has developed advanced multimodal cryo-EM solutions that integrate X-ray and electron imaging technologies. Their Titan Krios platform incorporates correlative light and electron microscopy (CLEM) capabilities, allowing researchers to first identify regions of interest using fluorescence microscopy before transitioning to high-resolution cryo-EM imaging. FEI's technology enables seamless workflow integration between X-ray tomography for larger-scale structural information and cryo-EM for atomic-level details. Their Direct Electron Detection Device (DED) technology significantly improves signal-to-noise ratios in electron imaging, while their automated data collection software (EPU) optimizes the acquisition process across multiple imaging modalities. FEI has also pioneered energy-filtered TEM integration with X-ray analysis, providing complementary elemental composition data alongside structural information[1][2].
Strengths: Industry-leading integration of multiple imaging technologies; exceptional resolution capabilities; comprehensive workflow solutions from sample preparation to image processing. Weaknesses: High equipment costs limit accessibility; complex systems require specialized training; integration between different imaging modalities still faces technical challenges in perfect alignment and calibration.
Key Technical Innovations in X-Ray and Electron Correlation
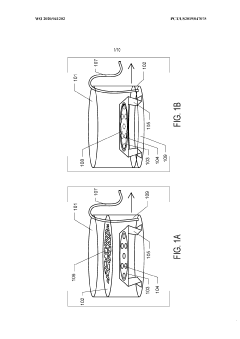
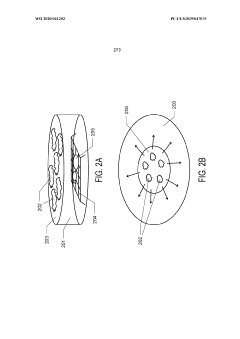
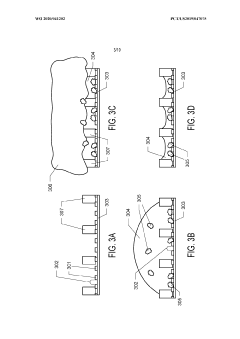
Thin-ice grid assembly for cryo-electron microscopy
PatentInactiveUS20160351374A1
Innovation
- A grid assembly for cryo-EM is developed, comprising two support members with electron-transparent layers and a rigid spacer layer, allowing precise control of ice thickness between them, enabling consistent vitrification and efficient imaging.
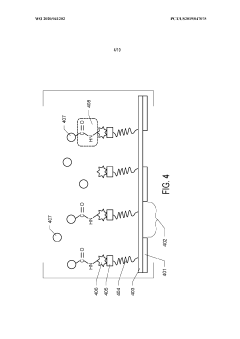
Graphene oxide affinity sample grids for cryo-em
PatentWO2020041202A1
Innovation
- The use of graphene oxide affinity grids with functionalized surfaces, including polyethylene glycol spacers, to immobilize samples away from the air-water interface and substrate interactions, enabling diverse target species capture and storage, and facilitating high-quality sample preparation for cryo-EM imaging.
Data Processing and Computational Methods for Multimodal Integration
The integration of multimodal cryo-EM data presents significant computational challenges that require sophisticated data processing methodologies. Current approaches focus on developing algorithms capable of handling heterogeneous data types from X-ray crystallography and electron microscopy simultaneously. These methods typically employ Bayesian statistical frameworks to combine structural information across modalities while accounting for different resolution scales and noise characteristics.
Key computational techniques include cross-modality registration algorithms that align X-ray diffraction patterns with electron microscopy images through feature detection and transformation estimation. These registration processes often utilize machine learning approaches such as convolutional neural networks to identify corresponding structural elements across imaging modalities despite their different contrast mechanisms and resolution profiles.
Data fusion techniques represent another critical component, where wavelet-based multi-resolution analysis enables the integration of high-resolution X-ray data with the contextual information provided by cryo-EM. Recent advances in this area have implemented tensor decomposition methods that preserve the unique characteristics of each modality while generating a unified structural representation.
Noise reduction presents a particular challenge in multimodal integration, as each imaging technique introduces distinct artifacts. Adaptive filtering algorithms specifically designed for multimodal data have emerged, employing modality-specific noise models to selectively enhance signal quality without compromising structural details. These approaches typically leverage the complementary nature of the noise distributions across modalities.
Computational efficiency remains a significant concern due to the massive datasets generated in multimodal imaging. Distributed computing frameworks have been developed to parallelize processing across high-performance computing clusters, with specialized data structures optimized for the sparse representation of structural information. Cloud-based solutions have gained popularity, offering scalable resources for the intensive computational requirements of multimodal reconstruction.
Validation methodologies for multimodal integration have evolved to include cross-validation techniques where portions of data from one modality are used to verify reconstructions primarily based on another. Statistical confidence metrics have been developed to quantify uncertainty in the integrated models, providing researchers with reliability assessments for different regions of the reconstructed structures.
Open-source software platforms such as RELION-X and cryoSPARC have recently incorporated multimodal processing capabilities, though these implementations remain in early stages. The computational community continues to work toward standardized formats and protocols for multimodal data exchange, which will be essential for broader adoption of these techniques across structural biology research.
Key computational techniques include cross-modality registration algorithms that align X-ray diffraction patterns with electron microscopy images through feature detection and transformation estimation. These registration processes often utilize machine learning approaches such as convolutional neural networks to identify corresponding structural elements across imaging modalities despite their different contrast mechanisms and resolution profiles.
Data fusion techniques represent another critical component, where wavelet-based multi-resolution analysis enables the integration of high-resolution X-ray data with the contextual information provided by cryo-EM. Recent advances in this area have implemented tensor decomposition methods that preserve the unique characteristics of each modality while generating a unified structural representation.
Noise reduction presents a particular challenge in multimodal integration, as each imaging technique introduces distinct artifacts. Adaptive filtering algorithms specifically designed for multimodal data have emerged, employing modality-specific noise models to selectively enhance signal quality without compromising structural details. These approaches typically leverage the complementary nature of the noise distributions across modalities.
Computational efficiency remains a significant concern due to the massive datasets generated in multimodal imaging. Distributed computing frameworks have been developed to parallelize processing across high-performance computing clusters, with specialized data structures optimized for the sparse representation of structural information. Cloud-based solutions have gained popularity, offering scalable resources for the intensive computational requirements of multimodal reconstruction.
Validation methodologies for multimodal integration have evolved to include cross-validation techniques where portions of data from one modality are used to verify reconstructions primarily based on another. Statistical confidence metrics have been developed to quantify uncertainty in the integrated models, providing researchers with reliability assessments for different regions of the reconstructed structures.
Open-source software platforms such as RELION-X and cryoSPARC have recently incorporated multimodal processing capabilities, though these implementations remain in early stages. The computational community continues to work toward standardized formats and protocols for multimodal data exchange, which will be essential for broader adoption of these techniques across structural biology research.
Sample Preparation Advancements for Dual-Modality Imaging
Sample preparation techniques for multimodal cryo-electron microscopy have evolved significantly in recent years, addressing the unique challenges of preparing specimens suitable for both X-ray and electron imaging modalities. Traditional sample preparation methods often optimize for a single imaging technique, creating incompatibilities when attempting dual-modality approaches. The advancement of these techniques represents a critical enabling factor for the successful integration of X-ray and electron microscopy data.
Recent innovations have focused on developing vitrification protocols that maintain sample integrity across both imaging platforms. Modified plunge-freezing techniques now incorporate specialized grids with fiducial markers visible in both modalities, allowing for precise correlation between datasets. These markers, typically gold nanoparticles or quantum dots, provide spatial registration points that facilitate computational alignment of the different imaging datasets.
Cryo-lamella preparation has emerged as a particularly promising approach for multimodal imaging. Using focused ion beam (FIB) milling, researchers can now prepare thin sections of vitrified samples that are optimized for both X-ray tomography and electron microscopy. The thickness control afforded by FIB milling allows for samples that balance the penetration requirements of X-rays with the resolution capabilities of electrons.
Environmental control during sample transfer between instruments represents another area of significant advancement. Specialized transfer systems now maintain samples at cryogenic temperatures throughout the imaging workflow, preventing devitrification and structural alterations that would compromise data quality. These systems incorporate vacuum-insulated chambers and automated handling mechanisms to minimize exposure to environmental contaminants.
Contrast enhancement strategies have been developed specifically for multimodal applications. Selective staining protocols now utilize compounds that provide differential contrast in X-ray and electron imaging, enhancing complementary features in each modality. Phase-contrast agents that function effectively across both techniques have shown particular promise in visualizing low-density cellular components.
Time-resolved sample preparation techniques represent the cutting edge of multimodal cryo-EM. These methods synchronize rapid freezing with dynamic cellular processes, allowing researchers to capture transient states for subsequent multi-technique analysis. Microfluidic platforms integrated with flash-freezing capabilities have enabled millisecond-scale temporal resolution while maintaining sample compatibility with both imaging modalities.
The standardization of these preparation protocols remains an ongoing challenge, with efforts underway to establish reproducible workflows that can be widely adopted across research facilities. Collaborative initiatives between microscopy centers are working to develop best practices and quality control metrics specific to multimodal sample preparation.
Recent innovations have focused on developing vitrification protocols that maintain sample integrity across both imaging platforms. Modified plunge-freezing techniques now incorporate specialized grids with fiducial markers visible in both modalities, allowing for precise correlation between datasets. These markers, typically gold nanoparticles or quantum dots, provide spatial registration points that facilitate computational alignment of the different imaging datasets.
Cryo-lamella preparation has emerged as a particularly promising approach for multimodal imaging. Using focused ion beam (FIB) milling, researchers can now prepare thin sections of vitrified samples that are optimized for both X-ray tomography and electron microscopy. The thickness control afforded by FIB milling allows for samples that balance the penetration requirements of X-rays with the resolution capabilities of electrons.
Environmental control during sample transfer between instruments represents another area of significant advancement. Specialized transfer systems now maintain samples at cryogenic temperatures throughout the imaging workflow, preventing devitrification and structural alterations that would compromise data quality. These systems incorporate vacuum-insulated chambers and automated handling mechanisms to minimize exposure to environmental contaminants.
Contrast enhancement strategies have been developed specifically for multimodal applications. Selective staining protocols now utilize compounds that provide differential contrast in X-ray and electron imaging, enhancing complementary features in each modality. Phase-contrast agents that function effectively across both techniques have shown particular promise in visualizing low-density cellular components.
Time-resolved sample preparation techniques represent the cutting edge of multimodal cryo-EM. These methods synchronize rapid freezing with dynamic cellular processes, allowing researchers to capture transient states for subsequent multi-technique analysis. Microfluidic platforms integrated with flash-freezing capabilities have enabled millisecond-scale temporal resolution while maintaining sample compatibility with both imaging modalities.
The standardization of these preparation protocols remains an ongoing challenge, with efforts underway to establish reproducible workflows that can be widely adopted across research facilities. Collaborative initiatives between microscopy centers are working to develop best practices and quality control metrics specific to multimodal sample preparation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!